Abstract
We report results from a study exploring the combination of romidepsin, bortezomib, and dexamethasone for the treatment of patients with multiple myeloma (MM) previously treated with > 1 prior therapy. The primary objective was to determine the maximum tolerated dose (MTD) of the combination using a novel accelerated dose-escalation schedule in patients with relapsed or refractory MM. The secondary objective was to determine overall response (OR), time to progression (TTP), and overall survival (OS). The MTD identified was bortezomib 1.3 mg/m2 (days 1, 4, 8, and 11), dexamethasone 20 mg (days 1, 2, 4, 5, 8, 9, 11, and 12), and romidepsin 10 mg/m2 (days 1, 8, and 15) every 28 days. Thrombocytopenia (64%) was the most common ≥ grade 3 hematologic toxicity. Peripheral neuropathy occurred in 76% of patients (n = 19) (≥ grade 3, 8%; 95% confidence interval [CI] 1%-26%). Maintenance romidepsin 10 mg/m2 (on days 1 and 8 of a 28-day cycle) proved feasible, with 12 patients receiving a median of 7.5 cycles (range: 1-29). An OR (M-protein) of > minor response (MR) was seen in 18 of 25 patients (72%); 2 (8%) had complete remissions (CRs) and 13 (52%) had partial responses (PRs), including 7 (28%) with very good PRs (VGPRs). The median TTP was 7.2 (95% CI: 5.5-19.6) months, and the median OS was > 36 months. This regimen shows activity with manageable toxicity and warrants further evaluation. This trial was registered at www.clinicaltrials.gov as NCT00431990.
Introduction
Multiple myeloma (MM) remains an incurable plasma cell malignancy. Bortezomib (Velcade), a specific and reversible inhibitor of the 26S proteasome, has activity in MM and is often combined with dexamethasone, immunomodulatory agents, and conventional and high-dose chemotherapy.1-6 Apart from directly inducing apoptosis, it has been widely regarded that the major consequence of proteasome inhibition is the accumulation of inhibitor-κB (IκB), an inhibitor of the transcription factor nuclear factor κB (NFκB), resulting in a net inhibition of the production of IL-6 and other growth factors that promote plasma cell growth.7 More recent data have suggested that bortezomib also induces transcriptional repression of class I histone deacetylases (HDAC1, HDAC2, and HDAC3) by caspase 8–dependent degradation of Sp1.8
The dysregulation of histone acetylation has been shown to be an important pro-oncogenic process in several malignancies, including MM.9-11 HDACs are enzymes that remove acetyl groups from the histones, resulting in the compaction of chromatin and the prevention of gene transcription. HDAC inhibitors reverse this process, leading to the transcription of previously silenced genes responsible for cellular differentiation and the induction of apoptosis. HDAC inhibitors can also target nonhistone proteins, which can affect both nuclear and cytoplasmic targets.9 Romidepsin (depsipeptide, FK228, Istodax) is a cyclic tetrapeptide HDAC inhibitor that was approved by the Food and Drug Administration (FDA) in the United States in 2009 for relapsed cutaneous T-cell lymphoma (CTCL). As a single agent, it is typically administered on a day 1, 8, and 15 schedule at a dose of 14 mg/m2 over 4 hours. It has demonstrated activity against several MM cell lines, and the anti-MM activity is associated with up-regulation of the tumor suppressors p21 and p5312 ; down-regulation of antiapoptotic molecules, including Bcl-2, Bcl-XL, BAX, and MCL-1; and induction of apoptosis in a dose-dependent fashion.9,11 As discussed in recent reviews by San Miguel et al9 and Dickinson et al,13 additional mechanisms are likely to be important target processes in MM, such as: up-regulation of p53, the disruption of the function of HSP90 and tubulin with associated dysfunction of the endoplasmic reticulum and aggresome formation, and cell-cycle arrest (mediated via the p21 and AKT pathways). Until recently, romidepsin was thought to have activity that was restricted to class I HDACs. However, recent evidence suggests that the spectrum of romidepsin activity is broader. For example, Kikuchi et al have shown that at higher doses (> 2nM) romidepsin has activity against both class 1 and class 2 HDACs, as shown by the acetylation of tubulin (HDAC6).8
As single agents, the efficacy of HDAC inhibitors in relapsed and refractory MM in previous pilot studies has been modest, with 1 study demonstrating 1 minor response (MR) in 10 patients and stable disease in several more14 treated with vorinostat (suberoylanilide hydroxamic acid). One study showed partial responses (PRs) in 1 of 15 patients treated with ITF2357.15 Similarly, 4 of 12 patients treated with romidepsin achieved disease stabilization with favorable tolerability.16
There is clear scientific rationale for combining a proteasome inhibitor with an HDAC inhibitor to simultaneously target multiple cellular processes to overcome drug resistance. Indeed, the synergistic effects of the bortezomib/HDAC inhibitor combination have been demonstrated in vitro, including synergistic effects on the aggresome, oxidative injury, and the induction of plasma cell apoptosis.17-19 Furthermore, the combination of bortezomib and romidepsin is able to overcome bortezomib resistance induced by overexpression of HDAC1.8 These preclinical data provide strong rationale for the use of this combination.
To date, there have been no published trials on combining the HDAC inhibitor romidepsin with proteasome inhibitors. In a phase 1 study of vorinostat in combination with bortezomib for relapsed and refractory MM, Badros et al demonstrated that the maximum tolerated dose (MTD) of oral Vorinostat was 400 mg once daily for 8 days (days 4-11), with bortezomib 1.3 mg/m2 administered on days 1, 4, 8, and 11 of a 21-day cycle. Response (PR or better) was seen in 9 of 21 (42%) of evaluable patients (including 2 very good PR [VGPR] and 7 PR).20 We sought to evaluate the tolerability and clinical efficacy of the combination of romidepsin, bortezomib, and dexamethasone in relapsed or refractory MM.
The potential for overlapping toxicities was anticipated, particularly fatigue and thrombocytopenia. The rate of grade 3 and 4 thrombocytopenia with bortezomib in the relapsed/refractory setting is ∼ 30%.3 There are limited data on the toxicity of romidepsin in patients with MM, with the only study of 13 patients demonstrating grade 3 thrombocytopenia in 23%.16 However, this is in keeping with the larger CTCL and peripheral T-cell lymphoma studies demonstrating grade 3 thrombocytopenia of 6%-24%.21,22 Furthermore, QTc prolongation is a potential class effect of HDAC inhibitors, including romidepsin. However, 3 romidepsin clinical trials have shown no absolute QTc values > 480 milliseconds and no QTc changes > 60 milliseconds with careful management of electrolytes and appropriate monitoring.23
To avoid the use of subtherapeutic doses of romidepsin and bortezomib in patients with advanced MM, we used a novel accelerated dose escalation schedule in which cohorts of one new patient per dose level were enrolled during the initial accelerated stage of the trial.24-27 Accelerated titration designs are more aggressive than standard approaches and therefore may be associated with a greater risk of unpredictable toxicity to individual patients. However, they have the capacity to significantly reduce the number of “undertreated” patients. Herein we report the results of our phase 1/2 trial of combination romidepsin and bortezomib with dexamethasone in patients with relapsed or relapsed and refractory MM using a novel accelerated dosing design.
Methods
Patient eligibility
Eligible patients were 18 years of age or more, had at least 1 previous therapy for MM, and required further treatment because of relapsed or refractory disease. Patients were required to have measurable disease, which was defined as one of either serum monoclonal protein of ≥ 5 g/L, urine Bence Jones protein of ≥ 200 mg/24 hours, serum-free light chain of > 100 mg/L, or a measureable soft tissue plasmacytoma. Patients previously treated with bortezomib were eligible if the response to bortezomib was > 6 months. Patients were not selected on the basis of cytogenetic or other clinical risk–based criteria. Additional eligibility criteria included a Karnofsky performance status of 80% or greater; anticipated life expectancy of at least 3 months; adequate BM reserve (a minimum peripheral absolute neutrophil count of 0.75 × 109/L, platelet count of 50 × 109/L, and hemoglobin of 75 g/L); adequate liver function (aspartase aminotransferase and alanine aminotransferase of up to 2.5 times the upper limit of normal, bilirubin up to 1.5 times the upper limit of normal); adequate cardiac function (ventricular ejection fraction of at least 45% by gated radionuclide study); creatinine clearance of at least 20 mL/min; and normal serum calcium, potassium, and magnesium levels (state supplements allowed). Patients on drugs that could cause prolongation of the QTc interval or inhibit CYP3A4 or those with National Cancer Institute-Common Terminology Criteria for Adverse Events (CTCAE) ≥ grade 3 neuropathy, congestive heart failure, uncontrolled ischemic heart disease, cardiac arrhythmias requiring antiarrhythmic medications other than calcium channel or β-blockers, QTc interval of 480 milliseconds or above, any past history of sustained ventricular tachyarrhythmia, or pregnant or lactating women were excluded. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki and the protocol was approved by the ethics committee of the Peter MacCallum Cancer Center. This trial was registered at www.clinicaltrials.gov as NCT00431990.
Drug administration and study design
The primary objective of the study was to determine the maximum tolerated dose (MTD) and safety profile of romidepsin when administered with bortezomib and dexamethasone in patients with relapsed or refractory MM. The secondary objective was to determine the efficacy of this combination in terms of overall response rate, time to progression (TTP), and overall survival (OS). Dose-limiting toxicities (DLTs) were defined as platelet count < 25 × 109/L, > CTCAE grade 2 neutropenia on day 1 of cycle 2 despite G-CSF support for 5 days or more, or febrile neutropenia, or > grade 2 nonhematologic toxicity that was considered of adequate clinical significance by the treating physician enough to warrant a dose reduction of bortezomib and/or romidepsin.
We also sought to reduce the toxicity risk to the participants by including a definition of “moderate toxicity” in the rules defining the end of the accelerated phase of dose escalation. Moderate toxicities were defined as platelet count between 25-50 × 109/L, grade 2 nausea or diarrhea lasting for 2 days or more despite optimum supportive therapy, grade 3 fatigue, other grade 2 treatment–related nonhematologic toxicities, and any toxicity resulting in > 3 weeks (and up to 4 weeks) of treatment interruption due to treatment-related toxicities.
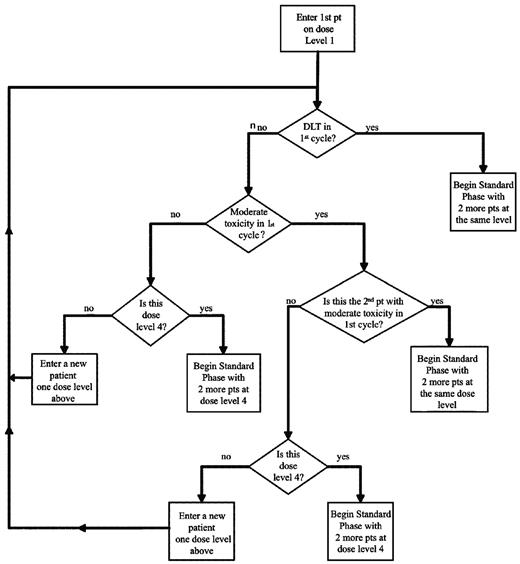
The trial consisted of 2 phases of romidepsin dose-escalation (Table 1 and Figure 1). All patients initially received bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11), dexamethasone (20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12), and romidepsin (8-14 mg/m2 on days 1, 8, and 15). Because romidepsin was given on day 15 of the cycle, a cycle length of 28 days was chosen to allow sufficient time for any over overlapping hematologic toxicities, particularly thrombocytopenia, to recover before commencement of the next cycle. During the initial accelerated phase, one patient was entered per cohort and was started at romidepsin 8 mg/m2 (Figure 1). Provided that no DLTs or moderate toxicities were observed before day 1 of cycle 2, the next patient was enrolled in the next dose level. If any moderate toxicity occurred, the next patient was enrolled at the same dose level. If < 2 patients experienced moderate toxicities in cycle 1, the next patient was enrolled at one dose level higher of romidepsin at 10 mg/m2, then 12 mg/m2, to a maximum dose of 14 mg/m2 (Table 1). The accelerated phase ended when one patient had experienced DLT or 2 patients had experienced moderate toxicity during cycle 1.
Dose escalation schedule 1
| Level . | Dose . | ||
|---|---|---|---|
| Bortezomib, mg/m2 . | Dexamethasone, mg . | Romidepsin, mg/m2 . | |
| 1 | 1.3 | 20 | 8 |
| 2 | 1.3 | 20 | 10 |
| 3 | 1.3 | 20 | 12 |
| 4 | 1.3 | 20 | 14 |
| Level . | Dose . | ||
|---|---|---|---|
| Bortezomib, mg/m2 . | Dexamethasone, mg . | Romidepsin, mg/m2 . | |
| 1 | 1.3 | 20 | 8 |
| 2 | 1.3 | 20 | 10 |
| 3 | 1.3 | 20 | 12 |
| 4 | 1.3 | 20 | 14 |
Accelerated phase cycle 1 dose escalation schema. Only toxicities experienced during cycle 1 apply. Dose level 4 is the highest level.
Accelerated phase cycle 1 dose escalation schema. Only toxicities experienced during cycle 1 apply. Dose level 4 is the highest level.
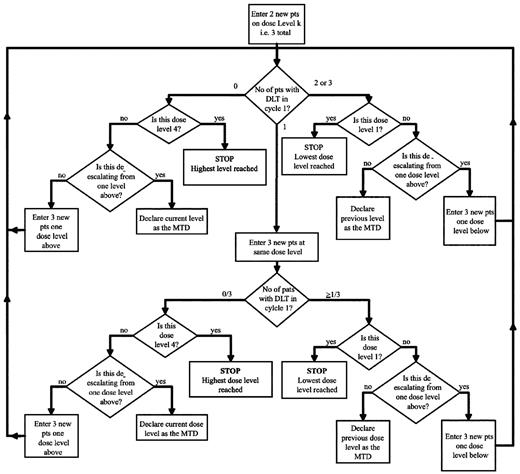
In the subsequent standard dose escalation phase (Figure 2), patients were entered in cohorts of 3, starting at the dose level of the last patient on the accelerated phase. If < 2 of 3 patients in the first cohort experienced DLT in cycle 1, then the next dose level below was investigated in the following cohort of 3 patients. However, if > 1 patient in this initial cohort experienced DLT in cycle 1, the cohort was further expanded to a total of 6 patients. Provided that DLT occurred in < 33% of patients in any given cohort, the dose of romidepsin was escalated in the following cohort of 3 patients until the MTD was determined or to a maximum of 14 mg/m2. The MTD was defined as the highest dose level studied at which the incidence of DLT was < 33%.
Standard phase cycle 1 dose escalation schema. Only toxicities experienced during cycle 1 apply. Level k is the dose level of the last patient entered in the accelerated phase in cycle 1.
Standard phase cycle 1 dose escalation schema. Only toxicities experienced during cycle 1 apply. Level k is the dose level of the last patient entered in the accelerated phase in cycle 1.
Before the determination of MTD, intrapatient dose escalation of romidepsin was permitted after every 2 cycles by 2 mg/m2 increments to a maximum of 14 mg/m2 (or MTD once determined), provided there had been no requirement to reduce bortezomib and/or romidepsin from the start of protocol treatment and the safety data from the next romidepsin dose level had been reviewed by the data-monitoring committee and deemed safe. After the determination of MTD, an additional 15 patients were accrued at the MTD to obtain further clinical efficacy data.
Patients were eligible for maintenance romidepsin therapy if they had stable disease or better after 8 cycles or complete remission (CR; see below) plus 2 cycles, or developed bortezomib-related neuropathy that required cessation of drug and achieved PR or better. Maintenance was given as single-agent romidepsin at the same dose as the last dose of induction therapy on day 1 and 8 of a 4-week cycle for the first 12 months and then every 2 months until disease progression.
Response assessment
Patients were assessed for response after every 2 cycles of therapy. Patients who stopped study treatment before the first response assessment were defined as having a response of “no change” (NC; or progressive disease if this could be determined). The definition of response was based on M-protein response criteria, with CR documented according to European Group for Blood & Marrow Transplantation criteria in conjunction with soft tissue plasmacytoma response assessment,28 and designated the overall study response criteria. The overall response (OR) was defined in 2 ways: (1) as CR + PR + MR as per the overall study response criteria, and (2) as defined by the uniform criteria (UC) with OR defined as stringent(s) CR + CR + VGPR + PR.29
Drug administration
Romidepsin was supplied by Celgene (initially by Gloucester Pharmaceuticals) and was and administered intravenously over 4 hours on days 1, 8, and 15 of a 28-day cycle. Bortezomib was supplied by Janssen Cilag and was administered as an intravenous bolus over 3-5 seconds on days 1, 4, 8, and 11 of a 28-day cycle. Dexamethasone was supplied as 4-mg tablets, and 20 mg was given orally on the day of bortezomib infusions and on the following day.
Supportive care
All patients were given valacyclovir 500 mg orally daily for herpes prophylaxis, sulfamethoxazole and trimethoprim for Pneumocystis jiroveci pneumonii prophylaxis, and bisphosphonate therapy as per our institutional practice. All patients were required to have adequate serum potassium (not < 4mM) and magnesium levels (not < 0.85mM) before each infusion of romidepsin. G-CSF support was allowed to maintain neutrophil counts above 1.0 × 109/L on day 1 of each cycle of treatment for up to 5 days before a dose reduction of romidepsin was required. G-CSF support was recommended during febrile neutropenia and for neutropenic patients with additional risk factors such as age > 65 years, severe lymphopenia, extensive mucosal damage, or rapidly advancing disease.
Statistical analyses
Two-sided Fisher exact tests were used to assess associations between categorical variables. Confidence intervals were exact, 2-sided with a confidence coefficient of 95%. TTP and OS were estimated for all patients using the Kaplan-Meier product limit method. Times were censored at the study close-out date for patients who were still being followed without having experienced the relevant event by the close-out date or the date of last contact for patients lost to follow-up without a prior event.
Results
Patients and demographics
Twenty-five patients were enrolled into the study between November 22, 2006 and February 16, 2009 (26 months) and all were assessable for toxicity. The characteristics of these patients are summarized in Table 2. There were 6 patients with previous exposure to bortezomib, with a median interval to retreatment of 13.7 months before date of registration to this study (range 1.9-28.1 months).
Patient demographics at study registration
| Total patients enrolled . | 25 . |
|---|---|
| Male:female | 2:3 |
| Median age, y (range) | 58 (30-78) |
| Karnofsky performance status, n (%) | |
| 100% | 11 (48%) |
| 90% | 7 (30%) |
| 80% | 6 (26%) |
| Unknown | 2 |
| β2-microglobulin, mg/L, median (range) | 3.4 (1.7-14.3) |
| ≤ 3.5 mg/L, n (%) | 14 (56%) |
| > 3.5-5.5 mg/L, n (%) | 6 (24%) |
| > 5.5 mg/L, n (%) | 5 (20%) |
| International staging at study entry, n (%) | |
| I | 13 (52%) |
| II | 7 (28%) |
| III | 5 (20%) |
| Past lines of treatment, n, median (range) | 2 (1-3) |
| Time from diagnosis, y, median (range) | 2.9 (0.1-9.8) |
| Previous treatment, n (%) | |
| Bortezomib-containing regimen | 6 (24%) |
| Thalidomide-containing regimen | 12 (48%) |
| Lenalidomide-containing regimen | 1 (4%) |
| Previous autologous stem cell transplantation | 12 (48%) |
| Cytogenetics performed [metaphases] Abnormality detected, n (%) | 15 (60%) [11 (44%)] 5 (20%) |
| FISH performed [successful] abnormality detected, n (%) | 14 (56%) [9 (36%)] 4 (16%) |
| 13q del | 2 Karyotype + 3 FISH only |
| t(4;14) or t(14;16) | 0 |
| 17p del | 0 |
| Total patients enrolled . | 25 . |
|---|---|
| Male:female | 2:3 |
| Median age, y (range) | 58 (30-78) |
| Karnofsky performance status, n (%) | |
| 100% | 11 (48%) |
| 90% | 7 (30%) |
| 80% | 6 (26%) |
| Unknown | 2 |
| β2-microglobulin, mg/L, median (range) | 3.4 (1.7-14.3) |
| ≤ 3.5 mg/L, n (%) | 14 (56%) |
| > 3.5-5.5 mg/L, n (%) | 6 (24%) |
| > 5.5 mg/L, n (%) | 5 (20%) |
| International staging at study entry, n (%) | |
| I | 13 (52%) |
| II | 7 (28%) |
| III | 5 (20%) |
| Past lines of treatment, n, median (range) | 2 (1-3) |
| Time from diagnosis, y, median (range) | 2.9 (0.1-9.8) |
| Previous treatment, n (%) | |
| Bortezomib-containing regimen | 6 (24%) |
| Thalidomide-containing regimen | 12 (48%) |
| Lenalidomide-containing regimen | 1 (4%) |
| Previous autologous stem cell transplantation | 12 (48%) |
| Cytogenetics performed [metaphases] Abnormality detected, n (%) | 15 (60%) [11 (44%)] 5 (20%) |
| FISH performed [successful] abnormality detected, n (%) | 14 (56%) [9 (36%)] 4 (16%) |
| 13q del | 2 Karyotype + 3 FISH only |
| t(4;14) or t(14;16) | 0 |
| 17p del | 0 |
Dose escalation
Table 2 summarizes the progress of the accelerated and standard phases of the dose escalation. Three patients were treated in the accelerated escalation phase, each receiving bortezomib 1.3 mg/m2 and romidepsin 8, 10, and 12 mg/m2. The patient receiving romidepsin at 12 mg/m2 experienced DLTs during cycle 1 (platelets < 25 × 109/L and atrial flutter in the setting of hypokalemia). In cohort 4, 2 patients entered the standard dose escalation phase at a romidepsin dose of 12 mg/m2, including 1 patient who died during cycle 1 from an intracerebral hemorrhage in the setting of rapidly progressive disease and grade 4 thrombocytopenia. This event was deemed to be disease related and the cohort was expanded to 6 patients, but when another patient experienced grade 3 fatigue, constipation, and bowel obstruction, this dose level was closed early and cohort 5 was commenced at the lower dose level of 10 mg/m2 and all patients were dose reduced to this level. The MTD was determined after cohort 5 as: bortezomib 1.3 mg/m2 and romidepsin 10 mg/m2, and a further 15 patients were treated at this dose in the expansion cohort. Therefore, a total of 19 patients were treated at the MTD, including 1 in the accelerated phase (cohort 2), 3 in standard escalation (cohort 5), and 15 in the expansion phase.
Adverse events
All 25 patients experienced treatment-related adverse events. Of these, 22 patients (88%; 95% confidence interval [CI]: 69%-97%) had at least one ≥ grade 3 toxicity that was possibly, probably, or definitely related to study treatment; these patients are summarized in Table 3. Eighteen patients (72%) experienced at least one hematologic toxicity, most commonly thrombocytopenia. Fifteen patients (60%) experienced nonhematologic toxicities, most commonly fatigue. Twenty-two patients experienced ≥ grade 3 toxicities, 4 (16%) of which occurred at dose level 3 (bortezomib 1.3 mg/m2 and romidepsin 12 mg/m2) before MTD determination. At the MTD, 16 of 19 patients (84%) had ≥ grade 3 hematologic toxicity, the most common being nonneutropenic fever/infection requiring hospital admission.
More than or equal to grade 3 adverse events at least possibly related to treatment
| Adverse event . | All patients (N = 25), n (%) [95% CI] . | Patients treated at MTD (n = 19*), n (%) [95% CI] . | ||||
|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Any hematological | 9 (36%) [18%-57%] | 9 (36%) [18%-57%] | 7 (37%) [16%-62%] | 6 (32%) [15%-57%] | ||
| Thrombocytopenia | 40% [21c61%] | 24% [9%-45%] | 42% [20%-67%] | 16% [3%-40%] | ||
| Anaemia | 24% [9%-45%] | 12% [3%-31%] | 21% [6%-46%] | 11% [1%-33%] | ||
| Neutropenia | 24% [9%-45%] | 12% [3%-31%] | 26% [9%-51%] | 11% [1%-33%] | ||
| Febrile neutropenia | 4% [0%-20%] | 0% [0%-18%] | ||||
| Any nonhematological | 8 (32%) [15%-54%] | 6 (24%) [9%-45%] | 1 (4%) [0%-20%] | 5 (26%) [9%-51%] | 5 (26%) [9%-51%] | |
| Neuropathy: sensory | 8% [1%-26%] | 5% [0%-26%] | ||||
| Fatigue (asthenia lethargy malaise) | 12% [3%-31%] | 8% [1%-26%] | 5% [0%-26%] | 5% [0%-26%] | ||
| Gastrointestinal | 4% [0%-20%] | 0% [0%-18%] | ||||
| Constipation | 4% [0%-20%] | 0% [0%-18%] | ||||
| Diarrhea | 4% [0%-20%] | 5% [0%-26%] | ||||
| Vomiting | 4% [0%-20%] | 5% [0%-26%] | ||||
| Dizziness | 4% [0%-20%] | 5% [0%-26%] | ||||
| Infection with < 2 neutrophils | 8% [1%-26%] | 4% [0%-20%] | 5% [0%-26%] | 5% [0%-26%] | ||
| Elevated creatinine | 4% [0%-20%] | 0% [0%-18%] | ||||
| Hyperkalemia | 8% [1%-26%] | 11% [1%-33%] | ||||
| Hypokalemia | 4% [0%-20%] | 0% [0%-18%] | ||||
| Hyponatremia | 20% [7%-41%] | 16% [3%-40%] | ||||
| Hyperglycemia | 8% [1%-26%] | 4% [0%-20%] | 11% [1%-33%] | 5% [0%-26%] | ||
| Elevated ALT | 8% [1%-26%] | 5% [0%-26%] | ||||
| Hypocalcemia | 4% [0%-20%] | 0% [0%-18%] | ||||
| Hypercalcemia | 4% [0%-20%] | 0% [0%-18%] | ||||
| Pain | 4% [0%-20%] | 5% [0%-26%] | ||||
| Hemorrhage CNS | 4% [0%-20%] | 0% [0%-18%] | ||||
| Adverse event . | All patients (N = 25), n (%) [95% CI] . | Patients treated at MTD (n = 19*), n (%) [95% CI] . | ||||
|---|---|---|---|---|---|---|
| Grade 3 . | Grade 4 . | Grade 5 . | Grade 3 . | Grade 4 . | Grade 5 . | |
| Any hematological | 9 (36%) [18%-57%] | 9 (36%) [18%-57%] | 7 (37%) [16%-62%] | 6 (32%) [15%-57%] | ||
| Thrombocytopenia | 40% [21c61%] | 24% [9%-45%] | 42% [20%-67%] | 16% [3%-40%] | ||
| Anaemia | 24% [9%-45%] | 12% [3%-31%] | 21% [6%-46%] | 11% [1%-33%] | ||
| Neutropenia | 24% [9%-45%] | 12% [3%-31%] | 26% [9%-51%] | 11% [1%-33%] | ||
| Febrile neutropenia | 4% [0%-20%] | 0% [0%-18%] | ||||
| Any nonhematological | 8 (32%) [15%-54%] | 6 (24%) [9%-45%] | 1 (4%) [0%-20%] | 5 (26%) [9%-51%] | 5 (26%) [9%-51%] | |
| Neuropathy: sensory | 8% [1%-26%] | 5% [0%-26%] | ||||
| Fatigue (asthenia lethargy malaise) | 12% [3%-31%] | 8% [1%-26%] | 5% [0%-26%] | 5% [0%-26%] | ||
| Gastrointestinal | 4% [0%-20%] | 0% [0%-18%] | ||||
| Constipation | 4% [0%-20%] | 0% [0%-18%] | ||||
| Diarrhea | 4% [0%-20%] | 5% [0%-26%] | ||||
| Vomiting | 4% [0%-20%] | 5% [0%-26%] | ||||
| Dizziness | 4% [0%-20%] | 5% [0%-26%] | ||||
| Infection with < 2 neutrophils | 8% [1%-26%] | 4% [0%-20%] | 5% [0%-26%] | 5% [0%-26%] | ||
| Elevated creatinine | 4% [0%-20%] | 0% [0%-18%] | ||||
| Hyperkalemia | 8% [1%-26%] | 11% [1%-33%] | ||||
| Hypokalemia | 4% [0%-20%] | 0% [0%-18%] | ||||
| Hyponatremia | 20% [7%-41%] | 16% [3%-40%] | ||||
| Hyperglycemia | 8% [1%-26%] | 4% [0%-20%] | 11% [1%-33%] | 5% [0%-26%] | ||
| Elevated ALT | 8% [1%-26%] | 5% [0%-26%] | ||||
| Hypocalcemia | 4% [0%-20%] | 0% [0%-18%] | ||||
| Hypercalcemia | 4% [0%-20%] | 0% [0%-18%] | ||||
| Pain | 4% [0%-20%] | 5% [0%-26%] | ||||
| Hemorrhage CNS | 4% [0%-20%] | 0% [0%-18%] | ||||
n = 19 including 1 in accelerated phase, 3 in standard escalation, and 15 in expansion phase.
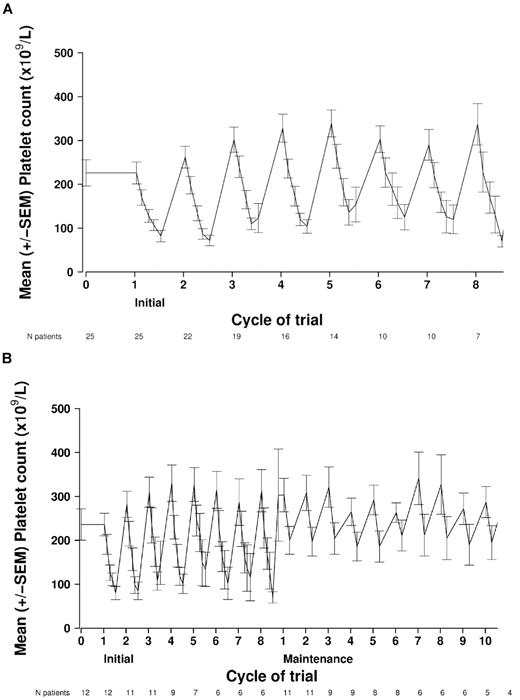
Eighteen patients (72%; 95% CI: 51%-88%) experienced ≥ grade 3 hematologic toxicity. As predicted, the most common ≥ grade 3 toxicity was thrombocytopenia, which occurred in 64% (grade 3, 40%; grade 4, 24%) of the total 25 patients. Before MTD determination, 3 of 5 patients who were on romidepsin 12 mg/m2 + bortezomib 1.3 mg/m2 in the standard dose escalation phase experienced grade 4 thrombocytopenia. At the MTD, 58% (11 of 19 patients) had ≥ grade 3 thrombocytopenia, but no bleeding complications resulted. In keeping with the thrombocytopenia pattern previously observed when both romidepsin and bortezomib were used as single agents, the thrombocytopenia rapidly resolved before the beginning of the next cycle (Figure 3A).30,31 The mean nadir of the platelet count was 48 × 109/L and occurred on cycle 2, day 15 of induction. During maintenance, the mean nadir of the platelet count of 155 × 109/L occurred on cycle 11, day 22 (Figure 3B).
Kinetics of thrombocytopenia. Mean ± SEM platelet values from each treatment day during induction (romidepsin, bortezomib, and dexamethasone) and maintenance therapy (romidepsin). (A) Kinetics of thrombocytopenia during induction (all patients). The mean nadir of the platelet count (48 × 109/L) occurred on cycle 2, day 15 of induction. (B) Kinetics of thrombocytopenia during induction and maintenance in patients who went on to receive maintenance romidepsin (n = 12). Mean ± SEM platelet values from each treatment day during maintenance therapy (romidepsin). The mean nadir of the platelet count (154 × 109/L) occurred on cycle 3, day 22.
Kinetics of thrombocytopenia. Mean ± SEM platelet values from each treatment day during induction (romidepsin, bortezomib, and dexamethasone) and maintenance therapy (romidepsin). (A) Kinetics of thrombocytopenia during induction (all patients). The mean nadir of the platelet count (48 × 109/L) occurred on cycle 2, day 15 of induction. (B) Kinetics of thrombocytopenia during induction and maintenance in patients who went on to receive maintenance romidepsin (n = 12). Mean ± SEM platelet values from each treatment day during maintenance therapy (romidepsin). The mean nadir of the platelet count (154 × 109/L) occurred on cycle 3, day 22.
Overall, 9 patients (36%) experienced ≥ grade 3 neutropenia; one patient with grade 4 and one patient with grade 3 neutropenia received romidepsin 12 mg/m2. However, there were no episodes of neutropenic sepsis and only one patient required G-CSF support on 9 single days (day 1 of cycles 2, 4, 5, and 7; days 8 and 11 of cycle 6; and days 8 and 11 of cycle 8) to allow continued treatment. Six patients experienced grade 3 anemia and 3 patients experienced grade 4 anemia (36%).
Peripheral neuropathy, mainly sensory, was experienced by 76% of all patients. Sensory neuropathy was mainly ≤ grade 2 and was manageable with bortezomib dose reduction. The worst grades of sensory neuropathy that were at least possibly related to study treatment are shown in Table 4. The incidence of serious, ≥ grade 3 peripheral neuropathy was 8% (95% CI 1%-26%). Five patients (20%) developed bortezomib-related neuropathy (3 with grade 2 and 2 with grade 3) that did not improve with dose interruption. No herpes zoster reactivation occurred.
Worst grades of sensory neuropathy
| Worst grade . | n (% [95% CI]) . | No. treated at MTD . |
|---|---|---|
| 0 | 6 (24% [9%-45%]) | 4 (21% [6%-46%]) |
| 1 | 8 (32% [15%-54%]) | 7 (37% [16%-62%]) |
| 2 | 9 (36% [18%-57%]) | 7 (37% [16%-62%]) |
| 3 | 2 (8% [1%-26%]) | 1 (5% [0%-26%]) |
| 4 | 0 (0% [0%-14%]) | 0 (0% [0%-18%]) |
| Worst grade . | n (% [95% CI]) . | No. treated at MTD . |
|---|---|---|
| 0 | 6 (24% [9%-45%]) | 4 (21% [6%-46%]) |
| 1 | 8 (32% [15%-54%]) | 7 (37% [16%-62%]) |
| 2 | 9 (36% [18%-57%]) | 7 (37% [16%-62%]) |
| 3 | 2 (8% [1%-26%]) | 1 (5% [0%-26%]) |
| 4 | 0 (0% [0%-14%]) | 0 (0% [0%-18%]) |
Overall, 12 patients (48%) eventually required bortezomib dose reduction or discontinuation (9 patients to 1.0 mg/m2 and 1 patient to 0.7 mg/m2 and 2 patients discontinued bortezomib and moved on to maintenance romidepsin only) because of peripheral neuropathy (n = 8), fatigue (n = 1), or physician's discretion (n = 3) after a median of 3.5 treatment cycles (range 1-8). Three patients required romidepsin dose reduction to 8 mg/m2 (n = 2: 1 from 12 mg/m2 and 1 from 10 mg/m2) or 10 mg/m2 (n = 1; from 12 mg/m2), 1 patient because of grade 4 fatigue, 1 patient because of grade 3 increased alanine aminotransferase, and 1 patient because of neuropathy. Overall, 25 patients underwent a median of 5 treatment bortezomib/romidepsin/dexamethasone cycles (range 1-8; 7 patients completed 8 cycles) and 12 patients underwent a median of 7.5 maintenance romidepsin cycles (range 1-29). Overall, patients received a median number of 8 cycles of therapy (range 1-33). Six patients commenced maintenance early, 5 because of neuropathy and 1 who attained a CR.
Efficacy
The median duration of follow-up was 21.8 months (range 12.7-39.6). Response was assessed by an intention-to-treat analysis and is summarized in Table 5. Four patients were not assessable for response: 2 died before the first assessment from pulmonary embolism or intracranial hemorrhage, 1 patient withdrew from study, and 1 patient progressed before the first assessment. These patients were assumed to have a response of NC or worse for estimation of the overall response rate. A response of MR or better was seen in 18 of 25 patients (72%; 95% CI: 51%-88%); 2 (8%) patients achieved CR, 13 (52%) PR, and 3 MR. In addition, 2 patients (8%) had stabilization of disease. One patient progressed at the first assessment after 1 treatment cycle. Median time to best response was 3.4 (range 1.6-12.3) months (Table 6). In comparison, when measured by UC, 15 patients (60%; 95% CI: 39%-79%) achieved an OR, with CR + VGPR achieved in 9 patients (36%): 2 (8%) CR, 7 (28%) VGPR, 6 (24%) PR, and a further 5 patients (20%) with disease stabilization (Table 7).
Summary of DLTs and moderate toxicities during dose escalation
| Cohort . | Phase . | Bortezomib, mg/m2 . | Romidepsin, mg/m2 . | n . | Toxicity . |
|---|---|---|---|---|---|
| 1 | Accelerated | 1.3 | 8 | 1 | None in cycle 1 |
| 2 | Accelerated | 1.3 | 10 | 1 | None in cycle 1 |
| 3 | Accelerated | 1.3 | 12 | 1 | DLTs at cycle 1: platelet count < 25 × 109/L; febrile neutropenia |
| 4 | Standard escalation | 1.3 | 12 | 4 | 2 patients with DLTs at cycle 1: (1) Intracerebral hemorrhage grade 5 in the setting of PD and grade 4 thrombocytopenia (2) Bowel obstruction |
| 5 | Standard escalation | 1.3 | 10 | 3* | No DLTs |
| Cohort . | Phase . | Bortezomib, mg/m2 . | Romidepsin, mg/m2 . | n . | Toxicity . |
|---|---|---|---|---|---|
| 1 | Accelerated | 1.3 | 8 | 1 | None in cycle 1 |
| 2 | Accelerated | 1.3 | 10 | 1 | None in cycle 1 |
| 3 | Accelerated | 1.3 | 12 | 1 | DLTs at cycle 1: platelet count < 25 × 109/L; febrile neutropenia |
| 4 | Standard escalation | 1.3 | 12 | 4 | 2 patients with DLTs at cycle 1: (1) Intracerebral hemorrhage grade 5 in the setting of PD and grade 4 thrombocytopenia (2) Bowel obstruction |
| 5 | Standard escalation | 1.3 | 10 | 3* | No DLTs |
PD indicates progressive disease.
The next cohort was in the expansion phase (n = 15) at this dose level.
Overall response by overall study response criteria
| Response . | Overall study response criteria . | |||
|---|---|---|---|---|
| All patients (N = 25), % (95% CI) . | Patients in expansion phase (N = 15), % (95% CI) . | |||
| Best response | ||||
| CR | n = 2 | 8 (1-26) | n = 1 | 7 (0-32) |
| PR | n = 13 | 52 (31-72) | n = 7 | 47 (21-73) |
| MR | n = 3 | 12 (3-31) | n = 2 | 13 (2-40) |
| NC | n = 2 | 8 (1-26) | n = 2 | 13 (2-40) |
| PD | n = 1 | 4 (0-20) | n = 1 | 7 (0-32) |
| Not evaluable* | n = 4 | 16 (5-36) | n = 2 | 13 (2-40) |
| Overall response (CR + PR + MR) | n = 18 | 72 (51-88) | n = 10 | 60 (38-88) |
| Response . | Overall study response criteria . | |||
|---|---|---|---|---|
| All patients (N = 25), % (95% CI) . | Patients in expansion phase (N = 15), % (95% CI) . | |||
| Best response | ||||
| CR | n = 2 | 8 (1-26) | n = 1 | 7 (0-32) |
| PR | n = 13 | 52 (31-72) | n = 7 | 47 (21-73) |
| MR | n = 3 | 12 (3-31) | n = 2 | 13 (2-40) |
| NC | n = 2 | 8 (1-26) | n = 2 | 13 (2-40) |
| PD | n = 1 | 4 (0-20) | n = 1 | 7 (0-32) |
| Not evaluable* | n = 4 | 16 (5-36) | n = 2 | 13 (2-40) |
| Overall response (CR + PR + MR) | n = 18 | 72 (51-88) | n = 10 | 60 (38-88) |
Patients came off study prior to the first scheduled assessment prior to cycle 3.
PD indicates progressive disease.
Overall response by UC
| . | UC . | |||
|---|---|---|---|---|
| All patients (N = 25), % (95% CI) . | Patients in expansion phase (at MTD) (N = 15), % (95% CI) . | |||
| Response | ||||
| sCR | n = 0 | 0 (0-22) | n = 0 | 0 (0-22) |
| CR | n = 2 | 8 (1-26) | n = 1 | 7 (0-32) |
| VGPR | n = 7 | 28 (12-49) | n = 4 | 27 (8-55) |
| PR | n = 6 | 24 (9-45) | n = 3 | 20 (4-48) |
| Stable disease | n = 5 | 20 (7-41) | n = 4 | 27 (8-55) |
| PD | n = 1 | 4 (0-20) | n = 1 | 7 (0-32) |
| Not evaluable* | n = 4 | 16 (5-36) | n = 2 | 13 (2-40) |
| Overall response (sCR + CR + VGPR + PR) | n = 15 | 60 (39-79) | n = 8 | 53 (27-79) |
| . | UC . | |||
|---|---|---|---|---|
| All patients (N = 25), % (95% CI) . | Patients in expansion phase (at MTD) (N = 15), % (95% CI) . | |||
| Response | ||||
| sCR | n = 0 | 0 (0-22) | n = 0 | 0 (0-22) |
| CR | n = 2 | 8 (1-26) | n = 1 | 7 (0-32) |
| VGPR | n = 7 | 28 (12-49) | n = 4 | 27 (8-55) |
| PR | n = 6 | 24 (9-45) | n = 3 | 20 (4-48) |
| Stable disease | n = 5 | 20 (7-41) | n = 4 | 27 (8-55) |
| PD | n = 1 | 4 (0-20) | n = 1 | 7 (0-32) |
| Not evaluable* | n = 4 | 16 (5-36) | n = 2 | 13 (2-40) |
| Overall response (sCR + CR + VGPR + PR) | n = 15 | 60 (39-79) | n = 8 | 53 (27-79) |
sCR indicates stringent CR; and PD, progressive disease.
Patients came off study prior to the first scheduled assessment prior to cycle 3.
The overall response among 12 patients previously treated with the immunomodulatory drugs (IMiDs) thalidomide or lenalidomide was 33% (95% CI: 10%-65%), and among 13 patients not previously exposed to thalidomide or lenalidomide, it was 77% (95% CI: 46%-95%; P = .05). There was a trend for the group previously treated with an IMiD to be more heavily pretreated, with 10 of 12 versus 7 of 13 (P = .20) of the IMiD-treated group having received > 1 prior line of therapy. There was also a trend for patients previously treated with an IMiD to have a shorter time from last therapy, with 8 of 12 versus 4 of 13 patients starting study treatment within 6 months of the previous therapy (P = .12).
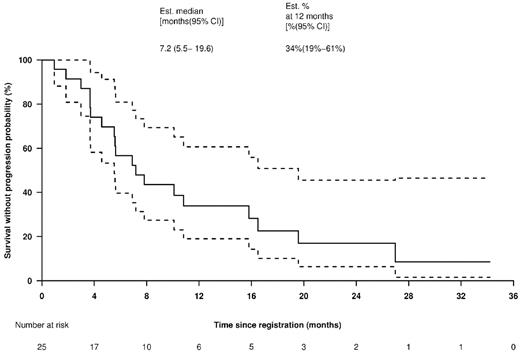
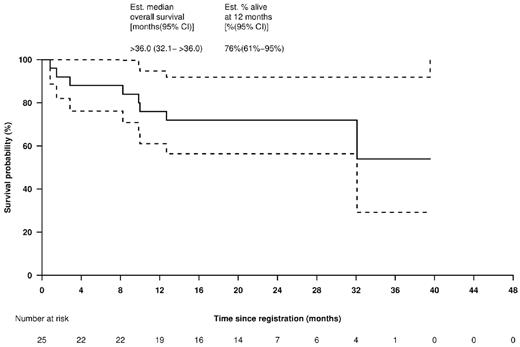
The median TTP was 7.2 (95% CI: 5.5-19.6) months (Figure 4). The median OS was estimated at > 3 (95%CI: 2.67 to > 3) years (Figure 5). In the 19 patients treated at the MTD, the median TTP was 7.0 (95% CI: 5.5 to > 20) months (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and, again, the median OS was estimated at > 3 years (supplemental Figure 2).
TTP for all patients. Solid line shows TTP Kaplan-Meier curve and dotted lines indicate 95% CIs.
TTP for all patients. Solid line shows TTP Kaplan-Meier curve and dotted lines indicate 95% CIs.
OS for all patients. Solid line shows OS Kaplan-Meier curve and dotted lines indicate 95% CIs.
OS for all patients. Solid line shows OS Kaplan-Meier curve and dotted lines indicate 95% CIs.
Discussion
The incurable nature of MM necessitates the need for the continued development of novel agents and novel combinations to overcome the growth of treatment-resistant plasma cell clones. Indeed, there are compelling scientific reasons for combining proteasome inhibitors and HDAC inhibitors in the treatment of relapsed MM. Despite there being 2 large, randomized trials of bortezomib with or without HDAC inhibitors (vorinostat and panobinostat) under way, there have been no phase 1 studies published on the combination of a proteasome inhibitor, an HDAC inhibitor, and dexamethasone.
In this phase 1/2 study, we have determined the MTD of the combination of bortezomib and the HDAC inhibitor romidepsin. We chose a novel accelerated dose escalation schedule in which cohorts of one new patient per dose level were used during the initial accelerated stage of the trial. Such a design has the capacity to reduce the number of patients treated at potentially subtherapeutic doses of drug. For example, the MTD-recommended dose of single-agent romidepsin in CTCL is 14 mg/m2 and, in this trial, we commenced romidepsin in cohort 1 well below that level at 8 mg/m2. Conversely, compared with the standard “3 + 3” phase 1 design, accelerated titration designs have the potential to expose patients in subsequent cohorts to a higher risk of toxicity (because of fewer patients in each cohort). In an attempt to offset this risk, we defined the end of the accelerated phase of dose-escalation as either 2 incident occurrences of moderate toxicity or one incident occurrence of DLT. In this study, only a single patient was treated below the final MTD.
As a single agent, the MTD of romidepsin in studies involving patients with solid tumors was 17.8 mg/m2 when given on days 1 and 5 of a 21-day cycle.32 Based on subsequent phase 2 studies, the Food and Drug Administration–approved dose for patients with CTCL was 14 mg/m2 on days 1, 8, and 15 of a 28-day cycle. In combination with bortezomib (1.3 mg/m2) and dexamethasone, we have demonstrated a lower romidepsin MTD of 10 mg/m2 on days 1, 8, and 15 of a 28-day cycle. Thrombocytopenia was the main DLT, which was not unexpected given that the thrombocytopenia induced by bortezomib and romidepsin demonstrate similar kinetics, the nadir of which occur on days 1030 and 11,31 respectively. In the present study, the nadir of the platelet count occurred on day 15 of cycle 2, similar to the day 11 of cycle 2 reported during the APEX study,33 and recovered to above baseline before the start of each induction cycle of therapy. We have recently demonstrated that this observed phenomenon is almost certainly driven by a paradoxical increase in circulating thrombopoietin during the periods of thrombocytopenia due to impaired platelet release driving an overshoot of circulating platelet levels on recovery platelet production.34 However, at the MTD, ≥ grade 3 thrombocytopenia was observed in 38% of treated patients; that value is only slightly higher than the 30% reported with single-agent bortezomib in the APEX trial.3 In the patients who went on to receive maintenance therapy, the degree of thrombocytopenia was less, but a similar pattern occurred, perhaps suggesting a similar underlying molecular etiology. The incidence of ≥ grade 3 neutropenia and anemia were acceptable and similar to those reported in previous phase 2 or 3 trials using bortezomib ± dexamethasone.3,33,35
Nonhematologic toxicities at the MTD were all manageable with standard approaches. Whereas the incidence of serious, ≥ grade 3 peripheral neuropathy (8%; 95% CI: 1%-26%) was similar to the previously reported 8%-12% with single-agent bortezomib,3 the overall incidence of peripheral neuropathy at any grade (76%; 95% CI: 55%-81%) was substantially higher than that reported in APEX (36%). This was mostly manageable with bortezomib dose reduction, but 20% of patients (n = 5) experienced > grade 2 neuropathy that did not improve with dose interruption. This may be the result of at least 50% of patients having been previously treated with regimens containing neurotoxic drugs such as bortezomib, thalidomide, and vincristine. However, it is reasonable to speculate that the higher incidence of peripheral neuropathy observed in our group of patients cotreated with romidepsin may have been due to disruption of bortezomib-induced, cytoprotective aggresome formation mediated by HDAC6. This also increases endoplasmic reticulum stress in peripheral neurons, which exacerbates peripheral neuropathy while increasing antitumor cytotoxicity.36 In other studies combining HDAC inhibition with bortezomib, less neuropathy has been suggested, although this may have been because of qualitative differences between the agents.37,38 Peripheral neuropathy is the most important toxicity limiting the duration of bortezomib therapy in a significant number of patients. The incidence of bortezomib-induced neuropathy is significantly less with weekly IV39 or subcutaneous administration40 and, although neither was accepted practice at the time we designed our study, these would be attractive alternative treatment strategies that should be explored in the future.
Patients received a median of 5 (range 1-8) cycles of the bortezomib-romidepsin and dexamethasone combination and 12 received a median of 7.5 (range 1-29) cycles of romidepsin as maintenance therapy, demonstrating the long-term tolerability of this regimen.
Sixty percent of our patients achieved at least a PR by modified M-protein criteria (36% CR + VGPR by UC), which is substantially higher than that reported for either bortezomib ± dexamethasone (30%-38%)3,33,35 or HDAC inhibitor alone.15,41 Of the 6 patients who previously relapsed on bortezomib, 4 established disease stability or improvement to PR (1 PR, 1 MR, and 2 NC), with a median TTP of 5.6 months despite having rapidly progressive disease at the time of study entry.
Responses to this combination were durable, with a median TTP of 7.2 months and 3 of the patients achieving a TTP exceeding 20 months. The median survival for our cohort has yet to be reached but is > 36 months, compared with 29.8 months in the pivotal APEX bortezomib study. The 1-year survival rate was 76% (95% CI: 61%-95%) in a group of heavily pretreated patients. Overall, our results compare favorably with the APEX study, in which similar patient characteristics were reported. Our results are also in agreement with preclinical data, and indicate that the addition of romidepsin to the bortezomib/dexamethasone combination has encouraging activity and manageable toxicity in relapsed and relapsed/refractory MM and so warrants further evaluation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This was an investigator-initiated trial with financial support from Celgene (formerly Gloucester Pharmaceuticals). S.J.H. receives research support from the Vaccari Foundation. R.J. receives research support from the National Health and Medical Research Council of Australia, the Victorian Cancer Agency, and the Leukemia Foundation of Australia.
Authorship
Contribution: S.J.H. and H.M.P. designed and managed the study, analyzed the data, and wrote the manuscript; H.Q. and E.L. analyzed the data and wrote the manuscript; J.D. managed the data; S.R. managed the study and contributed to the manuscript; J.F.S., H.J., D.S.R., and M.D. contributed to the study and to the manuscript; R.J. and P.N. contributed to the data interpretation and to the manuscript; and J.N. provided romidepsin and contributed to the study design and to the manuscript.
Conflict of interest disclosure: J.N. is an employee of Celgene Corporation. S.J.H. and H.M.P. receive research funding from and have been members of advisory boards for Celgene Corporation and Janssen Cilag. J.F.S. and D.S.R. receive research funding from and have been members of advisory boards for Celgene Corporation. P.N. and H.Q. receive research funding from Celgene Corporation.
Correspondence: Dr Simon Harrison MBBS, MRCP(uk), FRCPath(uk), FRACP, PhD, Haematologist, Director of Clinical Aphaeresis, Honorary Senior Fellow, Melbourne University, Peter MacCallum Cancer Centre, Locked Bag 1, A'Beckett St, Melbourne, Victoria 8006, Australia; e-mail: simon.harrison@petermac.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal