Abstract
Abstract 4708
Systemic inflammation is well appreciated to play a role in the pathogenesis of acute graft-versus-host disease (aGVHD). Recent studies have focused on homeostatic mechanisms that counteract inflammation and heal damaged tissues. These include the generation of lipoxins, resolvins, and protectins, eicosanoid molecules collectively referred to as the anti-inflammatory and pro-resolving lipid mediators. These lipid mediators have biologic activities known to antagonize mechanisms underlying the development of aGVHD, including downregulation of pro-inflammatory cytokines such as TNFα. Resolvins and protectins are derived from the Ω3-polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), respectively. Diets enriched for Ω3-PUFAs (high fish oil) are protective against inflammatory conditions, whereas “Western” diets with higher Ω6-PUFA promote inflammation. Furthermore, the anti-inflammatory properties of acetylsalicylic acid (ASA) are increasingly being attributed to its ability to enhance the generation of aspirin-triggered lipoxins, epimers of endogenously produced lipoxins.
We hypothesized that a diet enriched for Ω3-PUFAs, ASA ingestion or the injection of a purified aspirin triggered lipoxin would mitigate the development of aGVHD and provide a survival advantage in a major histocompatibility mismatched mouse model of aGVHD.
The C56BL/6→(C57BL/6 × DBA/2)F1-hybrid mouse model was used to induce lethal aGVHD. Five groups (n=10) were established and treated as follows:
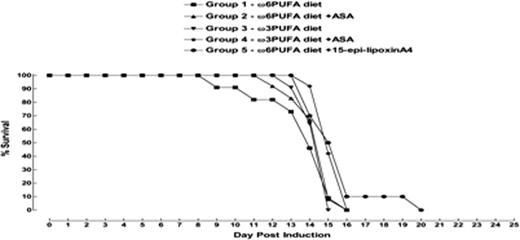
Group 1: Ω6 PUFA diet (control)
Group 2: Ω6 PUFA diet + ASA (0.02 mg ASA/g of feed)
Group 3: Ω3 PUFA diet
Group 4: Ω3 PUFA diet + ASA (0.02 mg ASA/g of feed)
Group 5: Ω6 diet + 15-epi-LxA4 (200μg/kg i.v. on day 0 and 200μg/kg i.p. on day +7)
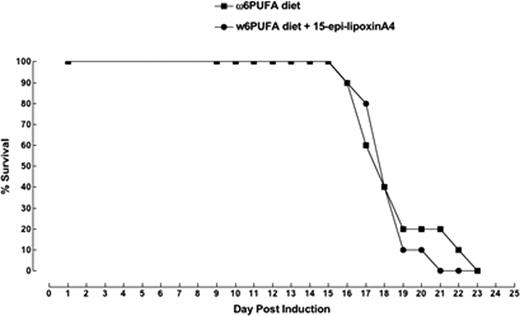
Recipients were fed the diets for 8-weeks before transplantation. The Ω6-diet (control) was enriched to 2% total FA with arachidonic acid (ARASCO, Martek). The Ω3-diet was enriched to 1% each of EPA (ROPUFA-30, DSM Nutritionals) and DHA (DHASCO, Martek). Chromatography was used to confirm the fatty acid (FA) composition of each diet, and the FA content (Ω6 and Ω3 tissues stores) of the mouse livers (n=4/diet) after 8-weeks of feeding. Diets were the same for each group pre- and post-transplant. Using identical methodology, a second experiment was performed in which the recipients were treated as follows: Ω6-diet plus 15-epi-LxA4 100 mcg/kg/day, i.p. daily from day 0 until death (n=10). Survival post-transplant was the primary outcome. Mice were euthanized when they showed signs of GVHD-associated morbidity and had reached their humane endpoint as defined in our animal protocol.
Compared to mice fed the Ω6 or Ω6 plus ASA diets, the Ω3 and Ω3 plus ASA diets resulted in a higher hepatic content of both EPA and DHA after 8-weeks of feeding, suggesting differential tissue stores of these Ω3-PUFAs immediately before transplant in the mice fed the Ω3-diets (mean EPA as % of total FA: 0.22% and 0.19% vs. 0.92% and 1.03%, respectively; mean DHA as % of total FA: 1.58% and 1.79% vs. 6.99% and 8.15%, respectively: p<0.05). No significant survival differences were seen post- transplant in groups 1–4 (figure 1). Similarly, mice injected with 200 mcg/kg/day of 15-epi-LxA4 at days 0 and 7 (Group 5) failed to show a significant survival advantage, although a non-significant trend towards improved survival by a couple of days was suggested (figure 1). Since no dosing strategy for 15-epi-LxA4 is known, we repeated the experiment using a lower (100 mcg/kg/day) but more frequent (daily) dosing of 15-epi-LxA4. No significant improvements in survival were observed compared to the control group (figure 2).
We were unable to demonstrate a significant improvement in survival in any of the treatment groups in this model of aGVHD. Possible reasons for this may include a suboptimal dosing strategy (frequency and/or cumulative dose) or the intense systemic inflammatory response that develops in this mouse model of acute GVHD. Alternatively, the treatments used in this study may be insufficient to have any therapeutic benefit on the relevant outcomes such as survival from aGVHD, when administered as stand-alone treatments. Since little is known about these interventions in transplantation, we continue to explore different combinations and dosing strategies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal