Abstract
Abstract 3086
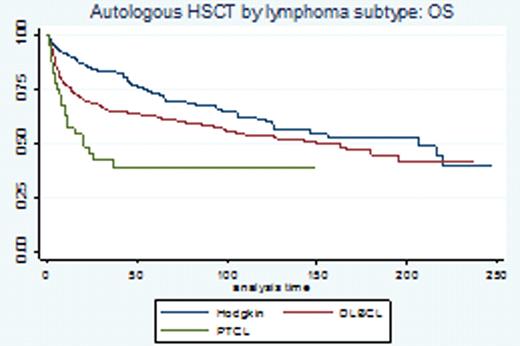
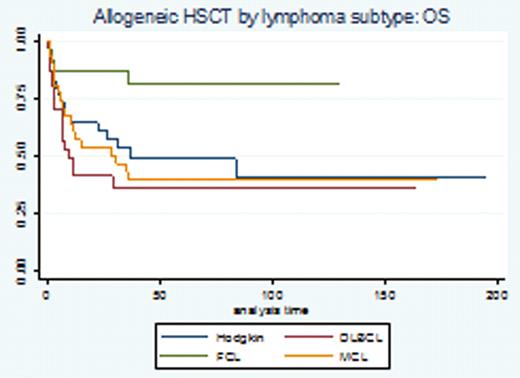
The use of high dose therapy (HDT) and autologous stem cell transplantation (ASCT) remains the standard of care in chemo-sensitive relapsed diffuse large cell lymphoma (DLBCL) and Hodgkin Lymphoma (HL). The use of chemoimmunotherapy (rituximab based combinations in B-cell NHL) and dose-dense/-intensive regimens in the frontline setting seems to affect our ability to salvage patients with HDT-ASCT after relapse. The development of non-myeloablative transplantation has allowed us to explore this modality in patients with relapsed NHL in spite of a typically more advanced median age at presentation. However the role and benefit of each modality (auto vs allo) remains controversial and long-term survival follow-up data with large consecutive series are needed. For this analysis we report the outcomes of 938 consecutive lymphoma patients who underwent hematopoietic stem cell transplantation at our center. Methods: Utilizing Kaplan Meier survival and Cox regression analyses, we conducted a retrospective cohort analysis to describe the survival experience of 938 lymphoma patients who underwent either autologous (n=770) or allogeneic (n=168) transplantation between 1990–2011. The primary study endpoint was OS assessed by chart review and SSDI database. Secondary endpoints: comparison of allogeneic stem cell source (PB vs. BM source), comparison of radioimmunotherapy (RIT) as pre-conditioning for ASCT in CD 20 (+) NHL, and comparison of donor types (allo sib vs. URD vs. mismatched URD). The proportional hazards assumption was met for this analysis. Results: Survival data on 938 patients (median age at transplant 51, range 17–77) were analyzed, representing 408 failure events and 60353 total months at risk. Baseline characteristics included: sex (57% male); ethnicity (82% Caucasian, 8% Hispanic, 7% African American); performance status (97% ECOG 0–1); diagnosis: DLBCL: 432 pts (46%), HD: 245 pts (26%), MCL: 94 pts (9%), FCL grI-II: 76 pts (8%), PTCL: 66 pts 7%, other subtypes 4%); allo donor (sib 49%, URD 31%, mismatched URD 20%), allo stem cell source (72% PB, 24% BM, 4% cord) and allo transplant intensity (51% myeloablative vs 49% non-myeloablative). Results: The median OS for patients who underwent ASCT was 149 months (52 months median follow up) and 39 months (16 months median follow up) for patients who underwent allogeneic transplant. OS KM curves by selected disease subtype are represented in figure 1a-b. We performed a COX regression analysis to address outlined secondary endpoints: in univariate analysis patients undergoing allogeneic transplantation with a mismatched unrelated donor had an inferior OS (p=.02 vs. matched sib donor). The OS was similar when comparing sib-matched allogeneic donors vs. MUD donors (HR 1.4, p=.14). No difference in OS was detected when comparing allogeneic stem cells harvested from peripheral blood vs. bone marrow source (HR.7, p=.1). For the subset of patients with CD20 (+) NHL undergoing autologous HSCT, no difference in OS was detected whether radio-immunotherapy (n=38) was administered immediately prior to HSCT (HR.6, p=.3).Conclusions: This is a large and consecutive series of lymphoma patients who underwent ASCT or allogeneic stem cell transplantation in the relapse setting. In the ASCT group: long term follow up results show continuous pattern of relapse and our ongoing studies are looking at consolidation/immune based maintenance strategies (lenalidomide) post transplantation to improve patient's outcome. In the allogeneic setting, a subset of patients with DLBCL, HL or MCL and FL can enjoy very long disease-free intervals and potentially be cured with no significant difference when comparing sib-matched allogeneic donors vs. matched URD (HR 1.4, p=.14). Our incidence of GVH has been dramatically reduced with the implementation of our donor TH2 amplification strategy (Blood Nov 2010; 116: 521) as recently shown.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal