Abstract
Abstract 3704
Obinutuzumab (GA101) is the only type II glycoengineered and humanized monoclonal anti-CD20 antibody to enter clinical trials. The efficacy and safety of GA101 in combination with cyclophosphamide/doxorubicin/vincristine/prednisone (G-CHOP) or fludarabine/cyclophosphamide (G-FC) in patients with CD20+ relapsed/refractory B-cell follicular lymphoma has been evaluated in an open-label, multicenter, randomized Phase Ib study (BO21000). Response rates were similar for G-CHOP (94%) and G-FC (93%) (Davies et al, EHA 2011). Studies in patients with CD20+ malignancies treated with GA101 monotherapy have suggested that responding patients eliminate GA101 more slowly than nonresponders (Meneses-Lorente et al, ASH 2010). Here we describe results from BO21000, exploring GA101 pharmacokinetics (PK), exposure and response in patients receiving concomitant chemotherapy.
Patients received 6–8 cycles of G-CHOP or 4–6 cycles of G-FC. The choice of chemotherapy and number of cycles were at the discretion of the investigator. After stratification by chemotherapy backbone, patients were randomized to receive one of two GA101 dosing regimens: 1,600 mg on Days 1 and 8 of Cycle 1, then 800 mg on Day 1 of subsequent cycles (1,600/800 mg; G-CHOP, n=14; G-FC, n=14), or 400 mg on Days 1 and 8 of Cycle 1 and on Day 1 of subsequent cycles (400/400 mg; G-CHOP, n=14; G-FC, n=14). G-CHOP or G-FC were administered every 21 or 28 days, respectively. Serum samples were taken prior to and directly after each infusion, for all treatment cycles, between Days 1 and 21 of Cycle 1 (G-CHOP) and Days 1 and 28 of Cycle 1 (G-FC), and for several days (up to 32) after the final cycle. Serum levels of GA101 were measured by ELISA.
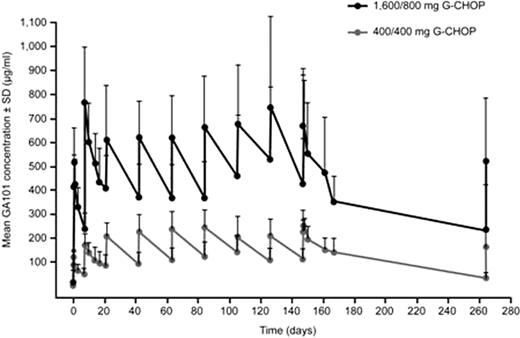
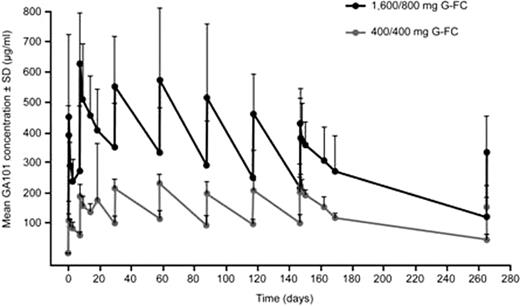
Similar approximate Cmax values of 580 and 600 μg/ml in the 1,600/800 mg G-FC and G-CHOP cohorts, respectively, were seen in Cycle 3; by the end of treatment these were approximately 400 μg/ml (Cycle 6, G-FC) and 675 μg/ml (Cycle 8, G-CHOP). Treatment cycle duration for G-CHOP was shorter than for G-FC; thus, lower GA101 serum concentrations in the G-FC cohort would be anticipated. In contrast, Cmax and Cmin values for the 400/400 mg cohorts were similar for both treatment arms, being around 200 μg/ml and 100 μg/ml, respectively.
GA101 serum concentrations in patients with complete response and partial response (1,600/800 mg G-CHOP)
GA101 serum concentrations in patients with complete response and partial response (1,600/800 mg G-CHOP)
In conclusion, GA101 serum concentrations were higher in the 1,600/800 mg cohorts compared with the 400/400 mg cohorts when dosed with either G-CHOP or G-FC. Furthermore, GA101 serum concentrations appeared to be higher in the 1,600/800 mg G-CHOP cohort compared with the 1,600/800 mg G-FC cohort. Response data indicate that patients with the best clinical response have higher GA101 serum concentrations and appear to eliminate GA101 more slowly than other patients. This is consistent with observations from monotherapy studies and may reflect the impact of shrinking tumor burden upon the clearance of GA101, which will be subject to further analyses.
Carlile:Roche: Employment. Meneses-Lorente:Roche: Employment. Wassner-Fritsch:Roche: Employment. Hourcade-Potelleret:Roche: Employment. Wenger:Roche: Employment. Cartron:Roche: Consultancy, Honoraria. Vitolo:Roche: Membership on an entity's Board of Directors or advisory committees. Morschhauser:Roche: Honoraria; Celgene: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal