Abstract
Asparaginase is a standard and critical component in the therapy of childhood acute lymphoblastic leukemia. Asparagine synthetase (ASNS) and the basic region leucine zipper activating transcription factor 5 (ATF5) and arginosuccinate synthase 1 (ASS1) have been shown to mediate the antileukemic effect of asparaginase and to display variable expression between leukemia cells that are resistant and sensitive to treatment. Fourteen polymorphisms in the regulatory and coding regions of these genes were investigated for an association with acute lymphoblastic leukemia outcome. Lower event-free survival (EFS) was associated with ATF5 T1562C, tandem-repeat ASNS polymorphism, derived haplotype, and ASS1 G1343T and G34T substitutions (P ≤ .03). Associations were limited to patients who received Escherichia coli asparaginase. Variations that sustained correction for multiple testing (ATF5 T1562C, P = .005; ASNS tandem-repeat and related haplotype, P ≤ .01) were subsequently analyzed in the replication cohort. The E coli–dependent association of the ATF5 T1562 allele with reduced EFS was confirmed (P = .01). A gene-reporter assay showed that the haplotype tagged by T1562 had higher promoter activity (P ≤ .01). The remaining regulatory polymorphisms also appeared to affect ATF5 function; 2 additional high-activity haplotypes were identified (P ≤ .02) and were further corroborated by quantitative mRNA analysis in lymphoblastoid cell lines. The ATF5-regulated increase in ASNS expression in response to more efficacious E coli–induced asparagine depletion may explain our observed results.
Introduction
Acute lymphoblastic leukemia (ALL) is the most frequent malignancy of childhood. The introduction of multiagent treatment protocols has led to a remarkable increase in overall survival (OS) and event-free survival (EFS). Nevertheless, for a subpopulation of patients, resistance to the chemotherapeutics used within these protocols remains an obstacle to successful treatment. Asparaginase is a standard component in the therapy of childhood ALL.1 Asparagine is required by all cells for survival and is normally produced by the enzyme asparagine synthethase (ASNS). Malignant lymphoblasts are thought to have low ASNS levels and therefore to depend on extracellular sources of asparagine for their rapid growth. Depletion of asparagine by asparaginase selectively kills leukemia cells by decreasing protein biosynthesis.2 A significant improvement in OS and EFS was seen for children with ALL who were assigned to receive asparaginase during postremission consolidation compared with those who did not receive asparaginase.3 Associations between treatment outcome and asparaginase dose intensity4 and between an inferior outcome and the use of asparaginase preparation with a shorter half-life5 have been reported. Asparaginase intolerance can manifest as allergic reactions, pancreatitis, and abnormalities of hemostasis.6,7 Such asparaginase-associated complications may also affect treatment outcomes. Patients who experienced a dose-limiting asparaginase toxicity had a significantly worse disease-free survival than those who were able to tolerate their intended doses.4,5
The polymorphisms of the genes mediating asparaginase antileukemia effect can underlie observed variability. ASNS, which catalyzes the transfer of an amino group to aspartic acid to form asparagine, may counteract the asparaginase effect. In vitro experiments conducted in leukemia cell lines and patient lymphoblasts suggested that elevated ASNS activity might be a cause of asparaginase resistance.8-12 The basic region leucine zipper activating transcription factor (ATF) can up-regulate ASNS transcription.13 ATF5 has been shown to activate ASNS transcription after nutrition deprivation and to be differentially expressed between leukemic cells that are resistant and sensitive to asparaginase.13-15 Arginosuccinate synthase 1 (ASS1) is an enzyme at the crossroad of the urea and nitric oxide cycles, which catalyzes the conversion of aspartate and citrullin into argininosuccinate. The latter can serve as a source for de novo synthesis of asparagine.16 Microarray analysis identified up-regulation of ASS1 in cell lines with resistance to asparaginase.13
In the present study, we report the analysis of the polymorphisms in ASNS, ATF5, and ASS1 genes and their association with ALL disease outcomes in 2 patient populations, and provide a functional assessment of ATF5 polymorphisms that significantly affect disease outcome in ALL.
Methods
Study population and end points
Our study population consisted of 318 Caucasian children (97.5% of French-Canadian origin from a similar geographic region) diagnosed with ALL at the Hospital Sainte-Justine (referred to herein as the HSJ group or test group) between January 1989 and July 2005. The patients underwent treatment with the Dana-Farber Cancer Institute ALL Consortium protocols DFCI 87-01, 91-01, 95-01, or 2000-01.4,5 Patients received 20-30 weeks of asparaginase during the intensification phase (protocol 87-01 patients received 20 weeks of Escherichia coli asparaginase 25 000 IU/m2/wk and protocol 91-01 patients received 30 weeks of the same asparaginase preparation). On protocol 95-01, patients were randomized to receive either E coli or Erwinia asparaginase for 20 weeks and on protocol 2000-01, patients were randomized to receive either conventional doses of E coli for 30 weeks or individualized doses starting from half the standard dose and then adjusting it subsequently according to asparaginase levels.5,17
An association of genotypes/haplotypes with ALL outcome was assessed by EFS and OS analysis.18 Children who had an induction failure, relapsed after achieving complete remission, or died were defined as having had an event. Given the difference that existed across treatment protocols in the duration of asparaginase treatment or asparaginase preparation used, the same analyses were performed following the stratification by the protocol and according to the type of asparaginase.
A validation set of white patients called the Dana-Farber Cancer Institute (DFCI) group was composed of a subset of patients who underwent treatment on the DFCI 95-01 and 2000-01 protocols in 9 remaining consortium institutions.5,17 This group was composed of 307 nonincident cases whose samples provided sufficient DNA to allow genotyping. To minimize confounding due to population stratification, only whites (self-reported, n = 267) were included in the analysis.
The characteristics of patients for both test and validations set are provided in Table 1.
Characteristics of ALL patients in the test (HSJ) and validation (DFCI) cohorts
| Characteristic . | HSJ . | DFCI . | ||
|---|---|---|---|---|
| n . | Frequency, % . | n . | Frequency, % . | |
| Sex | ||||
| Female | 142 | 44.7 | 123 | 46.1 |
| Male | 176 | 55.3 | 144 | 53.9 |
| Age, y | ||||
| ≥ 1 < 9.99 | 256 | 80.5 | 220 | 82.4 |
| ≥ 10 | 62 | 19.5 | 47 | 17.6 |
| WBCs, × 109/L | ||||
| < 50 | 263 | 82.6 | 216 | 80.9 |
| > 50 | 55 | 17.4 | 51 | 19.1 |
| Cell type | ||||
| B | 291 | 91.6 | 244 | 92.1 |
| T | 27 | 8.4 | 21 | 7.9 |
| Risk group | ||||
| Standard | 153 | 48.1 | 165 | 61.8 |
| High | 165 | 51.9 | 102 | 38.2 |
| Treatment protocol | ||||
| 87-01 | 29 | 9.1 | ||
| 91-01 | 63 | 19.8 | ||
| 95-01 | 114 | 35.8 | 91 | 34.1 |
| 2000-01 | 112 | 35.2 | 176 | 65.9 |
| Event | ||||
| Yes | 52 | 16.4 | 51 | 19.1 |
| No | 266 | 83.6 | 216 | 80.9 |
| Total | 318 | 100 | 267 | 100 |
| Characteristic . | HSJ . | DFCI . | ||
|---|---|---|---|---|
| n . | Frequency, % . | n . | Frequency, % . | |
| Sex | ||||
| Female | 142 | 44.7 | 123 | 46.1 |
| Male | 176 | 55.3 | 144 | 53.9 |
| Age, y | ||||
| ≥ 1 < 9.99 | 256 | 80.5 | 220 | 82.4 |
| ≥ 10 | 62 | 19.5 | 47 | 17.6 |
| WBCs, × 109/L | ||||
| < 50 | 263 | 82.6 | 216 | 80.9 |
| > 50 | 55 | 17.4 | 51 | 19.1 |
| Cell type | ||||
| B | 291 | 91.6 | 244 | 92.1 |
| T | 27 | 8.4 | 21 | 7.9 |
| Risk group | ||||
| Standard | 153 | 48.1 | 165 | 61.8 |
| High | 165 | 51.9 | 102 | 38.2 |
| Treatment protocol | ||||
| 87-01 | 29 | 9.1 | ||
| 91-01 | 63 | 19.8 | ||
| 95-01 | 114 | 35.8 | 91 | 34.1 |
| 2000-01 | 112 | 35.2 | 176 | 65.9 |
| Event | ||||
| Yes | 52 | 16.4 | 51 | 19.1 |
| No | 266 | 83.6 | 216 | 80.9 |
| Total | 318 | 100 | 267 | 100 |
Characteristics are shown for white patients of both cohorts.
Genotyping
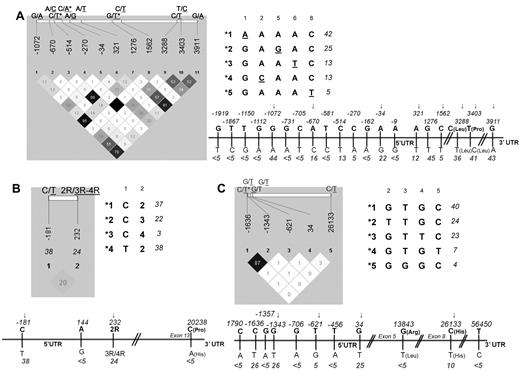
Thirty-five polymorphisms in the ASNS, ATF5, and ASS1 genes located in regulatory and coding gene regions were selected from the National Center for Biotechnology Information (NCBI) single nucleotide polymorphism (SNP) databases (http://www.ncbi.nlm.nih.gov/SNP). Selected polymorphisms were analyzed in 60 controls to estimate allele frequency, linkage disequilibrium (LD), and haplotype phase (Figure 1). Tag SNPs (sufficient to define common haplotypes) with frequency ≥ 5% were retained for the analysis in patients comprising 8 SNPs in ATF5, 4 in ASS1 and 2 in ASNS gene. Primers and probes used for amplification and genotyping of these polymorphisms are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). dbSNP numbers for the polymorphisms genotyped only in controls are given in supplemental Table 2. The subset of samples was genotyped in duplicate to ensure genotype reproducibility. Genotyping was performed in part by allele specific oligonucleotide hybridization as described previously19 and in part using Sequenom genotyping platform at Genome Quebec and McGill Innovation Center. The amplification was not equally successful for all loci analyzed explaining the minor difference in the total number of genotypes.
Statistics
The estimates of LD and haplotype phase in control individuals were obtained using Phase Version 2.0 software.20 The tag SNPs were selected based on LD information (r2 ≥ 0.87, Figure 1) using Haploview Version 4.2 software. Tag SNPs with a minor allele frequency (MAF) ≥ 5% were retained for further analysis in ALL patients. Association of genotypes with ALL outcome was assessed by EFS and OS. Survival differences, estimated by Kaplan-Meier analysis for patients with different genotypes, were assessed using a log-rank test. Times to an event or death were measured as the time between diagnosis and the event of interest. For censored cases, it represented the time from diagnosis to date last known alive without an event; for longer time durations, all times were truncated at 5 years posttreatment. The analyses stratified by treatment protocol and asparaginase preparation were also performed. A false discovery rate (FDR) correction was performed to adjust for multiple genetic variants tested using Q-value software and the bootstrap approach described in Storey et al.21 The Q value measures the minimum FDR that is incurred when calling that test significant. The P value cutoff when controlling FDR at level 0.1 using all observed P values was ≤ .01 (q = 0.07). The hazard ratio (HR) with a 95% confidence interval (95% CI) for genetic variants was estimated by Cox regression analysis. Cox regression was also used to estimate multivariable HR for the variant that remained significant after the replication step and after adjustment for multiple comparisons. The common prognostic factors (outlined in Table 1) categorized according to relapse risk prediction were included in the model.18 P < .05 was considered significant.
The power estimates are performed with Power and Precision Calculator Version 3.0 software for survival studies,22 and a sample size of 300 patients has 80% power to detect minimum HR of 2.0-2.5 (using dominant genetic model and variants ranging in frequency from 5%-30%) assuming a follow-up of 5 years and a relapse rate of 0.2.5
Gene-reporter assay and mRNA expression
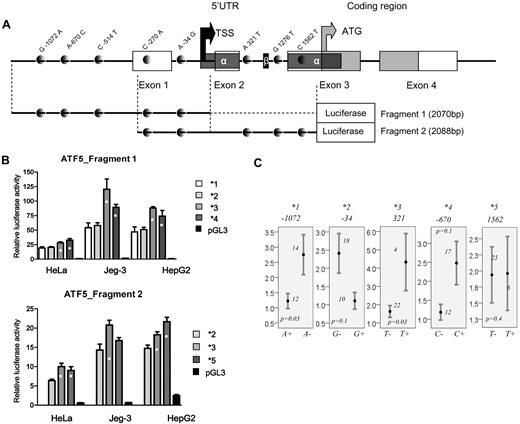
Two fragments corresponding to 2070 and 2088 bp (fragments 1 and 2, respectively, Figure 3A) of the ATF5 proximal promoter and 5′UTR region were amplified from genomic DNA of individual with known genotype. Both fragments included a core promoter element from −105 to +3 relative to transcription start site.23 Fragments were cloned into the promoterless pGL3-basic vector (Promega). Constructs corresponding to 5 common haplotypes were sequenced to confirm the presence of the expected polymorphic sites. Human placental Jeg-3, cervical cancer HeLa, and hepatoma HepG2 (ATCC) were cotransfected with 100 ng of firefly luciferase plasmid containing the corresponding allelic construct—either a pool of 3 clones or 1 clone in which any base error was corrected by site-directed mutagenesis (Stratagene) and 0.5 ng of SV40-driven renilla luciferase pRL-SV40 plasmid to control transfection efficiency. Transfection was performed as described previously.18 The renilla luciferase activity of the control pRL-SV40 was used to normalize the results of the firefly luciferase activity of the allelic constructs (relative luciferase activity was expressed as mean ± SD). Similar experiments were performed with empty, promoterless, pGL3-basic plasmid (Promega), providing a negative control. Each experiment was performed in triplicate, and the difference in gene-reporter activity between haplotypes was assessed by 1-way ANOVA with post hoc correction for multiple comparisons.
Total RNA from lymphoblastic cell lines of CEPH families extracted with a QIAGEN kit was reverse transcribed using 2uM oligo-dT and random primers (mixed at 1:1) and M-MLV enzyme (Invitrogen) according to the protocol provided by the supplier. Quantitative PCR was carried out using the Syber Green detection system (Applied Biosystems), 8 ng of cDNA, and a pair of primers (final concentration 0.25uM) specific for ATF5: (F)-GGCTCCCTATGAGGTCCTTG and (R)-CAACTCGCTCAGTCATCCAG separated by an intron to avoid amplification of genomic DNA. The expression was measured by relative quantification normalized to B2-microglobulin using the primers (F)-TACTCTCTCTTTCTGGCCTG and (R)-GGATGGATGAAACCCAGACA, and the calculation was performed using the comparative cycle threshold (CT) method.24,25 Genotypes for lymphoblastoid cell lines were either retrieved from HapMap data (www.hapmap.org) or genotyping was performed as described in “Genotyping.”
Results
Thirty-five polymorphisms located in regulatory and coding regions of the ATF5, ASNS, and ASS1 genes were selected from the dbSNP database at NCBI. We initially genotyped these SNPs in 60 controls of Caucasian origin to estimate allelic frequency and LD. The MAF for all polymorphisms and Haploview LD display with pairwise R2 for the SNPs with MAF ≥ 5% are given for each gene in Figure 1. Fourteen tag polymorphisms were retained for the analysis in patients, including 8, 2, and 4 SNPs in the ATF5, ASNS, and ASS1 genes, respectively. Common haplotypes (with frequency ≥ 3%) based on all tag SNPs within the gene, or only those within the promoter and 5′UTR for ATF5, are also provided in Figure 1.
ATF5, ASNS, and ASS1 gene polymorphisms and derived haplotypes. Haploview LD displays, linear representation and derived haplotypes for the selected ATF5 (A), ASNS (B), and ASS1 (C) polymorphisms. The linear display refers to all initially selected SNPs, and haploview LD (with pairwise r2) refers to SNPs with MAF ≥ 5%. MAF in the control population is given below the position of each SNP. SNPs excluded from the analysis of ALL patients because of pairwise r2 ≥ 87% are indicated by asterisks, and those retained in the analysis are indicated by arrows. Haplotypes (with a frequency ≥ 3%) derived from tag SNPs are arbitrarily numbered. The frequency in controls is given next to each haplotype. Note that for ATF5, only the haplotypes in the promoter and 5′UTR are listed (the allele defining each haplotype is underlined) because of their correspondence with functional assay performed (see Figure 3).
ATF5, ASNS, and ASS1 gene polymorphisms and derived haplotypes. Haploview LD displays, linear representation and derived haplotypes for the selected ATF5 (A), ASNS (B), and ASS1 (C) polymorphisms. The linear display refers to all initially selected SNPs, and haploview LD (with pairwise r2) refers to SNPs with MAF ≥ 5%. MAF in the control population is given below the position of each SNP. SNPs excluded from the analysis of ALL patients because of pairwise r2 ≥ 87% are indicated by asterisks, and those retained in the analysis are indicated by arrows. Haplotypes (with a frequency ≥ 3%) derived from tag SNPs are arbitrarily numbered. The frequency in controls is given next to each haplotype. Note that for ATF5, only the haplotypes in the promoter and 5′UTR are listed (the allele defining each haplotype is underlined) because of their correspondence with functional assay performed (see Figure 3).
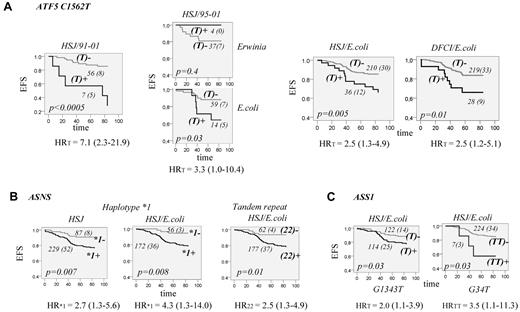
The association analysis between polymorphisms of the ATF5 gene and ALL outcome in HSJ patients (test group) revealed no significant association with EFS when all patients were analyzed. Given the difference of asparaginase preparation and duration of treatment across treatment protocols, the same analyses were performed after stratification by protocol. The association between EFS and C1562T polymorphism located in the 5′UTR region of the ATF5 gene was noted for the patients on the 91-01 protocol only. The carriers of the ATF5 T1562 allele had reduced EFS compared with individuals with the CC genotype (P < .0005, HR = 7.1, 95% CI = 2.3-21.9, Figure 2A). All patients of the test group who were treated with the 91-01 protocol received E coli asparaginase for 30 weeks, whereas on other protocols, patients had either a shorter duration of asparaginase treatment or were randomized to receive different asparaginase preparations or a different dose. This might suggest that an association between ATF5 T1562 polymorphism and EFS is dependent on asparaginase preparation and/or duration of treatment. To verify this, we next analyzed only patients on the 95-01 protocol, which included randomization on E coli or Erwinia asparaginase. For the analysis, the latter group also included patients who were switched to Erwinia asparaginase because of allergy to E coli asparaginase. The analysis revealed a significant association only for the patients who received the E coli preparation (P = .03, Figure 2A) and accordingly remained significant when patients who received E coli asparaginase on all protocols were included in the analysis (P = .005, Figure 2A, HR = 2.5, 95% CI = 1.3-4.9). The association sustained correction for multiple testing (P < .01, a cutoff value for FDR at level 0.1). The analysis was subsequently performed in the replication cohort (the DFCI group), which confirmed the association found. Carriers of the T1562 allele had lower EFS only in patients assigned to E coli asparaginase (P = .01, HR = 2.5, 95% CI = 1.2-5.1, Figure 2A). The association remained significant in both patient groups in multivariable analysis with inclusion of typical prognostic factors in the Cox regression model (Table 2). No association between T1562 and OS was noted in either the test or the replication group.
EFS for patients with ALL according to ATF5, ASNS, and ASS1 genotypes. (A) EFS according to genotypes of ATF5 C1562T polymorphism. EFS curves are shown for patients who were carriers (T+; dark gray) or not (T−; light gray) of the T1562 allele. EFS curves are presented for HSJ patients (test set) on the 91-01 protocol (HSJ/91-01, left panel), HSJ patients on 95-01 protocol (HSJ/95-01) randomized to E coli or Erwinia asparaginase (2 plots of the middle panel), and for all HSJ and DFCI patients (validation set) who were assigned to E coli asparaginase (2 plots on the right panel, HSJ/E coli and DFCI/E coli, respectively). The number of all patients in each curve (with the number of cases with an event in brackets) is indicated next to the curve. The EFS difference between patients with and without the T1562 allele is estimated by the log-rank test and the P value is indicated on each plot. Risk of an event for T allele carriers, expressed as a univariable HR with the 95% CI in brackets, is indicated below each plot. (B) EFS curves according to ASNS genotypes. EFS for all HSJ patients (left plot) and those assigned to E coli asparaginase (middle plot), with (+; dark gray line) and without (-; light gray line) ASNS haplotype *1. Haplotype *1 is defined in Figure 1B. EFS for HSJ patients (right plot) assigned to E coli asparaginase who are homozygous (22+; dark gray line) or not (22-; light gray line) for the 2R allele of the ASNS tandem repeat. The number of patients within each curve, the number of cases with an event, the P value, and the genotype-associated risk of an event is labeled as in panel A. (C) EFS curves according to genotypes of ASS1 G1343T and G34T polymorphisms. Left plot is EFS for HSJ patients assigned to E coli asparaginase who are carriers (T+; dark gray line) or not (T-; light gray line) of the ASS1 T1343 allele and the right plot is EFS for HSJ patients assigned to E coli asparaginase who are carriers (TT+, dark gray line) or not (TT-, light gray line) of the ASS1 TT34 genotype. The number of cases within the curve, the number of patients with an event, the P value, and the genotype-associated risk of an event is labeled as in panel A.
EFS for patients with ALL according to ATF5, ASNS, and ASS1 genotypes. (A) EFS according to genotypes of ATF5 C1562T polymorphism. EFS curves are shown for patients who were carriers (T+; dark gray) or not (T−; light gray) of the T1562 allele. EFS curves are presented for HSJ patients (test set) on the 91-01 protocol (HSJ/91-01, left panel), HSJ patients on 95-01 protocol (HSJ/95-01) randomized to E coli or Erwinia asparaginase (2 plots of the middle panel), and for all HSJ and DFCI patients (validation set) who were assigned to E coli asparaginase (2 plots on the right panel, HSJ/E coli and DFCI/E coli, respectively). The number of all patients in each curve (with the number of cases with an event in brackets) is indicated next to the curve. The EFS difference between patients with and without the T1562 allele is estimated by the log-rank test and the P value is indicated on each plot. Risk of an event for T allele carriers, expressed as a univariable HR with the 95% CI in brackets, is indicated below each plot. (B) EFS curves according to ASNS genotypes. EFS for all HSJ patients (left plot) and those assigned to E coli asparaginase (middle plot), with (+; dark gray line) and without (-; light gray line) ASNS haplotype *1. Haplotype *1 is defined in Figure 1B. EFS for HSJ patients (right plot) assigned to E coli asparaginase who are homozygous (22+; dark gray line) or not (22-; light gray line) for the 2R allele of the ASNS tandem repeat. The number of patients within each curve, the number of cases with an event, the P value, and the genotype-associated risk of an event is labeled as in panel A. (C) EFS curves according to genotypes of ASS1 G1343T and G34T polymorphisms. Left plot is EFS for HSJ patients assigned to E coli asparaginase who are carriers (T+; dark gray line) or not (T-; light gray line) of the ASS1 T1343 allele and the right plot is EFS for HSJ patients assigned to E coli asparaginase who are carriers (TT+, dark gray line) or not (TT-, light gray line) of the ASS1 TT34 genotype. The number of cases within the curve, the number of patients with an event, the P value, and the genotype-associated risk of an event is labeled as in panel A.
Risk of event in ALL patients associated with the ATF5 T1562 allele in multivariable analysis
| Variable . | HSJ test group . | DFCI validation group . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Sex | 1.6 | 0.8-3.1 | .2 | 1.6 | 1.0-3.8 | .06 |
| Age, y | 1.4 | 0.5-3.8 | .5 | 2.1 | 1.2-3.7 | .01 |
| WBCs, × 109/L | 1.5 | 0.5-4.3 | .4 | 1.9 | 0.8-4.7 | .1 |
| Cell type | 0.3 | 0.03-2.0 | .2 | 0.8 | 0.2-2.7 | .7 |
| Risk | 0.6 | 0.3-1.5 | .3 | 0.8 | 0.3-2.1 | .6 |
| Treatment protocol | 1.4 | 1.0-1.9 | .04 | 1.7 | 0.9-3.3 | .1 |
| ATF5 T1562 carrier | 2.3 | 1.1-4.7 | .03 | 2.2 | 1.0-4.9 | .05 |
| Variable . | HSJ test group . | DFCI validation group . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Sex | 1.6 | 0.8-3.1 | .2 | 1.6 | 1.0-3.8 | .06 |
| Age, y | 1.4 | 0.5-3.8 | .5 | 2.1 | 1.2-3.7 | .01 |
| WBCs, × 109/L | 1.5 | 0.5-4.3 | .4 | 1.9 | 0.8-4.7 | .1 |
| Cell type | 0.3 | 0.03-2.0 | .2 | 0.8 | 0.2-2.7 | .7 |
| Risk | 0.6 | 0.3-1.5 | .3 | 0.8 | 0.3-2.1 | .6 |
| Treatment protocol | 1.4 | 1.0-1.9 | .04 | 1.7 | 0.9-3.3 | .1 |
| ATF5 T1562 carrier | 2.3 | 1.1-4.7 | .03 | 2.2 | 1.0-4.9 | .05 |
The Cox regression model was used to estimate the risk of the ATF5 genotype in the presence of the prognostic factors shown in Table 1. HR and 95% CI are indicated. The analyses were limited to the patients who received E coli asparaginase. There was no difference in prognostic factor distribution between the E coli group and all patients in either test or validation set. For the analysis, the absence of the T1562 allele and categories typically associated with lower risk of relapse were considered as references (age ≥ 1 and < 9.99 years, WBC count < 50 × 109/L, B-cell type, standard risk group, female sex, and most recent treatment protocol).
The analysis of the ASNS gene polymorphisms in the test group showed a reduction in EFS for individuals who were homozygous for double repeat (2R) of the first intron tandem-repeat sequence. Reduction in EFS was also seen for the carriers of haplotype*1 (Figure 1B) composed of the C-181 allele of the promoter C → T variation and 2R sequence. The associations seemed to be limited to the patients who received E coli asparaginase (P = .008, HR = 2.5, 95% CI = 1.3-4.9 and P = .01, HR = 4.3, 95% CI = 1.3-14.0, respectively, Figure 2B). The analysis in the replication cohort revealed the same tendency only for the 2R2R genotype; however, the result was not significant (not shown).
The analysis of the ASS1 gene variations in the test group revealed a reduction in EFS for patients with a T allele of the promoter G-1343T polymorphism, as well for those with the TT genotype of 5′UTR G34T substitution. The associations were limited to patients who received E coli asparaginase (P = .03, HR = 2.0, 95% CI = 1.1-4.9 and HR = 3.5, 95% CI = 1.1-11.3, respectively, Figure 2C). The ASS1 associations did not remain significant after correction for multiple testing and were not further tested in the replication group.
Given the significant association of ATF5 C1562T in the test and replication groups, further analyses were focused on the functional assessment of C1562T and remaining polymorphisms in the promoter and 5′UTR of the gene. Five common haplotypes arbitrarily named *1 to *5 (with allelic composition depicted in Figure 1A) were tested in a luciferase assay using 2 different fragments. The fragment design relative to the genomic organization of ATF5 and polymorphism positions is given in Figure 3A. Each haplotype is defined by the single minor allele of the given polymorphism or the polymorphism in LD, depending on the fragment used. Haplotypes *1 to *4 were tested using the first fragment and are defined by the A-1072, G-34, T-514 (in LD with T321), and C-670 alleles, respectively. Reporter gene assay of the fragment 1 revealed 2 low-activity haplotypes (*1 and *2) and 2 high-activity haplotypes (*3 and *4, Figure 3B top panel, P ≤ .02). The quantitative mRNA analysis appears to corroborate the results of the gene-reporter assay. Lower levels were seen in carriers of A-1072 and G-34 alleles (P = .03 and 0.1, Figure 3C, respectively), and higher mRNA levels were seen in carriers of the T321and C-670 alleles (P = .03 and 0.1, respectively, Figure 3C). Haplotype *5 is defined by the T1562 allele (or A-270 in correlation) and was tested within the fragment 2 along with 1 low- (*2) and 1 high-expressing haplotype (*3). Haplotype *5 had promoter activity comparable to haplotype *3 and was significantly different from the low-expressing ones (P ≤ .01 in 2 cell lines, Figure 3B bottom panel). No difference in basal mRNA levels was seen in lymphoblastoid cell lines between carriers and noncarriers of T1562 allele (Figure 3C).
Genomic structure of ATF5 regulatory region and polymorphism-related function. (A) Genomic structure of 2 ATF5 regions used in the luciferase assay. Exonic and coding sequences are represented by open and gray boxes, respectively, and SNPs are represented by gray dots. Alternatively spliced 5′UTRα and 5′UTRβ, as determined by Watatani et al,32 are indicated by the dark gray and black boxes, respectively. Note that 2 SNPs in LD, C-514T and A321T SNPs, which defines haplotype *3, are present in the first and the second fragment, respectively; 2 other SNPs in LD (C-270A/C1562T), which define haplotype *5, are both present in fragment 2. The black and gray arrows indicate the transcription and translation start sites, respectively, estimated according to Wei et al23 and Watatani et al32 and reference sequence NM012068. (B) Relative promoter activity of ATF5 haplotype *1,*2,*3, and *4 derived from fragment 1 (top panel) and haplotypes*2,*3, and *5 derived from fragment 2 (bottom panel) in 3 cell lines (HeLa, Jeg3, and HepG2). The haplotype numbers correspond to the haplotypes of the proximal promoter and 5′UTR given in Figure 1A. The haplotypes *1, *2, *3, *4, and *5 are defined by A-1072, G-34, T-514 (or T321 in correlation), C-670, and T1562 (or A-270 in correlation), respectively. Haplotypes showing a significant increase in promoter activity (P ≤ .02, ANOVA posthoc) compared with low-expression haplotypes are indicated by asterisks. (C) Relative mRNA levels in HapMap lymphoblastoid cell lines for individuals who are carriers or not of indicated alleles of ATF5 polymorphisms, which are defining haplotypes *1 to *5, respectively. Mean values ± SE are given. The number of individuals represented by each bar and the P value obtained by the Mann-Whitney test are indicated on the plots.
Genomic structure of ATF5 regulatory region and polymorphism-related function. (A) Genomic structure of 2 ATF5 regions used in the luciferase assay. Exonic and coding sequences are represented by open and gray boxes, respectively, and SNPs are represented by gray dots. Alternatively spliced 5′UTRα and 5′UTRβ, as determined by Watatani et al,32 are indicated by the dark gray and black boxes, respectively. Note that 2 SNPs in LD, C-514T and A321T SNPs, which defines haplotype *3, are present in the first and the second fragment, respectively; 2 other SNPs in LD (C-270A/C1562T), which define haplotype *5, are both present in fragment 2. The black and gray arrows indicate the transcription and translation start sites, respectively, estimated according to Wei et al23 and Watatani et al32 and reference sequence NM012068. (B) Relative promoter activity of ATF5 haplotype *1,*2,*3, and *4 derived from fragment 1 (top panel) and haplotypes*2,*3, and *5 derived from fragment 2 (bottom panel) in 3 cell lines (HeLa, Jeg3, and HepG2). The haplotype numbers correspond to the haplotypes of the proximal promoter and 5′UTR given in Figure 1A. The haplotypes *1, *2, *3, *4, and *5 are defined by A-1072, G-34, T-514 (or T321 in correlation), C-670, and T1562 (or A-270 in correlation), respectively. Haplotypes showing a significant increase in promoter activity (P ≤ .02, ANOVA posthoc) compared with low-expression haplotypes are indicated by asterisks. (C) Relative mRNA levels in HapMap lymphoblastoid cell lines for individuals who are carriers or not of indicated alleles of ATF5 polymorphisms, which are defining haplotypes *1 to *5, respectively. Mean values ± SE are given. The number of individuals represented by each bar and the P value obtained by the Mann-Whitney test are indicated on the plots.
Discussion
Differences in susceptibility to asparaginase have long been attributed to variable levels of ASNS expression. In vitro experiments conducted in leukemia cell lines suggested that elevated ASNS levels might be a cause of asparaginase resistance8-10 and that ASNS inhibitors may suppress a proliferation of resistant cell lines.11 It has been shown that leukemic cells can up-regulate ASNS gene expression under nutrient stress caused by asparaginase.26 Using genome-wide expression profiling and patient samples, Stams et al found that asparaginase-resistant, TEL-AML1–negative B cells had significantly higher ASNS expression compared with sensitive ones.12 An increase in ASNS expression was predictive of inferior disease outcome. Other groups nevertheless reported an absence of association between ASNS expression and in vitro response to asparaginase when patient lymphoblasts were analyzed.27,28 Recently, Iwamoto et al reported that BM-derived mesenchymal cells express much higher levels of ASNS compared with leukemic lymphoblasts, suggesting that resistance to asparaginase may be mediated by mesenchymal cells.29 Inter-individual differences in ASNS expression levels were noted, which might be explained by a change in expression of ASNS gene or genes coding for the regulators of ASNS expression. The polymorphisms in corresponding genes may influence the expression affecting disease outcome in leukemia patients treated with asparaginase. We noted that ALL patients homozygous for tandem-repeat 2R allele, as well as carriers of the 2R-derived haplotype, had lower EFS. The tandem repeat located in the first intron of the ASNS gene was recently reported to function as a transcriptional enhancer element.30 However, we did not observe a relationship between mRNA levels in lymphoblastoid cell lines and either tandem-repeat polymorphism or haplotype *1 (not shown). Likewise, we did not replicate this finding in the DFCI cohort, arguing against the role of these variations in ALL outcome.
ASNS transcription can be up-regulated by ATF transcription factors. The increase of ATF levels, including ATF5, has been seen in experiments comparing the pattern of genome-wide expression between leukemia patient cells that are sensitive and resistant to asparaginase treatment.13,27 ATF5 regulates processes that are involved in cellular differentiation, the cell cycle, and apoptosis, acting through a variety of DNA-binding regulatory elements.31,32 ATF5 is a stress response transcription factor that responds to amino acid limitation.33 After nutrition deprivation, it acts as a transcriptional activator of ASNS,14 binding to the nutrient-sensing response unit of the ASNS gene. We showed herein that the several polymorphisms located in the regulatory region (including the 5′UTR and the 2-kb element upstream from the transcription start site) of the ATF5 gene affect promoter activity and mRNA levels, which was supported by in silico analysis of a loss or gain of a variety of transcription binding sites associated with respective minor alleles (supplemental Table 3). Low and high expression haplotypes have been distinguished, haplotypes tagged by the A-1072 allele and G-34 allele (the latter located in the −105/+3 core promoter element23 ), were associated with lower activity. In contrast, 3 other haplotypes defined, respectively, by C-670, T-514 (or T321 in LD), and T1562 (or A-270 in LD) alleles were associated with higher promoter activity. The T1562 variation was associated with reduced EFS in ALL in both the test and the replication cohorts. This association seemed to be limited to E coli asparaginase, which might be related to higher asparaginase activity. Indeed, E coli asparaginase treatment has been shown to have higher efficacy and toxicity compared with Erwinia asparaginase.5,17,34 More pronounced asparagine depletion may more readily result in ATF5 up-regulation, which in turn can be influenced by genetic variations that are particularly apparent in those with higher promoter activity, as in the case of T1562 allele. Although this is one possible explanation, it remains unclear why there was no association between ALL outcome and other high-activity ATF5 alleles analyzed individually or combined (the presence of at least one high activity haplotype was not associated with reduction in EFS). Care was taken to avoid confounding because of the population stratification by limiting the analysis to Caucasians. Given that the patients in the replication cohort originate from different institutions (and different geographic regions), the effect of population stratification cannot be completely ruled out. In addition, the T1562 variation was associated with an increase in promoter activity, but there was no relationship with basal mRNA level in lymphoblastoid cell lines. Differences in cell type, between in vitro and in vivo assay, and the low frequency of T allele or basal and drug-induced expression might account for observed discrepancy. The latter seems a particularly interesting explanation, which we intend to test in the future using the cell lines harboring the *5 haplotype. The next step would be to estimate the best genetic predictors through an analysis that combines T1562 with other previously identified event-predisposing variants in the same cohort.35-37
The mechanisms regulating ATF5 and the mechanism by which ATF5 regulates downstream genes are complex.23,31,32 In addition to a variety of transcription factor binding sites recently mapped by Wei et al,23 translation ATF5 efficiency is regulated by alternatively spliced 5′UTR α and β32 (shown in Figure 3A). 5′UTR-regulated translational repression is released by amino acid limitation only in the case of 5′UTR α. Among the tested polymorphisms, C1562T is the only one located in the 5′UTR α (Figure 3A); the remaining variations are either located in promoters or are spliced out from the 5′UTR. The contribution of A-270 (in correlation with T1562), to the higher promoter activity by introduction of the GATA-binding element (supplemental Table 3) is also possible.
Given ASS1 contribution to de novo asparagine synthesis, ASS1 remains an important candidate for modulation of a response to asparaginase. Microarray analysis identified up-regulation of ASS1 in cell lines with resistance to asparaginase. ASS1 mRNA suppression can restore drug sensitivity in resistant cell lines.13 Recent genome-wide analysis identified genes of aspartate metabolism as contributors to asparaginase sensitivity in vitro.38 Among the top-ranking genes identified in that study, an association of ASS1 polymorphisms with in vitro asparaginase sensitivity in ALL patient samples and HapMap cell lines was found.38 Given their intronic positions, these polymorphisms were not tested in our study. Among those that we analyzed, 2 SNPs, G34T and G1343T, which were associated with lower EFS in the test cohort, did not sustain correction for multiple testing and were not further analyzed in the replication cohort.
In conclusion, we report an association of the ATF5 C1562T 5′UTR variation with disease outcome in childhood ALL. The T1562 allele was associated with increased promoter activity and inferior disease outcome in 2 independent groups of patients assigned to E coli asparaginase treatment. Our results may be related to an ATF5-regulated increase in ASNS expression in response to more efficacious E coli–induced asparagine depletion. This study provides information that will further increase our knowledge regarding childhood ALL pharmacogenetics.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients and their parents who consented to participate in these genetic studies related to leukemia.
Canadian Institutes of Health Research, Leukemia and Lymphoma Society of Canada, Charles Bruneau Foundation, and Center d'excellence en Oncologie pédiatrique et en soins palliatifs supported this study. Dana-Farber Cancer Institute ALL treatment protocols are supported by a National Cancer Institute/National Institutes of Health grant (P01 CA 68484). M.K. and D.S. are scholars of the Fonds de la Recherche en Santé du Québec. The gene-reporter assay was carried out in part within the Genome Quebec/Genome Canada: Gene Regulators in Disease project.
National Institutes of Health
Authorship
Contribution: J.R., V.G., and M.L. performed the experiments; D.S., C.L., a.m., S.E.S., L.B.S., D.N., and J.L.K. contributed to sample and clinical data collection and processing; D.S. contributed to the design of gene-reporter experiments; J.R., V.G., C.B., and M.K. performed the data analysis; M.K. designed the research and drafted the manuscript; and all authors contributed to the interpretation of data and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maja Krajinovic, Centre de Recherche, CHU Sainte-Justine, 3175 chemin de la Côte-Ste-Catherine, Montréal, QC, H3T 1C5 Canada; e-mail: maja.krajinovic@umontreal.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal