Abstract
Abstract 2047
Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare acquired clonal hematopoietic stem cell disease. Patients may present with intermittent hemolysis, thrombotic events or cytopenias. The manifestations can be life threatening. Hematopoietic Cell Transplantation (HCT) is still the only curative therapy. Hegenbart et al reported early results in 7 patients with PNH with high risk features who received transplants from matched related (n=2) or unrelated donors (n=5) after conditioning with a fludarabine / total body irradiation (TBI) based regimen (Biol Blood Marrow Transplant 2003; 9: 689–697). Here we update the data and report outcomes in 12 additional patients.
Nineteen patients diagnosed with severe PNH underwent allogeneic HCT after a reduced intensity conditioning (RIC) regimen. Patients were treated at 9 centers. Eighteen patients had at least 1 high-risk feature (Table), 1 patient had none but underwent transplant because of severe hemolysis prior to the era of eculizumab. Conditioning consisted of fludarabine (30 mg/m2/d) on days −4 to −2 before HCT and 2 Gy (n=16) or 4 Gy (n=3) of TBI on day 0 (n=16) followed by treatment with mycophenolate mofetil and cyclosporine or tacrolimus. All patients received peripheral blood stem cells (PBSC). Donors were HLA-matched related (n=3), HLA-matched unrelated (n=12) or HLA-mismatched unrelated (n=4).
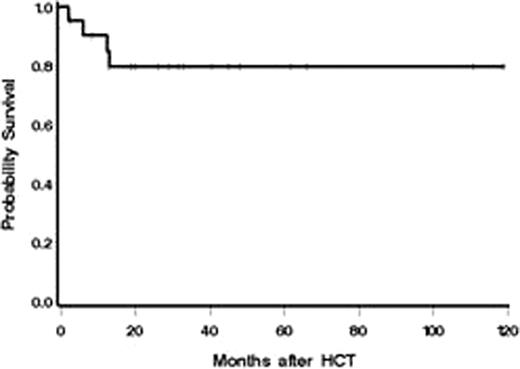
The follow-up period ranges between 2 months and almost 10 years (median 27.5 months) after HCT. Neutropenia (absolute neutrophil count<500/μl) developed in 15 of 19 patients after transplantation. The median time to neutrophil recovery in patients who engrafted was 16 days (range 0–29) with a median duration of neutropenia of 12 days (range 0–17, n=12). The median duration of thrombocytopenia (platelet count <20,000/μl) was 3 days (range, 0 to 10 days, n=12). All but 2 patients (89.5%) had a sustained engraftment. One patient experienced primary graft failure and remained neutropenic. He received a second nonmyeloablative HLA-matched unrelated PBSC transplant, 47 days after the first transplant, from a different donor, with 3 Gy TBI and 90mg/m2 fludarabine, and had sustained engraftment. The second patient experienced after engraftment, late graft failure 6 months after HCT. She underwent, 7 months after the first transplant, a second nonmyeloablative, HLA- matched unrelated PBSC transplant, from a different donor, with 4 Gy TBI and 150mg/m2 fludarabine, and had sustained engraftment. Eighteen patients were assessable for acute graft-versus-host disease (GvHD). The incidence of grades 2, 3, and 4 acute GvHD were 22% (4/18), 17% (3/18), and 5% (1/18), respectively. Chronic GvHD developed in 78% (14/18) of evaluable patients. Fifteen patients (78.9%) are alive at the time of last follow-up (Fig.). None of the 15 patients still alive has any evidence of relapse, clinically nor by flow cytometry. Four patients died (21.1%) of complications (necrotizing pancreatitis of unknown cause, pseudomonas infection during treatment of chronic GvHD, hemorrhage after liver biopsy with subacute/chronic liver GvHD, intracerebral bleed). All 15 surviving patients have an ECOG performance status of 0 or 1.
Overall survival
The RIC regimen reported here is well tolerated and leads to a high rate of successful engraftment. The graft-versus-host effect seems to be sufficient to eradicate the PNH clone. This regimen may be equally effective in medically fit PNH patients to eradicate the PNH clone while reducing treatment related complications of higher intensity conditioning regimens.
1st 3 authors contributed equally.
Patient and disease characteristics
| Characteristic . | Number (n=19) . | . | Percentage . |
|---|---|---|---|
| Sex | |||
| Male | 5 | 26 | |
| Female | 14 | 74 | |
| Age, years | |||
| Median | 34 | ||
| Range | 19–67 | ||
| Number of high risk features1 | |||
| 0 | 1 | 5 | |
| 1 | 10 | 63 | |
| 2 | 7 | 26 | |
| 3 | 1 | 5 | |
| Thrombosis | |||
| Yes | 10 | 47 | |
| No | 9 | 53 | |
| Treatment prior to transplant | |||
| Steroids | 16 | 84 | |
| CSP | 8 | 42 | |
| ATG2 | 5 | 16 | |
| MMF | 1 | 5 | |
| Azathioprine | 1 | 5 | |
| Danazol | 1 | 5 | |
| IVIG3 | 1 | 5 | |
| Eculizumab | 2 | 10 | |
| Myelodysplasia | |||
| Yes | 6 | 26 | |
| No | 13 | 74 | |
| HCT-CI4 | |||
| 0-1 | 6 | ||
| 2 | 3 | ||
| 3+ | 6 | ||
| Not available | 4 |
| Characteristic . | Number (n=19) . | . | Percentage . |
|---|---|---|---|
| Sex | |||
| Male | 5 | 26 | |
| Female | 14 | 74 | |
| Age, years | |||
| Median | 34 | ||
| Range | 19–67 | ||
| Number of high risk features1 | |||
| 0 | 1 | 5 | |
| 1 | 10 | 63 | |
| 2 | 7 | 26 | |
| 3 | 1 | 5 | |
| Thrombosis | |||
| Yes | 10 | 47 | |
| No | 9 | 53 | |
| Treatment prior to transplant | |||
| Steroids | 16 | 84 | |
| CSP | 8 | 42 | |
| ATG2 | 5 | 16 | |
| MMF | 1 | 5 | |
| Azathioprine | 1 | 5 | |
| Danazol | 1 | 5 | |
| IVIG3 | 1 | 5 | |
| Eculizumab | 2 | 10 | |
| Myelodysplasia | |||
| Yes | 6 | 26 | |
| No | 13 | 74 | |
| HCT-CI4 | |||
| 0-1 | 6 | ||
| 2 | 3 | ||
| 3+ | 6 | ||
| Not available | 4 |
High risk features are occurrence of thrombosis (relative risk [RR] 10.2), evolution to pancytopenia (RR 5.5), myelodysplastic syndrome or acute leukemia (RR 19.1), age over 55 years at diagnosis (RR 4), more than one treatment (RR 2.1), and thrombocytopenia at diagnosis (RR 2.2).
Antithymocyte globulin.
Intravenous immunoglobulin.
Hematopoietic Cell Transplant-Comorbidity Index score.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal