Abstract
Abstract 2314
Venous thromboembolism (VTE) collectively describes the debilitating, painful and potentially fatal conditions of deep vein thrombosis (DVT) and pulmonary embolism (PE). High-risk surgical procedures can lead to VTE, and patients undergoing major orthopaedic surgery, such as total hip or knee replacement (THR and TKR, respectively), are in the highest risk category for VTE. In the absence of anticoagulant prophylaxis the estimated incidence of DVT following orthopaedic surgery ranges from 40–60%, and the overall risk of fatal PE has been estimated to be between 0.2 and 0.9%. Thromboprophylaxis, both mechanical and pharmacological, is current standard practice for the prevention of VTE in patients undergoing orthopaedic surgery.Currently available anticoagulant therapies such as low molecular weight heparins (LMWHs), which are most commonly used, fondaparinux, and warfarin have demonstrated efficacy but have a number of limitations. LMWHs and fondaparinux require parenteral administration and warfarin has a narrow therapeutic window which is difficult to attain.
Apixaban, rivaroxaban and dabigatran are new anti-coagulants for thromboprophylaxis after orthopaedic surgery and have the advantages of oral administration and no requirement for routine laboratory monitoring. We compared the efficacy and safety of apixaban versus other anti-coagulants for the prevention of VTE following total hip replacement and total knee replacement surgery.
We systematically searched MEDLINE, EMBASE, the Cochrane library and CINAHL up to July 2010 for randomised controlled trials (RCTs) evaluating apixaban, rivaroxaban, dabigatran, fondaparinux and low molecular weight heparins at European licensed doses. A series of direct and indirect comparisons and a network meta-analysis (NMA) were performed where there were sufficient data for analysis, using enoxaparin as the common control.
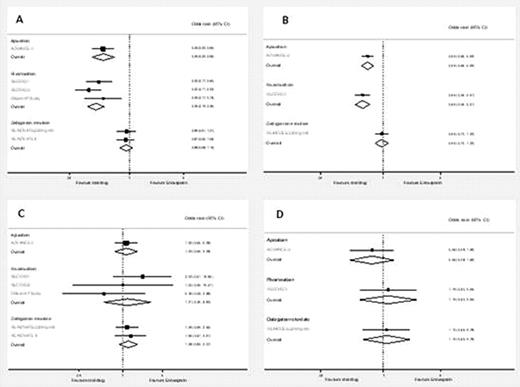
Indirect comparisons found that dabigatran 220mg od was significantly less efficacious than apixaban 2.5mg bd for the prevention of all VTE and all-cause death in THR patients (OR 2.51; 95% CI 1.50–4.21), and in TKR patients (OR 1.72; 95% CI 1.22–2.42). Rivaroxaban 10mg odwas slightly more effective than apixaban 2.5mg bd in both THR and TKR patients (OR 0.69; 95% CI 0.38–1.25, and OR 0.83; 95% CI 0.57–1.19, respectively), but the differences were not statistically significant. For the incidence of major bleeding the adjusted indirect comparison found that dabigatran 220mg od and rivaroxaban 10mg od had higher, but not statistically significant, bleeding rates compared with apixaban 2.5mg bd. Dabigatran versus apixaban: for THR patients OR 1.13; 95% CI 0.50–2.54 and for TKR patients OR 1.75; 95% CI 0.51–5.99. Rivaroxaban versus apixaban: for THR patients OR 2.48; 95%CI 0.44–13.8 and for TKR patients OR 1.86; 95% CI0.47–7.30. Trials comparing fondaparinux with enoxaparin were only available in THR patients, and for the outcomes any DVT and major bleeding. The adjusted indirect comparison found that fondaparinux 2.5mg od had higher but non-significant rates of any DVT (OR 1.29; 95% CI 0.69–2.43) and major bleeding (OR 1.22; 95% CI 0.56–2.67) compared with apixaban 2.5mg bd. The NMA showedno significant differences between the treatments for the outcomes evaluated.
Pooled estimates of the results of randomised controlled trials comparing the effects of apixaban, rivaroxaban and dabigatran versus enoxaparin on; the composite of all VTE and all-cause death for patients undergoing (A) total hip replacement and (B) total knee replacement and; major bleeding for patients undergoing (C) total hip replacement and (D) total knee replacement
Pooled estimates of the results of randomised controlled trials comparing the effects of apixaban, rivaroxaban and dabigatran versus enoxaparin on; the composite of all VTE and all-cause death for patients undergoing (A) total hip replacement and (B) total knee replacement and; major bleeding for patients undergoing (C) total hip replacement and (D) total knee replacement
Cohen:Pfizer Ltd: Consultancy. Pieter:Pfizer/BMS: Employment. Marchant:Pfizer Ltd: Employment. Mitchell:Pfizer Ltd: Consultancy. Orme:Pfizer Ltd: Consultancy. Simon:BMS: Employment. Sutton:Pfizer Ltd: Consultancy. Rublee:Pfizer Ltd: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal