Abstract
Abstract 36
Nephrotic syndrome (NS) is frequently accompanied by thromboembolic complications such as renal vein thrombosis, deep vein thrombosis and pulmonary embolism. However, the cause of the hypercoagulable state in NS patients is not understood. In contrast to quiescent cells, membrane-derived microparticles have exposed phosphatidylserine which may support procoagulant enzyme complexes in thrombosis.
The aim of this work was to measure the type and quantity of MPs that expose PS in NS patients, and to evaluate the associated procoagulant activity (PCA).
The subjects with membranous nephropathy (MN) or minimal change nephrotic syndrome (MCNS) were compared to healthy controls. Flow cytometry and confocal microscopy was used to evaluate microparticles. PCA was determined by clotting time and purified coagulation complex assays.
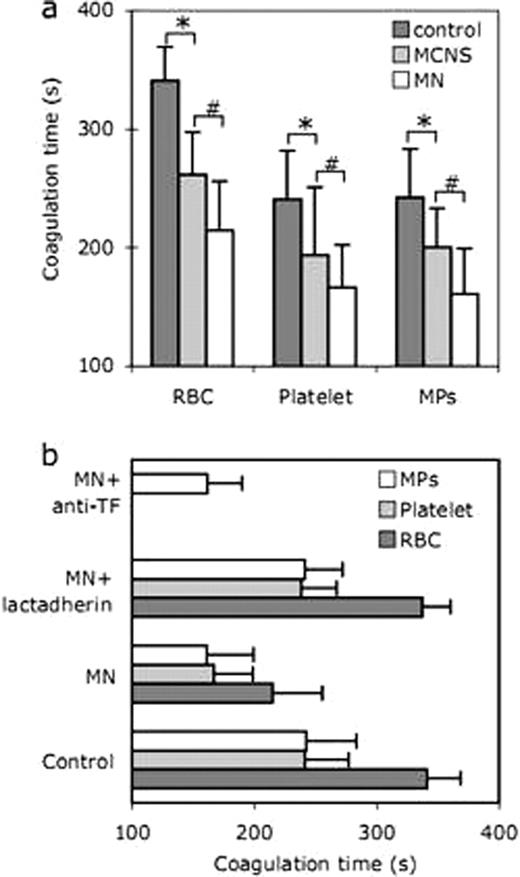
We found that the number of lactadherin+ MPs was significantly higher in each NS group MCNS (3230 ± 536)/MN (4642 ± 697) than that in the controls (1748 ± 239), furthermore, MPs in MN patients are significantly higher than ones in MCNS (P < 0.05; Table 1), which mostly derived from RBC and platelet membranes. The percentage of lactadherin+ RBCs was also significantly increased in each NS group, MN (9.7 ± 3.2%)/MCNS (5.1 ± 2.4%) patients compared to the controls (0.5 ± 0.2%). In addition, the mean percentage of lactadherin+ RBCs in MN showed significantly higher than that in MCNS (P < 0.05). In both NS groups, the percentage of lactadherin+ platelets was significantly higher than that in healthy control subjects (4.1 ± 1.1%) (P < 0.05 for both). Furthermore, patients in MN (11.5 ± 3.1%) also had significantly increased lactadherin+ platelets than in MCNS (7.3 ± 2.3%) (P < 0.05). By confocal laser-scanning microscope, nearly no staining by Alexa Fluro 488-lactadherin could be detected on RBCs or platelets membranes in healthy subjects, whereas a light green fluorescence on RBC and platelet with lactadherin accompanying vesiculation were observed in NS patients. PCA of RBCs/platelets from healthy individuals and NS patients was assessed by recalcification time assays, intrinsic, extrinsic FXa and prothrombinase assays. MPs shedding and PS exposure of RBCs/platelets were highly procoagulant in NS patients and blockade of PS with lactadherin inhibited over 90% of PCA. However, an anti-TF antibody had no significant inhibition effect (Figure 1).
Coagulation time and inhibition assay.(a) Coagulation times of RBCs/platelets/MPs in each group were evaluated. * and # indicate statistical significance (P < 0.05) from control and MN, respectively. (b) Coagulation times of RBCs/platelets/MPs of MN patients were detected in the absence or presence of 128 nM lactadherin or 40 μg/ml anti-TF. Data are displayed as mean ± SD for triplicate samples of independent experiments (n = 10). MPs: microparticles.
Coagulation time and inhibition assay.(a) Coagulation times of RBCs/platelets/MPs in each group were evaluated. * and # indicate statistical significance (P < 0.05) from control and MN, respectively. (b) Coagulation times of RBCs/platelets/MPs of MN patients were detected in the absence or presence of 128 nM lactadherin or 40 μg/ml anti-TF. Data are displayed as mean ± SD for triplicate samples of independent experiments (n = 10). MPs: microparticles.
MPs numbers per microliter of plasma in MCNS, MN and controls.
| . | Controls . | MCNS . | MN . |
|---|---|---|---|
| Total lactadherin+ MPs (/μl) | 1748 (1341–2014) | 3230* (2484–3819) | 4642*# (3499–5496) |
| Lactadherin+ CD41a+ | 1237 (944–1450) | 2363* (1888–2587) | 3470*# (2587–4144) |
| Lactadherin+ CD235a+ | 23 (16–32) | 174* (138–205) | 335*# (262–410) |
| Lactadherin+ CD142+ | 2.6 (0–4.2) | 2.7 (0–4.9) | 2.6 (0–6.8) |
| . | Controls . | MCNS . | MN . |
|---|---|---|---|
| Total lactadherin+ MPs (/μl) | 1748 (1341–2014) | 3230* (2484–3819) | 4642*# (3499–5496) |
| Lactadherin+ CD41a+ | 1237 (944–1450) | 2363* (1888–2587) | 3470*# (2587–4144) |
| Lactadherin+ CD235a+ | 23 (16–32) | 174* (138–205) | 335*# (262–410) |
| Lactadherin+ CD142+ | 2.6 (0–4.2) | 2.7 (0–4.9) | 2.6 (0–6.8) |
Corrected for the number of events with isotype controls,
P < 0.05 versus controls.
P < 0.05 versus MCNS.
This is the first study to show that loss of RBCs/platelets membrane phospholipid asymmetry with increased PS exposure and MPs release may contribute to the hypercoagulable state of NS patients. The extent of PS exposure on cells and MPs may be a marker of thromboembolic risk in these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal