Abstract
Abstract 829
During pregnancy, transplacental traffic of cells occurs and maternal B and T cells may become primed against human leukocyte antigens (HLA) that the fetus inherited from its father (inherited paternal antigens - IPA). Maternal T cells recognize these IPA targets and peptides of IPA minor histocompatibilty antigens (mHA). Anti-IPA memory T cells can survive in the mother for decades and may account for the lower relapse rates and improved survival in maternal haplo-identical hematopoietic stem cell (HSC) transplants compared with paternal grafts (Stern, et al. Blood 2008;112:2990). IPA-primed microchimeric maternal cells in CB, when transplanted, may have a similar effect on relapse. We hypothesized that CB graft recipients who share the same antigen(s) as their CB donor IPAs should have a lower incidence of relapse (and possibly a higher risk of GVHD) due to the presence of the primed maternal cells, than recipients who have no antigens shared with their graft IPAs. Maternal cells in CB units that have no IPA target (homozygous or matched to the mother's HLA) should not be sensitized. Like CB transplants with no shared IPA, these grafts should also portend a high relapse risk.
We carried out a retrospective, observational study in 1,155 patients with hematological malignancies who received single unit CB grafts from NYBC between 1993–2006, including 453 with acute lymphoblastic leukemia (ALL), 392 with acute myelogenous leukemia (AML) and 310 with other hematological malignancies (mostly CML, MDS and lymphomas). Primary study endpoints were relapse and grade III–IV acute GVHD; secondary endpoints were engraftment, mortality and treatment failure (relapse or death).
CB grafts were classified by the presence or absence of an IPA target at HLA-A, -B (intermediate resolution) and DRB1 (high resolution). Maternal HLA typing was available, permitting inferred identification of CB IPAs. Patients were classified by whether or not they shared the same antigen(s) as the CB IPAs or received a unit with no IPA targets (both designated as “no shared IPA target” transplants), as well as by the number of HLA mismatches, mismatch direction and match to non-inherited maternal HLA (NIMA). An example of a CB graft with 1 shared IPA target is shown below:
| HLA . | A . | B . | DRB1 . |
|---|---|---|---|
| Mother | 2, 23 | 38, 62 | 11, 13 |
| CB Unit | 2, 24 | 35, 38 | 13:01, 13:01 |
| Patient | 2, 68 | 35, 58 | 13:01, 13:01 |
| HLA . | A . | B . | DRB1 . |
|---|---|---|---|
| Mother | 2, 23 | 38, 62 | 11, 13 |
| CB Unit | 2, 24 | 35, 38 | 13:01, 13:01 |
| Patient | 2, 68 | 35, 58 | 13:01, 13:01 |
(IPAs: A24, B35, DRB1*13:01)
In this case, the patient shares the CB IPA B35, but does not share IPA A24. Hence, the patient's B35 is a potential target for anti-IPA primed maternal T cells. There is no shared IPA target at the A locus and maternal T cells would not be primed to the patient's A68. The CB donor is homozygous for DRB1*13:01, provided by both mother and father. The mother, therefore, has no DRB1 IPA target in the CB donor's cells.
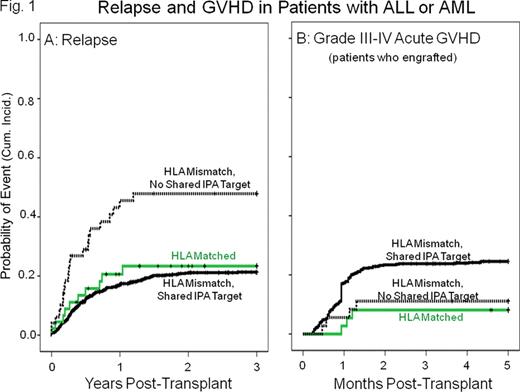
Results: Among 1,094 patients given HLA mismatched CB units, 1,030 shared antigens with one or more CB IPA targets and 64 (6%) had no shared IPA target. Transplants with a shared IPA target had a significantly lower incidence of relapse during the first 3 years post-transplantation compared to those that had no shared IPA target. This effect was detected in both ALL and AML (combined hazard ratio=0.4 in a multivariate analysis with other risk factors, P<0.001. Figure 1A), but not in patients with other malignancies. The lower relapse rate was strongest in patients with 1 HLA mismatch (HR=0.1, P<0.001) and was reflected in a lower treatment failure rate in this group (HR=0.5, P=0.012). There was a higher incidence of acute GVHD in patients with a shared IPA target, but the difference was not significant (Figure 1B). Maternal HLA match to the recipient (a control for possible effects of unprimed maternal cells) was not associated with relapse risk.
Our study found a significantly lower relapse rate in CB transplant recipients with AML or ALL who shared HLA with one or more of their CB donor IPA targets, providing strong indirect evidence that the relapse reducing effect is mediated by microchimerism with IPA-primed maternal cells. Expanding CB unit matching criteria to include identification of IPA targets (achieved by maternal HLA typing) and their presence in potential transplant recipients, should further improve CB unit selection for transplantation. An intriguing implication of our observation is the possibility that maternal chimerism might play a role in cancer surveillance in general.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal