Abstract
Abstract 2707
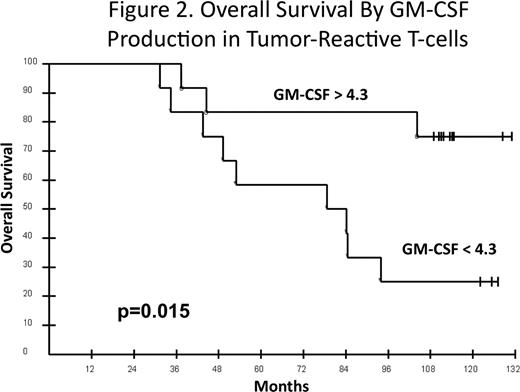
We hypothesized that immunotherapy with autologous tumor-derived idiotype (Id)-vaccine may improve the outcome of mantle cell lymphoma (MCL). Some murine lymphoma models have shown that Id-vaccine can induce an anti-tumor humoral response but others indicate that eradication of tumor requires a CD4+ and/or a CD8+ T-cell response. Antitumor T-cells may produce one or many cytokines. The Th1/Tc1 cytokines (IFNγ, IL-2, TNFα, GM-CSF) are commonly believed to mediate antitumor effects. However, a recent paper (Codarri et al. Nat Immunol 2011) proposes that production of GM-CSF by helper T-cells relies on activation of RORγt and that GM-CSF secretion is required for induction of autoimmune inflammation irrespective of helper T-cell polarization. We reported the results of Id-vaccine following DA-EPOCH-R in 26 untreated MCL patients (Neelapu et al Nat Med 2005) and found no association between PFS (19%) or OS (89%) and immune responses at the median of 46 months potential follow-up. We now present an 11-year follow-up and association between OS and antitumor immune responses. Study Design: DA-EPOCH-R was administered q3 weeks × 6, followed by 5 cycles of Id-vaccine beginning at least 12 weeks later to untreated MCL patients. Id protein was produced using hybridoma technology, conjugated to keyhole limpet hemocyanin (KLH), and administered together with GM-CSF × 5 over 6 months. Pre- and post-vaccine samples were tested in parallel to assess humoral and cellular immune responses. Anti-Id and anti-KLH antibody responses were determined by ELISA. KLH-specific cellular responses were determined by intracellular cytokine assay and cellular responses against autologous tumor cells were determined by cytokine induction and IFNγ ELISPOT assays. For cytokine induction assay, PBMCs were cultured with and without autologous tumor cells. After 6 days TNFα, IFNγ and GM-CSF were assessed in culture supernatants by ELISA. Normalized post-vaccine responses were calculated for each patient. Results: Characteristics of all 26 patients: median age 57 (r 22–73), PS 1 (0–2), male sex 73%, blastoid variant 15%, and MIPI (low-65%; intermediate-16%; high-19%). Responses to DA-EPOCH-R: CR-92%, PR-8%. Immune analyses were performed in 24 patients; vaccine could not be made in one patient and one patient progressed and did not have immune analyses. The associations between OS and MIPI scores and normalized immune responses (KLH and anti-Id antibody responses, frequency of KLH-specific CD4+ T-cell responses in PBMC (intracellular IL-2 and TNFα), antitumor cytokine responses and IFNγ ELISPOT) were determined. With 122 mos median potential follow-up (r 111–132), the median PFS is 24 mos and OS is 104 mos. MIPI was significantly associated with OS (Fig 1; p=0.01); median OS: low (not reached), intermediate (84 mos) and high (44 mos). There was no association between OS and KLH humoral response or KLH-specific CD4+ T cells. There was also no association between OS and Id-specific humoral response, IFNγ ELISPOT, or antitumor TNFα, or IFNγ cytokine responses. However, there was a significant association between antitumor GM-CSF production and OS (Fig 2). The median OS at the median GM-CSF normalized value (<4.3 versus >4.3) was 79 mos versus not reached, respectively (p=0.015 (unadjusted) and p=0.045 (bonferroni adjusted)). MIPI and GM-CSF were jointly assessed in a Cox model and showed a trend toward improved OS for higher GM-CSF (p=0.10) after adjusting for MIPI (p=0.20). Conclusions: With 10-year median potential follow-up, GM-CSF cytokine response mediated by antitumor T-cells was significantly associated with OS. Recent studies support the hypothesis that antitumor T-cells that produce significant amounts of GM-CSF are uniquely polarized and that non-GM-CSF producing T-cells do not induce antitumor effects even if they produce TNFα or IFNγ. This may explain why we did not observe an association between OS and TNFα or IFNγ cytokine responses or an anti-Id-antibody response. These results provide the first evidence that Id-vaccines may improve the survival of MCL following induction with immuno-chemotherapy and need to be confirmed in future trials.
Neelapu:Biovest International, Inc.: Research Funding. Kwak:Biovest International, Antigenics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Biovest International: Other remuneration; Antigenics, Xeme Biopharma: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal