Abstract
Abstract 4472
High dose melphalan with autologous stem cell transplant (ASCT) is a standard treatment for patients with multiple myeloma (MM) and AL amyloidosis. A standard part of the pre stem cell harvest (SCH)/SCT evaluation at most medical centers is a battery of infection screening tests. While the reactivation of hepatitis B with rituximab is well described, there are limited data on the outcomes or significance of abnormal test results in non-endemic areas in the context of ASCT. Moreover, it is unclear whether patients with plasma cell disorders (PCDs), with their dysregulation of humoral immunity, may have differing rates of abnormal test results on antibody based testing than patients without PCDs. Indeed, one study found hepatitis B seroconversions more commonly with MM post ASCT (Uhm et al, ASBMT 2007). We therefore conducted this retrospective study to examine the outcomes of abnormal infection screening in patients with PCDs and non PCDs undergoing SCH/SCT in the New York Metropolitan area.
We reviewed 639 consecutive patients who had autologous hematopoietic stem cell harvests (SCH) done at Saint Vincent Hospital, NY from February, 2000 to March, 2010. Of these patients, 555 had PCDs including multiple myeloma and AL Amyloidosis. The 84 non PCDs included lymphoma and solid tumors patients were used as a comparator group.
We first reviewed the rate of positive infectious screening tests and found no difference between PCDs (55 out of 555, 9.9%) and non PCDs (9 out of 84, 10.7%). Of these patients with abnormal screening results, 40 and 7 patients respectively went on to ASCT. 11 patients had more than one infection screening test abnormality, making a total of 76 abnormal tests.
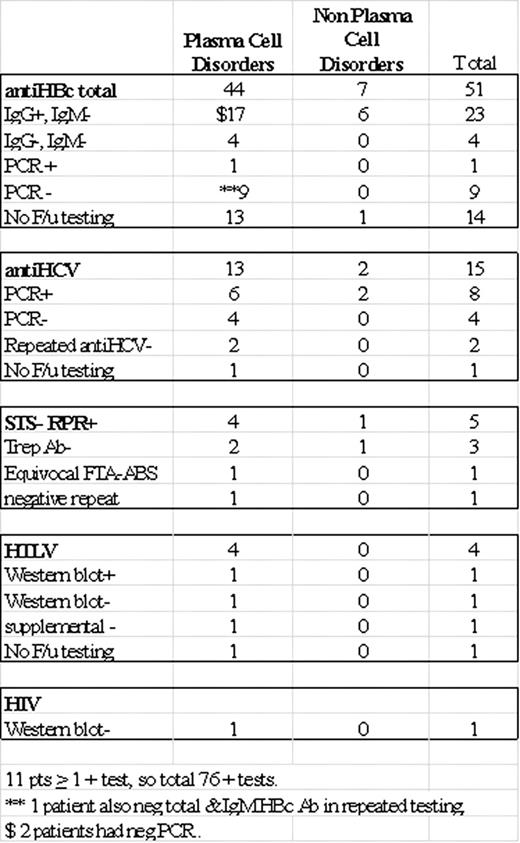
As shown in Table 1, 67.1% (51/76) were positive for Hep B core Ab, 19.7% (15/76) for Hepatitis C Ab, 6.5 % (5/76) for syphilis, 5.2 % (4/76) for HTLV and 1.3% (1/76) for HIV.
Amongst the 44 patients with PCDs and positive anti-HBc total, 39% (17) were positive antiHBc Ig G antibody but negative for IgM, representing chronic carriers. 9% (4) were negative by both antiHBc Ig M and IgG, which may represent false positive tests, and this pattern was not seen in any of the 7 patients with non PCDs. Of the PCD patients who had Hepatitis B PCR testing, 1 showed chronic active hepatitis and 9 had undetectable viral load. 68.5% of positive Hepatitis B screening patient went on to SCT (including the patient with PCR + but IgM was negative) regardless of the status of test results without any treatment for hepatitis B.
Of the 13 PCD patients with positive anti-HCV Ab screening, 46% (6) had active viral replication by PCR and 30% (4) had with undetectable viral load by PCR. 2 patients had negative repeat HCV testing. Only one patient with chronic active HCV infection (146,1200,000 IU/ml) by PCR got treatment by IFN and Ribavirin pretransplant and did not get ASCT. 66.6% of positive Hepatitis C screening patient went on to SCT.
Five patients had RBC transfusions during the year preceding infection screening (3 with positive antiHBc total antibody, 1 with HCV antibody and 1 with HTLV antibody). One patient had IVIG transfusion 7 days before screening. There appeared to be no relationship to abnormal screening tests and MM subtype or hypogammaglobulinemia.
Of those patients who went on to SCT, transient grade 1 transaminitis during transplant or by day 100 was found in 4 patients (6%) with positive hepatitis B screening. One patient had transient asymptomatic hepatomegaly. We observed no serious hepatic complications in chronic hepatitis B and C carriers without active disease and ASCT appears to be feasible and safe without any anti-viral treatment.
With the exception of a possible excess of false positive hepatitis B testing in patients with PCDs, there are no other differences in rates of abnormal infection screening tests between PCDs and nonPCDs. The use of more specific viral testing like PCR and Western blot or highly sensitive immunoassay should be considered to avoid false positive results in the analysis of antibody based tests to avoid both unnecessary delays of SCT and anti-viral treatment. In the absence of clinical hepatitis and active viral replication, treatment for chronic Hepatitis B and C carriers does not appear to be required preASCT.
Jagannath:Celgene: Membership on an entity’s Board of Directors or advisory committees; Merck: Membership on an entity’s Board of Directors or advisory committees; Millennium: Membership on an entity’s Board of Directors or advisory committees.
Screening Tests and Repeated or Confirmatory Testing Results in Patients with Plasma cell disorders and Non plasma cell disorders
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal