We thank Theurich et al for their interest in our article published in the February issue of BLOOD and would like to address several of the points raised in their commentary.1 Like Theurich et al, we too were surprised to find that peripheral blood B cells did not promote CD40L expression on TCR activated CD4 T cells. We explored this observation further and reported that neither CD40 activated nor EBV activated and immortalized B-cell populations could promote CD40L expression. This was also the case for the Ramos and Raji B cell lines. Theurich et al have chosen to focus on the inability of activated primary B cells to promote CD40L expression, suggesting that the use of a soluble anti-CD40 antibody, rather than multimeric CD40L, to activate primary B cells resulted in suboptimal B-cell activation. We wish to first correct the authors' representation of our B-cell activation conditions by noting that anti-CD40 antibody was not used alone, but rather in combination with rhIL-21. These conditions have been previously reported by Ettinger et al and others to induce B-cell activation and differentiation into antibody-secreting plasma cells.2 Furthermore, activation and differentiation of B cells with this combination is essentially the same as that obtained using rhCD40L and rhIL-21.3 The results reported here by Theurich et al using B cells activated for 7 days with cell (NIH3T3) bound CD40L are no different than those we have reported using our activation conditions. In actuality, it appears that less than half of the 7.2% of activated (CD107a+) T cells shown in Figure 1C of their data express CD40L. Thus the authors' results are entirely consistent with our observation that multiple activated B-cell populations have only a minimal capacity to promote CD40L expression on T cells.4
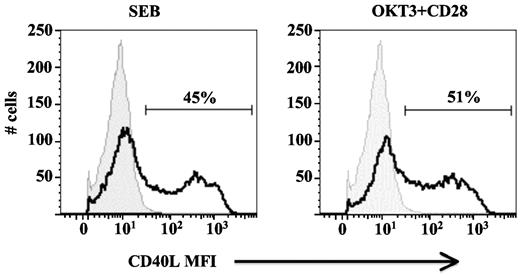
Theurich et al also commented on the use of α-CD3/α-CD28 antibodies to stimulate CD4 T cells, suggesting that the nature of this activation method may not be reflective of a physiologic TCR response. Again, we wish to correct the authors' representation of our data by noting that we do not preactivate our T cells; CD40L expression is measured 6 hours after the initial TCR stimulation of primary cells. We would also like to bring to the attention of the authors our previous publication examining the physiologic distribution of responding T cells to CD3/CD28 stimulation, in which we show that both naive and memory CD4+ T-cell subsets exhibit significant induction of CD40L expression (both early and late), as do Th1 and Th2 polarized CD4+ T cells.5 We also previously evaluated the role of TCR signaling in CD40L expression using Staphylococcal Enterotoxin-B (SEB) to crosslink HLA and TCR rather than α-CD3/α-CD28 antibodies. As we now show in Figure 1, SEB induces levels of CD40L expression similar to that observed with antibodies. Furthermore, as we published previously, and as pointed out by the authors in their Figure 1C, PBMC do not express CD40L in the absence of a TCR signal.
SEB induction of CD40L expression in PBMCs. PBMCs were plated in the presence and absence of SEB or plate-bound α-CD3 and soluble α-CD28 antibodies for 6 hours and stained for CD4 and CD40L expression. The CD4+ lymphocyte gate is shown. The data from stimulated cells (black open histograms) are overlaid on that from unstimulated cells (grey filled histograms). The percentage of CD40L+ CD4+ cells is indicated. Data is representative of 3 independent donors.
SEB induction of CD40L expression in PBMCs. PBMCs were plated in the presence and absence of SEB or plate-bound α-CD3 and soluble α-CD28 antibodies for 6 hours and stained for CD4 and CD40L expression. The CD4+ lymphocyte gate is shown. The data from stimulated cells (black open histograms) are overlaid on that from unstimulated cells (grey filled histograms). The percentage of CD40L+ CD4+ cells is indicated. Data is representative of 3 independent donors.
Thus, while we agree with Theurich et al that α-CD3 antibody delivers a potent TCR stimulus in the absence of specific antigen, we wish to emphasize that despite the potency of such TCR signaling, induction of CD40L expression remains dependent on a costimulatory signal, unequivocally demonstrating the critical role CD14hi monocytes (and mDC) play in this process.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jack A. Ragheb, MD, PhD, Division of Therapeutic Proteins, Office of Biological Products, Center for Drug Evaluation and Research, Food and Drug Administration, National Institute of Allergy and Infectious Disease, National Institutes of Health, Bethesda, MD 20892; e-mail: jr50b@nih.gov.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal