Abstract
Abstract 2407
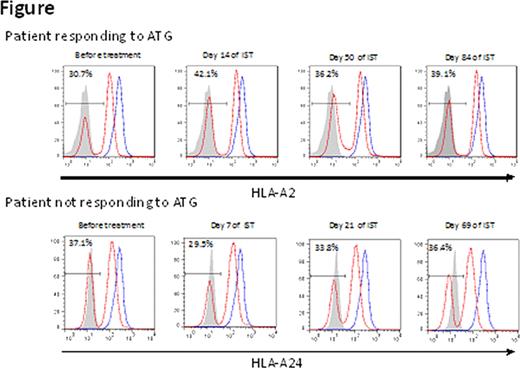
Hepatitis-associated aplastic anemia (HAA), a subset of acquired AA which accounts for 5% of all AA, is thought to have an immune-mediated pathogenesis. Although the attack by CD8+ T cells may be responsible for both hepatitis and bone marrow failure, the exact mechanisms underlying the development of HAA, as well as the reason for the time lag from its onset to that of hepatitis remain unclear. We recently found HLA-class I allelic loss from leukocytes due to copy-number neutral LOH in chromosome 6p (6pLOH) in 15% of AA patients, and identified four class I alleles (HLA-A*02:01, A*02:06, A*31:01, and B*40:02) that are overrepresented in AA, based on the finding that the missing HLA haplotype of 6pLOH(+) patients frequently contained one of the four alleles. Such immunologically-selected hematopoiesis caused by HLA allelic loss may be more common in patients with HAA than in non-HAA patients because HAA presents with homogenous pathophysiology, and a kinetic analysis of the HLA-missing leukocytes may help to understand the immune pathophysiology of HAA. Objectives/Methods: To gain insight into the immune pathophysiology of HAA, 17 patients with HAA (11 males and 6 females) aged 1 to 63 (median 23 years) whose 10 HLA allelic data were available were analyzed for this study. Blood samples from seven of the 17 patients were examined for the presence of 6pLOH and leukocytes lacking HLA-A antigens using Affymetrix® 500K SNP arrays and flow cytometry (FCM) using allele-specific monoclonal antibodies. For three HAA patients whose blood samples before therapy were available, peripheral blood was serially examined for the presence of HLA-A missing leukocytes. The frequency of high risk HLA alleles in HAA patients were compared to those of non-HAA patients and of 6,629 registries from the Japan Marrow Donor Program who had received an unrelated allogeneic bone marrow transplant. Results: 6pLOH was detected in 4 (57%) of the 7 HAA patients, which was more frequently than in non-HAA patients (12%, P=5.1 × 10−4). Granulocytes with unilateral HLA-A expression were detectable in all four patients at a frequency of 23% to 99%. The loss of HLA-A expression in the 6pLOH(+) cases was found in multiple lineages of leukocytes, including granulocytes, monocytes, B cells, and to a lesser extent, in T cells and NK cells. The missing HLA haplotypes of the 4 patients included at least one of the four high risk alleles. The frequency of patients possessing one or more of the high risk alleles was 77% in HAA patients, 64% in non-HAA patients, and 52% in the control population (P=0.03 for HAA vs. control; P=0.25 for HAA vs. non-HAA; P=2.0 × 10−8 for AA vs. control). In one patient who developed severe hepatitis and AA simultaneously, HLA-A missing leukocytes were not detectable before ATG, but all granulocytes that appeared 16 days after ATG therapy lacked an HLA-A antigen. On the other hand, in two patients who developed AA 1 month and 2 months after the onset of hepatitis, HLA-missing leukocytes were detectable before therapy at a frequency of 31% and 37%. The percentages remained unchanged in both a patient who responded to ATG (Figure) and another non-responding patient. Conclusions: HLA allelic loss from leukocytes and the high risk class I alleles were more strongly associated with HAA than idiopathic AA, suggesting that cytotoxic lymphocyte (CTL) attack against hematopoietic stem cells (HSCs) is involved in the pathogenesis of HAA. A 2-step mechanism may explain the time lag between hepatitis and the onset of AA; CTLs simultaneously attack hepatocytes and HSCs, but it takes several weeks or months until the secondary immune response can suppress HSCs enough to develop pancytopenia, because subsequent cytokine secretion is needed to cause BM failure.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal