Abstract
Abstract 478
The US community-based, phase 3b randomized, open-label, multicenter UPFRONT trial compares the efficacy and safety of three bortezomib (VELCADE®, Vc)-based regimens, VcD (Vc-dexamethasone), VcTD (Vc-thalidomide-dexamethasone), and VcMP (Vc-melphalan-prednisone), followed by weekly Vc maintenance, in elderly, newly diagnosed, transplant-ineligible multiple myeloma (MM) patients. This is the first phase 3 study of VcD and VcTD in this patient population.
Patients with symptomatic, measurable MM were randomized (1:1:1) to receive 49 weeks of therapy: 24 weeks (eight 21-day cycles) of induction with VcD, VcTD, or VcMP (VcD: Vc 1.3 mg/m2, days 1, 4, 8, 11; D 20 mg, days 1, 2, 4, 5, 8, 9, 11, 12 [cycles 1–4]), days 1, 2, 4, 5 [cycles 5–8]); VcTD: Vc as before; T 100 mg/day, days 1–21; D as before); VcMP: Vc as before; M 9 mg/m2 and P 60 mg/m2, days 1–4, every other cycle), followed by 25 weeks (five 35-day cycles) of maintenance with weekly Vc 1.6 mg/m2, days 1, 8, 15, 22. Patients in the VcTD arm received concomitant prophylaxis with aspirin, full-dose warfarin, or low-molecular weight heparin unless medically contraindicated. The primary endpoint was progression-free survival (PFS); secondary endpoints included overall response rate (ORR), complete response (CR)/near CR (nCR) and very good partial response (VGPR) rates, overall survival (OS), and safety. Best confirmed responses were assessed by investigators per modified International Myeloma Working Group (IMWG) criteria. Adverse events (AEs) were graded by NCI-CTCAE v3.0. PFS and OS were estimated by Kaplan–Meier methodology. For the first time, we report results from the entire cohort of 502 randomized patients (VcD, n=168; VcTD, n=167; VcMP, n=167), who completed up to a maximum of 13 cycles of treatment.
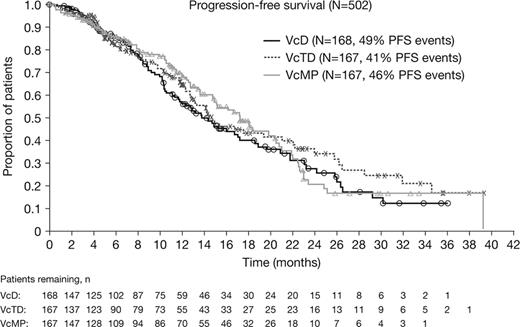
Patients in the VcD, VcTD, and VcMP arms had a median age of 74.5, 73.0, and 72.0 years, respectively, and 71%, 62%, and 72% had ISS stage II/III disease. Patients received a median of 8 (VcD), 6 (VcTD), and 7 (VcMP) treatment cycles; 50%, 38%, and 42% of patients, respectively, received Vc maintenance. Response and safety data are summarized in the table. All three Vc-based induction regimens exhibited substantial activity, with ORR of 73% (VcD), 80% (VcTD), and 69% (VcMP) during the treatment period. After a median follow-up of 21.8 months, no significant difference in PFS was observed between the treatment arms; median PFS was 13.8 months (VcD), 14.7 months (VcTD), and 17.3 months (VcMP), respectively (Figure). 1-year OS estimates were 87.4% (VcD), 86.1% (VcTD), and 88.9% (VcMP). Rates of grade ≥3 AEs, serious AEs (SAEs), and discontinuations due to AEs during the treatment period were highest for the VcTD arm. The most common grade ≥3 AEs across all three arms during the treatment period were neuropathy peripheral (23%), fatigue (10%), and diarrhea (9%). Grade ≥3 pneumonia was reported in 10% (VcD), 6% (VcTD), and 6% (VcMP) of patients. AEs of deep vein thrombosis/pulmonary embolism were reported in 8% (VcD), 7% (VcTD), and 2% (VcMP) of patients. Compared with rates during induction, Vc maintenance produced little additional toxicity; across all three treatment arms, only 5% of patients experienced grade ≥3 peripheral neuropathy during cycles 9–13. One second primary malignancy (lung neoplasm) was reported in the VcMP arm.
VcD, VcTD, and VcMP induction followed by weekly Vc maintenance produced similar activity in elderly, newly diagnosed, transplant-ineligible MM patients. Patients in the VcD doublet arm appear to have similar long-term outcomes to patients in the VcTD and VcMP triplet arms.
| . | VcD . | VcTD . | VcMP . | |||
|---|---|---|---|---|---|---|
| Best confirmed response rates during the treatment period | ||||||
| Response, % | n=146 | n=133 | n=144 | |||
| ORR (≥PR) | 73 | 80 | 69 | |||
| CR/nCR* | 30 | 40 | 33 | |||
| VGPR | 7 | 11 | 8 | |||
| PR | 36 | 29 | 29 | |||
| Stable disease | 15 | 2 | 15 | |||
| Progressive disease | 2 | <1 | 3 | |||
| Not evaluable | 10 | 17 | 12 | |||
| Safety profile during induction (I; cycles 1–8) and during maintenance (M; cycles 9–13)† | ||||||
| I (n=165) | M (n=82) | I (n=158) | M (n=60) | I (n=163) | M (n=69) | |
| AE, % | ||||||
| Grade ≥3 AEs | 74 | 9 | 84 | 8 | 82 | 3 |
| SAEs | 48 | 11 | 53 | 12 | 47 | 9 |
| Discontinuation of all study drugs due to AEs | 24 | 11 | 35 | 3 | 30 | 9 |
| Deaths (within 30 days of last dose) | 5 | 0 | 6 | 0 | 5 | 0 |
| . | VcD . | VcTD . | VcMP . | |||
|---|---|---|---|---|---|---|
| Best confirmed response rates during the treatment period | ||||||
| Response, % | n=146 | n=133 | n=144 | |||
| ORR (≥PR) | 73 | 80 | 69 | |||
| CR/nCR* | 30 | 40 | 33 | |||
| VGPR | 7 | 11 | 8 | |||
| PR | 36 | 29 | 29 | |||
| Stable disease | 15 | 2 | 15 | |||
| Progressive disease | 2 | <1 | 3 | |||
| Not evaluable | 10 | 17 | 12 | |||
| Safety profile during induction (I; cycles 1–8) and during maintenance (M; cycles 9–13)† | ||||||
| I (n=165) | M (n=82) | I (n=158) | M (n=60) | I (n=163) | M (n=69) | |
| AE, % | ||||||
| Grade ≥3 AEs | 74 | 9 | 84 | 8 | 82 | 3 |
| SAEs | 48 | 11 | 53 | 12 | 47 | 9 |
| Discontinuation of all study drugs due to AEs | 24 | 11 | 35 | 3 | 30 | 9 |
| Deaths (within 30 days of last dose) | 5 | 0 | 6 | 0 | 5 | 0 |
nCR includes patients IF+ as well as patients IF- but in whom confirmatory BM biopsy was not performed;
The M column represents first onset of an AE during maintenance
Niesvizky:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millennium Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Onyx: Research Funding. Flinn:Millennium Pharmaceuticals, Inc.: Research Funding. Rifkin:Celgene: Speakers Bureau; Amgen: Speakers Bureau; Onyx: Membership on an entity's Board of Directors or advisory committees; Millennium Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Charu:GSK: Research Funding; Celgene: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; Bristol-Myers Squibb: Equity Ownership; Pfizer: Equity Ownership. Neuwirth:Millennium Pharmaceuticals, Inc.: Employment. Corzo:Millennium Pharmaceuticals, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal