To the editor:
In the February issue of Blood, Chakrabarty and colleagues present data suggesting that early activation of CD4+ T cells, measured by CD40L expression, is significantly induced by CD14hi monocytes, myeloid dendritic cells and monocytic cell lines in vitro. In contrast, B cells (resting, activated or EBV immortalized) are reported to lack this capacity.1
This is rather surprising, as over the last decade B cells have increasingly been recognized as potent antigen presenting cells. If sufficiently activated, human and murine B cells prime naive and expand memory T cells in vitro and in vivo.2-4 They furthermore play a key role in T cell-mediated auto- and allo-immunity.5,6
For 2 reasons the experimental model used by Chakrabarty et al seems to be suboptimal to address the above aspect: First, CD3/CD28 preactivated T cells are used which represents a nonphysiologic, antigen-independent T-cell signal. Furthermore, it remains unclear whether the T-cell subpopulations responding to this stimulus represent a physiologic distribution and whether consecutive activation by different APCs is affected. Second, the B-cell stimulation model also is nonphysiologic as it uses a soluble CD40 monoclonal antibody and not a cell-bound CD40-ligand. At least in our hands, this approach leads to suboptimal activation (A.S.-V., unpublished results, 2010).
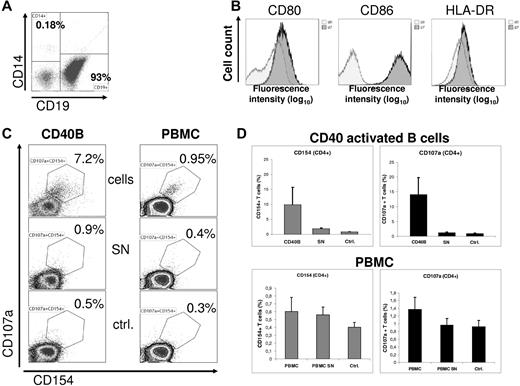
Here, we use a classic allogeneic mixed lymphocyte reaction (MLR) as a model for antigen-dependent T-cell activation using CD40L activated B cells as APCs. As shown in Figure 1A, enriched human peripheral B cells (CD19+ 93%; CD14+ < 0.2%) were activated on CD40L-expresssing NIH3T3 cells as described previously7 and showed a significant up-regulation of the costimulatory and major histocompatibility molecules (Figure 1B). Such CD40-activated B cells (CD40B) significantly induce early activation of CD4+ T cells measured by CD40L (CD154) expression after 5 hours of cocultivation (T:B ratio 2:1). T-cell activation depends on a cell-bound factor, as CD40B cell-free supernatants do not induce CD40L expression (Figure 1C). Early activation of T cells is further documented by significant up-regulation of CD107a. For both parameters, the mean frequency of CD154+ and CD107a+ activated T cells (10% and 14% respectively, Figure 1D) is within the range observed for early T-cell induction in a nonHLA matched setting.
CD40 activated human B cells can efficiently induce early T-cell activation. (A) Human B cells were enriched (EasySep, StemCell Technologies) from buffy coats of 4 healthy donors. Informed consent and approval of the institutional review board were previously obtained. A representative flow cytometry analysis of the 4 experiments is shown indicating high enrichment of B cells (> 90%) and efficient depletion of monocytes (< 0.2%). (B) Enriched B cells were activated over 7 days on a monolayer of NIH3T3 cells constitutively expressing the human CD40 ligand. Surface expression of CD80, CD86 and HLA-DR is significantly up-regulated after 7 days of coculture demonstrated by flow cytometry analysis (dark histograms). (C) A mixed lymphocyte reaction of CD40-activated B cells (CD40B) with allogeneic T cells (left column) was performed over 5 hours. Monensin was added after 1 hour. Corresponding cell free supernatants (SN) and culture media (ctrl) were used for T-cell cultures as controls. Subsequent analyses of CD40L (CD154) and CD107a expression on CD4+ T cells as early activation markers were performed by flow cytometry. Analog experiments were done with none-manipulated peripheral blood mononuclear cells (PBMC, right column). Supernatants from PBMC were taken from overnight cultures. Results are representatives of 4 experiments of each condition (CD40B versus PBMC). (D) Results of the coculture experiments are shown separately as means of the relative numbers of CD40L+ or CD107a+ T cells (n = 4 CD40B experiments; n = 4 PBMC experiments). SD is indicated for each column.
CD40 activated human B cells can efficiently induce early T-cell activation. (A) Human B cells were enriched (EasySep, StemCell Technologies) from buffy coats of 4 healthy donors. Informed consent and approval of the institutional review board were previously obtained. A representative flow cytometry analysis of the 4 experiments is shown indicating high enrichment of B cells (> 90%) and efficient depletion of monocytes (< 0.2%). (B) Enriched B cells were activated over 7 days on a monolayer of NIH3T3 cells constitutively expressing the human CD40 ligand. Surface expression of CD80, CD86 and HLA-DR is significantly up-regulated after 7 days of coculture demonstrated by flow cytometry analysis (dark histograms). (C) A mixed lymphocyte reaction of CD40-activated B cells (CD40B) with allogeneic T cells (left column) was performed over 5 hours. Monensin was added after 1 hour. Corresponding cell free supernatants (SN) and culture media (ctrl) were used for T-cell cultures as controls. Subsequent analyses of CD40L (CD154) and CD107a expression on CD4+ T cells as early activation markers were performed by flow cytometry. Analog experiments were done with none-manipulated peripheral blood mononuclear cells (PBMC, right column). Supernatants from PBMC were taken from overnight cultures. Results are representatives of 4 experiments of each condition (CD40B versus PBMC). (D) Results of the coculture experiments are shown separately as means of the relative numbers of CD40L+ or CD107a+ T cells (n = 4 CD40B experiments; n = 4 PBMC experiments). SD is indicated for each column.
To compare CD40B cells with the APC capacity of PBMC and to exclude a stimulatory effect of contaminating monocytes, we performed corresponding experiments with primary nonmanipulated PBMC and PBMC derived supernatants. Under our experimental conditions, we obtained results distinctly different from those of Chakrabarty and colleagues, who reported > 60% activated T cells after cocultivation with PBMC.1 In contrast, our data show only a weak up-regulation of CD154 and CD107a in CD4+ T cells (0.6% and 1.4%) after cocultivation with PBMC (Figure 1D). This represents a 10 to 16-fold difference. Of note, the tested PBMC contained a physiologic average of 9% CD14+ monocytes (50% were CD16low, data not shown).
It has been previously demonstrated that CD14+CD16+ monocytes represent a more effective APC subpopulation compared with CD14hiCD16− monocytes.8 In the light of our experiments, we feel that the discrepancy of the data reported by Chakrabarty and colleagues1 compared with the literature can at least partially be explained by the experimental system.
Finally, our results indicate that activated B cells indeed can induce early up-regulation of CD40L and CD107a in T cells and therefore serve as potent APCs in T-cell activation.
Authorship
A.S.-V. and M.v.B.-B. contributed equally to this work.
Acknowledgments: This work was funded by the Else Kröner-Fresenius-Stiftung grant.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sebastian Theurich, MD, University Hospital Cologne, Department I of Internal Medicine, Laboratory for Tumor and Transplantation Immunology, Kerpenerstr 62, 50937 Cologne, Germany; e-mail: sebastian.theurich@uk-koeln.de.