Abstract
Aberrant activation of the Wnt pathway plays a pathogenetic role in various tumors and has been associated with adverse outcome in acute lymphoblastic leukemia (ALL). LEF1, a key mediator of Wnt signaling, has been linked to leukemic transformation, and recurrent mutations of LEF1 have been identified in pediatric T-ALL. Here we evaluated the prognostic significance of LEF1 expression in B-precursor ALL patients. LEF1 expression was determined by quantitative real-time RT-PCR in 282 adult B-precursor ALL patients treated on 06/99 and 07/03 GMALL trials. Patients were grouped into quartiles (Q1-Q4) according to LEF1 expression levels (LEF1 high, Q4; n = 71; LEF1 low, Q1-Q3; n = 211). Patients with high LEF1 expression had a significantly shorter relapse-free survival (RFS) compared with low LEF1 expressers (5-year RFS: LEF1 high, 27%; LEF1 low, 47%; P = .05). Importantly, high LEF1 expression was also associated with inferior RFS in standard-risk patients and was independently predictive for RFS (P = .02) in multivariate analyses for this subgroup. Thus, high LEF1 expression identifies B-precursor ALL patients with inferior RFS, supporting a pathogenetic role of Wnt signaling in ALL. Standard-risk patients with high LEF1 expression might benefit from early treatment modifications and new molecular therapies, including agents targeting the Wnt pathway.
Introduction
Long-term outcome of most adult patients with B-precursor acute lymphoblastic leukemia (ALL) remains unsatisfactory, although intensified therapy regimens have resulted in high complete remission (CR) rates.1,2 During the last decade, overall survival (OS) of adult ALL patients has primarily been improved by risk-adapted treatment stratification based on clinical and molecular risk factors and by the use of tyrosine kinase inhibitors in BCR-ABL–positive patients. In the large, heterogeneous group of standard-risk patients without established risk factors, the relapse rate is still approximately 40% to 50% and not predictable with pretreatment markers.3,4 Prospective evaluation of minimal residual disease (MRD) has improved individual risk assessment of standard-risk patients; however, the identification of new prognostic factors is of particular interest for this subgroup of ALL. Moreover, characterization of aberrant signaling pathways with prognostic relevance in standard-risk B-precursor ALL might elucidate molecular mechanisms of therapy resistance and may help to develop novel targeted therapies for these patients.
Lymphoid enhancer factor 1 (LEF1) is a member of the LEF/T-cell factor family of transcription factors and a key mediator of the canonical Wingless-type (Wnt) pathway.5 Wnt activation results in the accumulation of β-catenin, which translocates to the nucleus and forms a complex with LEF/T-cell factor DNA binding proteins, thereby enhancing the transcription of target genes. Wnt downstream target genes (eg, MYC, CCND1) are involved in the regulation of central cellular processes, such as differentiation, cell cycle, proliferation, and survival.6 Activation of the Wnt pathway has been implicated in leukemic transformation7-9 and was shown to promote proliferation and survival of leukemic cells in vitro.9-11 In ALL patients, a frequent down-regulation of Wnt antagonists sFRP1, sFRP2, sFRP4, sFRP5, WIF1, DKK3, and HDPR1 by promoter hypermethylation has been observed.12 This aberrant methylation profile was associated with activation of the Wnt pathway and with inferior long-term survival.
In normal hematopoiesis, LEF1 plays a crucial role in the development of B- and T-lymphocytes as well as neutrophilic granulocytes.13-15 In different hematologic malignancies, including lymphomas,16,17 chronic lymphocytic leukemia,18 ALL, and acute myeloid leukemia (AML),19-21 LEF1 was found to be highly expressed. In vitro studies revealed a prosurvival effect of LEF1 in an AML1-ETO–positive leukemic cell line,9 primary chronic lymphocytic leukemia cells,18 and murine T-cell lymphomas.17 Moreover, high expression of LEF1 was demonstrated to induce B-lymphoblastic leukemia and myeloid leukemia in a mouse model.20 Gutierrez et al recently identified recurrent inactivating monoallelic and biallelic microdeletions and truncating mutations of the LEF1 gene in pediatric T-ALL.22 The subgroup of LEF1-inactivated T-ALL patients was characterized by a younger age and a trend toward a better OS.
Given the functional role of LEF1 in ALL and its putative prognostic impact, we evaluated the prognostic significance of LEF1 mRNA expression in 282 adult patients with B-precursor ALL enrolled on multicenter treatment protocols of the German Multicenter ALL (GMALL) study group in the context of known risk factors.
Methods
Patients
We analyzed 282 adult patients with newly diagnosed B-precursor ALL that were enrolled between 1999 and 2006 on GMALL multicenter trials (GMALL 06/99, n = 138; GMALL 07/03, n = 144).23 Samples with sufficient diagnostic material available were selected from consecutive patients. Clinical and molecular features and outcome of our patient cohort were comparable with those from the total study population.24,25 Both study protocols included intensive chemotherapy, radiotherapy, and autologous or allogeneic stem cell transplantation (SCT) according to risk-adapted stratification. The GMALL 07/03 protocol also contained rituximab for CD20+ patients. The following risk factors were used: WBC count more than 30/nL, late CR (> 3 weeks), pro-B ALL, t(9;22)/BCR-ABL, and t(4;11)/MLL-AF4. Risk groups were assigned as follows: standard-risk (no risk factor), high-risk (≥ 1 risk factor), and very high-risk (presence of t(9;22)/BCR-ABL). High-risk and very high-risk patients with a sibling or matched unrelated donor were scheduled for allogeneic SCT in first CR (CR1). Details of the study protocols are available in the European Leukemia Trial Registry.26 The studies were approved by the ethics board of the Johann Wolfgang Goethe University Frankfurt/Main, Germany. Informed consent was given according to the Declaration of Helsinki.
Diagnostic analyses
Immunophenotypic analyses were performed by flow cytometry on fresh pretreatment BM and PB samples at the GMALL central reference laboratory at Charité (Berlin, Germany).27 A cell-surface antigen was defined positive when fluorescence intensity of at least 20% of cells exceeded fluorescence of negative control.
Determination of BCR-ABL and MLL-AF4 were performed at the GMALL central reference laboratory at Charité (Berlin, Germany), as previously described.27,28 Analysis of MLL-AF4 was only done in CD10− samples because the presence of MLL-AF4 is nearly exclusively restricted to this subgroup in B-precursor ALL.29 BAALC expression levels were measured as reported previously.30
Real-time RT-PCR assays
RNA isolation and synthesis of complementary DNA from diagnostic BM samples were done as previously described.30 Quantitative real-time RT-PCR was done for each sample in duplicate. Multiplex PCR was performed with the housekeeping gene GUSB (β-glucuronidase), and the comparative cycle threshold (Ct) method was used to determine the relative expression levels of LEF1. The mean of the cycle number difference of the 2 replicates (ΔCt = GUS − LEF1) was calculated, expressed as 2μ(ΔCt). Amplifications were carried out on the Rotor Gene real-time PCR 3000 system (Corbett Research) at 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. GUS and LEF1 were coamplified using 2 μL cDNA, 1× master mix (IQ Mix, Bio-Rad), 600nM of each primer, and 350nM of each probe. Primers for GUS and LEF1 were intron spanning: GUS probe, 5′-HEX-CCAGCACTCTCGTCGGTGACTGTTCA-BHQ1; GUS forward, 5′-GAAAATATGTGGTTGGAGAGCTCATT; GUS reverse, 5′-CCGAGTGAAGATC- CCCTTTTTA; LEF1 probe, 5′FAM-CCAGATTCTTGGCAGAAGGTGGCAT-TAMRA; LEF1 forward, 5′-AATGAGAGCGAATGTCGTTGC; and LEF1 reverse, 5′-GCTGTCTTTCTTTCCGTGCTA. LEF1 forward and reverse primer sequences were obtained from a public database (http://pga.mgh.harvard.edu/primerbank; Primer Bank ID 7705917a1). Each assay included positive and negative controls. RNA from the cell line BE-13 was measured in each run to calibrate between runs. In all samples, amplification of GUS reached the threshold within 30 cycles.
Mutational analyses of LEF1
We performed mutational analyses of LEF1 exons 2 and 3, as the reported hotspot regions in T-ALL.22 Sufficient genomic DNA was available from 40 patients of the 282 patients' cohort. Patients' clinical and molecular characteristics were comparable with those from the total study population. Genomic DNA was isolated from pretreatment BM samples using Trizol reagent (Invitrogen) following the manufacturer's instructions. DNA fragments spanning the entire LEF1 exons 2 and 3 were amplified by PCR using AmpliTaq Gold (Applied Biosystems) and the following primers: exon 2 forward, 5′-TTTTCTTTCTTTTGGGTGTGG; exon 2 reverse, 5′-AAATTGCACCCCTTATCTGC; exon 3 forward, 5′-AAAGGGAAGTCAGTGCATCATT; and exon 3 reverse, 5′-ACAAATCAATTTGCACTTCTGAAC. DNA sequencing was performed on purified PCR products.
Statistical analyses
Patients were grouped into quartiles according to LEF1 expression levels (Q1-Q4, each quartile containing 25% of patients) and divided into high LEF1 (Q4; n = 71) and low LEF1 (Q1-Q3; n = 211) based on the trend observed in clinical outcome after performing a Cox regression analysis for relapse-free survival (RFS) with LEF1 quartile grouping as the independent variable. In this model, patients in the highest LEF1 quartile showed a substantially different RFS compared with the remaining patients with lower LEF1 expression levels. The differences in regression coefficients with SE for each quartile were as follows: Q1 versus Q4, −0.61 (SE 0.44), P = .17; Q2 versus Q4, −1.3 (SE 0.60), P = .03; Q3 versus Q4, −1.0 (SE 0.50), P = .05. LEF1 expression ranged between 0 and 1.24 with the following median expression for each quartile: 0.02 (Q1), 0.08 (Q2), 0.19 (Q3), and 0.42 (Q4).
Remission was determined after completion of induction chemotherapy. CR was defined by a granulocyte count of at least 1.5/nL, a platelet count of at least 100/nL, the absence of PB blasts, a BM cellularity of at least 20% with maturation of all cell lines and < 5% blasts, and the absence of extramedullary leukemia. Primary therapy failure was defined as persistence of PB blasts or > 5% blasts in BM. Relapse was defined as the reappearance of PB blasts, > 5% blasts in BM, or appearance of extramedullary leukemia after achievement of CR. OS was measured from the beginning of therapy until date of death or last follow-up. RFS was determined from the date of first CR until relapse. Patients without reported relapse were censored by the end of follow-up. Patients with withdrawal in CR were censored at the respective dates. Patients who received SCT in CR1 were censored at the time of transplantation for RFS analyses. OS analyses were performed both with inclusion of SCT patients as well as with censoring of SCT patients at the time of transplantation. The median follow-up for all living patients was 42.6 months.
Survival curves were calculated by the Kaplan-Meier method with the log-rank comparing differences between survival curves. Clinical features across groups were compared using the χ2 or a 2-sided Fisher exact test for categorical data and the nonparametric Mann-Whitney U test for continuous variables. A P value ≤ .05 (2-sided) was considered significant. Multivariate analyses were performed using the Cox proportional hazards model for survival and with logistic regression for the relapse rate, both with stepwise forward selection including the following variables in the full model: LEF1 expression (Q4 vs Q1-Q3), age (10-year increase), WBC (10/nL increase), CD20 (positive vs negative), BCR-ABL (presence vs absence), MLL-AF4 (presence vs absence), and immunophenotype (pro-B vs common/pre-B). For OS analysis, also BAALC expression was integrated as a continuous variable in the Cox model because this marker was of prognostic relevance for OS, but not for RFS in B-precursor ALL.30 SPSS software package (Version 18.0 for Windows; SPSS) was used for calculations.
Results
LEF1 expression with respect to clinical and molecular characteristics
We determined LEF1 mRNA expression in 282 patients with newly diagnosed B-precursor ALL. For statistical analyses, patients were divided into high and low LEF1 expression groups (Q4 vs Q1-Q3). High LEF1 expression was significantly associated with CD20 positivity (P = .009) and inversely associated with the expression of myeloid markers (P = .001; Table 1). No other significant correlations between LEF1 expression levels and clinical, molecular features, immunophenotypic subgroups, or the GMALL risk groups were found (Table 1). Of note, there were not enough patient samples assayed for MRD to allow correlation of LEF1 expression and MRD. In the standard-risk group, high LEF1 expression was again significantly associated with the absence of expression of myeloid markers (P = .01). No significant differences were observed regarding CD20 positivity or other patients' characteristics and LEF1 expression.
Clinical and molecular features with respect to LEF1 expression in B-precursor ALL (overall cohort)
| Characteristic . | LEF1 low (n = 211) . | LEF1 high (n = 71) . | P . |
|---|---|---|---|
| Age, y | .39 | ||
| Median | 37 | 39 | |
| Range | 15-64 | 16-65 | |
| Sex, % | .34 | ||
| Male | 50 | 56 | |
| WBC, × 109/L* | .58 | ||
| Median | 15 | 14 | |
| Range | 0.8-594 | 0.5-581 | |
| > 30 × 109/L, % | 37 | 38 | .89 |
| CNS involvement, no. (%) | 1.0 | ||
| Yes/total | 8/156 (5) | 2/52 (4) | |
| Molecular genetics | |||
| BCR-ABL+, no. (%) | 82 (39) | 35 (49) | .13 |
| MLL-AF4+, no. (%) | 21 (10) | 2 (3) | .08 |
| BAALC, high %† | 54 | 39 | .54 |
| CD34+, % | 76 | 75 | .87 |
| CD20+, % | 30 | 48 | .009 |
| Myeloid markers, %‡ | 48 | 27 | .001 |
| Immunophenotypic subtype, no. (%) | .20§ | ||
| Common ALL | 138 (66) | 45 (63) | |
| Pre-B ALL | 45 (21) | 21 (30) | |
| Pro-B ALL | 28 (13) | 5 (7) | |
| GMALL risk groups, %‖ | .25§ | ||
| Standard-risk | 70/210 (33) | 21/70 (30) | .77 |
| High-risk | 59/210 (28) | 14/70 (20) | .43 |
| Very high-risk | 82/210 (39) | 35/70 (50) | .33 |
| Allogeneic SCT in CR1, % | 37 | 36 | 1.0 |
| Characteristic . | LEF1 low (n = 211) . | LEF1 high (n = 71) . | P . |
|---|---|---|---|
| Age, y | .39 | ||
| Median | 37 | 39 | |
| Range | 15-64 | 16-65 | |
| Sex, % | .34 | ||
| Male | 50 | 56 | |
| WBC, × 109/L* | .58 | ||
| Median | 15 | 14 | |
| Range | 0.8-594 | 0.5-581 | |
| > 30 × 109/L, % | 37 | 38 | .89 |
| CNS involvement, no. (%) | 1.0 | ||
| Yes/total | 8/156 (5) | 2/52 (4) | |
| Molecular genetics | |||
| BCR-ABL+, no. (%) | 82 (39) | 35 (49) | .13 |
| MLL-AF4+, no. (%) | 21 (10) | 2 (3) | .08 |
| BAALC, high %† | 54 | 39 | .54 |
| CD34+, % | 76 | 75 | .87 |
| CD20+, % | 30 | 48 | .009 |
| Myeloid markers, %‡ | 48 | 27 | .001 |
| Immunophenotypic subtype, no. (%) | .20§ | ||
| Common ALL | 138 (66) | 45 (63) | |
| Pre-B ALL | 45 (21) | 21 (30) | |
| Pro-B ALL | 28 (13) | 5 (7) | |
| GMALL risk groups, %‖ | .25§ | ||
| Standard-risk | 70/210 (33) | 21/70 (30) | .77 |
| High-risk | 59/210 (28) | 14/70 (20) | .43 |
| Very high-risk | 82/210 (39) | 35/70 (50) | .33 |
| Allogeneic SCT in CR1, % | 37 | 36 | 1.0 |
CNS indicates central nervous system.
N = 268.
Above median expression level.
Coexpression of CD13, CD33, CD65s, or CD15.
Overall P value for the frequency of the 3 immunophenotypes/risk groups across the LEF1 expression groups.
N = 280.
LEF1 expression and outcome in B-precursor ALL patients (overall cohort)
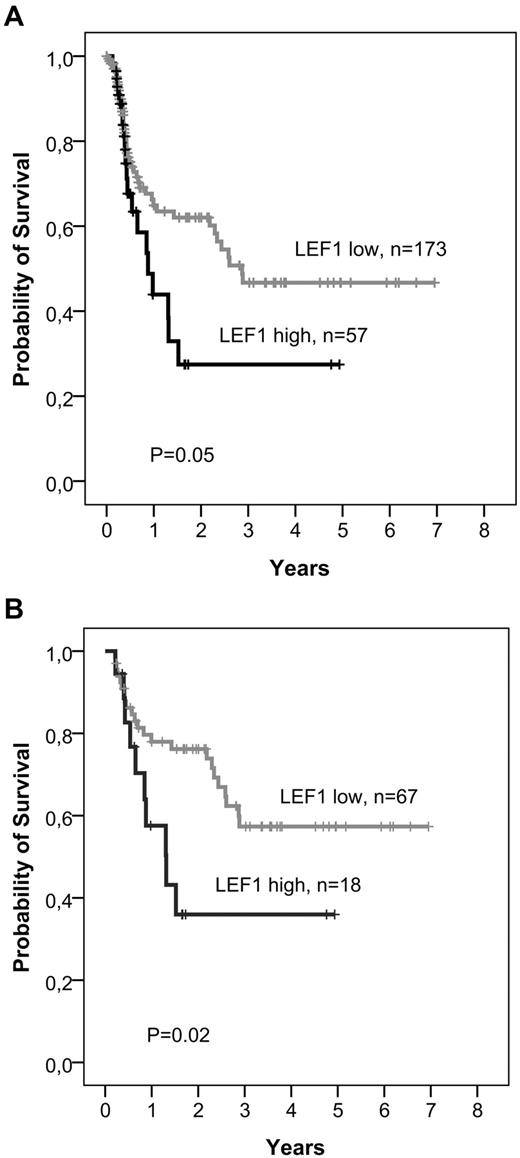
Patients with high compared to low LEF1 expression showed no significant differences regarding achievement of CR or the frequency of primary refractory disease (Table 2). High LEF1 expression was significantly associated with a higher relapse rate (LEF1 high, 74%; LEF1 low, 49%; P = .03; Table 2). In multivariate analyses, LEF1 expression was the only predictive variable for the relapse rate in the overall cohort (odds ratio = 3.4; 95% CI, 1.2-9.4; P = .02). High LEF1 expressers showed a significantly inferior RFS compared with low LEF1 expressers (5-year RFS: LEF1 high, 27%; LEF1 low, 47%; P = .05; Table 3; Figure 1A). On Cox regression analysis, LEF1 expression was not independently predictive for RFS in the overall cohort (Table 4). Patients with high LEF1 expression showed a trend toward an inferior OS (5-year OS: LEF1 high, 29%; LEF1 low, 47%; P = .06; Table 3) in the overall cohort of B-precursor ALL.
Clinical response according to LEF1 expression (overall cohort)
| Clinical outcome . | LEF1 low . | LEF1 high . | P . |
|---|---|---|---|
| Complete remission rate | |||
| CR/total, no. (%)* | 173/199 (87) | 57/68 (84) | .54 |
| Refractory disease | |||
| Refractory/total, no. (%)† | 16/189 (9) | 4/61 (7) | .79 |
| Relapse rate | |||
| Relapse/total, no. (%)‡ | 41/84 (49) | 20/27 (74) | .03 |
| Clinical outcome . | LEF1 low . | LEF1 high . | P . |
|---|---|---|---|
| Complete remission rate | |||
| CR/total, no. (%)* | 173/199 (87) | 57/68 (84) | .54 |
| Refractory disease | |||
| Refractory/total, no. (%)† | 16/189 (9) | 4/61 (7) | .79 |
| Relapse rate | |||
| Relapse/total, no. (%)‡ | 41/84 (49) | 20/27 (74) | .03 |
N = 267. For 15 patients, no remission status was available.
N = 250. Seventeen patients who died during induction therapy were excluded.
N = 111. Patients who received SCT in CR1 were excluded for evaluation of the relapse rate.
Long-term survival with respect to LEF1 expression
| Clinical outcome . | LEF1 low . | LEF1 high . | P . |
|---|---|---|---|
| RFS, relapse-free at 5 y, % (95% CI) | |||
| Overall cohort (N = 230) | 47 (35-59) | 27 (8-47) | .05 |
| Standard-risk (N = 85) | 57 (43-71) | 36 (12-61) | .02 |
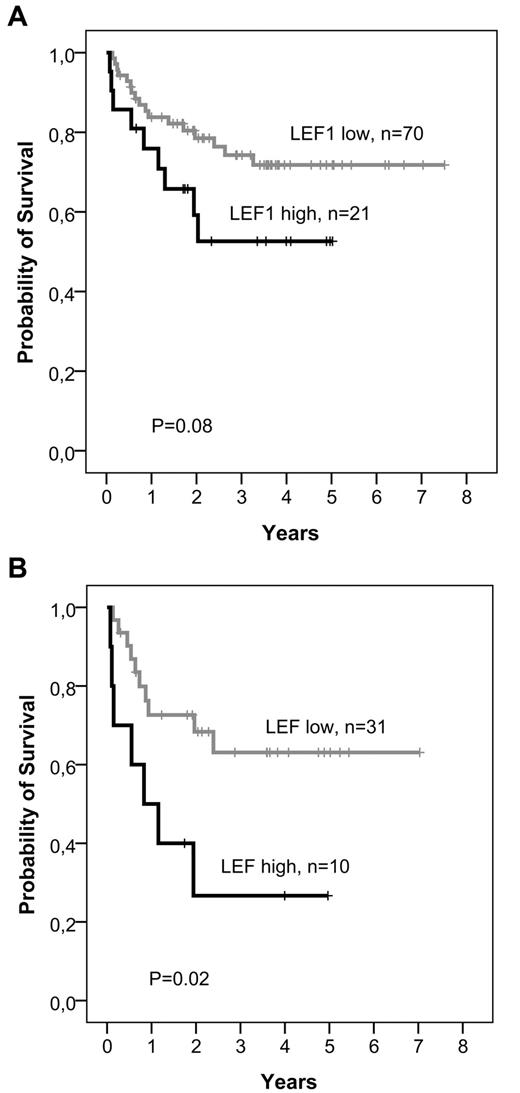
| OS, alive at 5 y, % (95% CI) | |||
| Overall cohort (N = 282) | 47 (38-56) | 29 (14-44) | .06 |
| Standard-risk (N = 91) | 72 (60-84) | 53 (29-77) | .08 |
| Standard-risk, age > 35 y (N = 41) | 63 (44-82) | 27 (12-42) | .02 |
| Clinical outcome . | LEF1 low . | LEF1 high . | P . |
|---|---|---|---|
| RFS, relapse-free at 5 y, % (95% CI) | |||
| Overall cohort (N = 230) | 47 (35-59) | 27 (8-47) | .05 |
| Standard-risk (N = 85) | 57 (43-71) | 36 (12-61) | .02 |
| OS, alive at 5 y, % (95% CI) | |||
| Overall cohort (N = 282) | 47 (38-56) | 29 (14-44) | .06 |
| Standard-risk (N = 91) | 72 (60-84) | 53 (29-77) | .08 |
| Standard-risk, age > 35 y (N = 41) | 63 (44-82) | 27 (12-42) | .02 |
Patients who received SCT in CR1 were censored at the time of transplantation.
Kaplan-Meier analyses of RFS with respect to LEF1 expression. Patients undergoing SCT in CR1 were censored at the time of transplantation. (A) Overall cohort. (B) Standard-risk patients.
Kaplan-Meier analyses of RFS with respect to LEF1 expression. Patients undergoing SCT in CR1 were censored at the time of transplantation. (A) Overall cohort. (B) Standard-risk patients.
Multivariate analyses for RFS
| Variable* . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| RFS, overall cohort | |||
| WBC | 1.1 | 1.0-1.1 | .02 |
| Age, y | 1.4 | 1.2-1.6 | < .001 |
| Immunophenotype | .04 | ||
| Common vs pro-B | 3.7 | 0.9-16 | .08 |
| Pre-B vs pro-B | 2.0 | 0.4-9.0 | .38 |
| RFS, standard risk | |||
| LEF1 expression, Q4 vs Q1-Q3 | 2.4 | 1.1-5.1 | .02 |
| Variable* . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| RFS, overall cohort | |||
| WBC | 1.1 | 1.0-1.1 | .02 |
| Age, y | 1.4 | 1.2-1.6 | < .001 |
| Immunophenotype | .04 | ||
| Common vs pro-B | 3.7 | 0.9-16 | .08 |
| Pre-B vs pro-B | 2.0 | 0.4-9.0 | .38 |
| RFS, standard risk | |||
| LEF1 expression, Q4 vs Q1-Q3 | 2.4 | 1.1-5.1 | .02 |
Variables considered for model inclusion were: LEF1 expression (Q4 vs Q1-Q3), BCR-ABL (presence vs absence), t(4;11) (presence vs absence), WBC (10/nL increase), age (10-year increase), immunophenotype (pro-B vs common/pre-B), and CD20 (positive vs negative).
No significant differences regarding deaths in induction therapy (P = .2) or deaths in CR (P = .3) were found between the LEF1 groups (data not shown).
LEF1 expression and survival in standard-risk B-precursor ALL
As identification of molecular markers is of particular importance for standard-risk ALL patients, we evaluated the prognostic significance of LEF1 expression in the standard-risk group as defined by GMALL (BCR-ABL− and MLL-AF4− patients with CR after first induction therapy and WBC ≤ 30/nL at initial diagnosis; n = 91). In this subgroup, patients with high LEF1 expression had a significantly inferior RFS compared to low LEF1 expressers (5-year RFS: LEF1 high, 36%; LEF1 low, 57%; P = .02; Table 3; Figure 1B). In multivariate analyses, LEF1 expression was the only factor with prognostic significance for RFS (P = .02; Table 4). As in the overall cohort, high LEF1 expression was associated with inferior OS without reaching statistical significance (5-year OS: LEF1 high, 53%; LEF1 low, 72%; P = .08; Table 3; Figure 2A). Cox regression analysis revealed a significant interaction between age and LEF1 expression (hazard ratio = 1.2; 95% CI, 1.0-1.5; P = .03), indicating that the impact of LEF1 expression on OS was most evident in standard-risk patients older than 35 years. In this subgroup of standard-risk patients over 35 years of age (n = 41), high LEF1 expression was significantly associated with inferior OS (5-year OS: LEF1 high, 27%; LEF1 low, 63%; P = .02; Table 3; Figure 2B). Moreover, LEF1 was the only significant factor for OS in this subgroup in multivariate models (hazard ratio = 2.7; 95% CI, 1.0-7.2; P = .02).
Kaplan-Meier analyses of OS according to LEF1 expression in standard-risk patients. Patients undergoing SCT in CR1 were censored at the time of transplantation. (A) Total group of standard-risk patients. (B) Standard-risk patients older than 35 years.
Kaplan-Meier analyses of OS according to LEF1 expression in standard-risk patients. Patients undergoing SCT in CR1 were censored at the time of transplantation. (A) Total group of standard-risk patients. (B) Standard-risk patients older than 35 years.
Data for OS refer to analyses done with censoring of SCT patients at the time of transplantation. However, similar results were obtained when patients who received allogeneic SCT in CR1 were not censored (data not shown).
In the subgroups of high-risk and very high-risk ALL, LEF1 expression had no significant impact on outcome (data not shown).
Mutational analyses of LEF1
Inactivating LEF1 mutations as mechanism of LEF1 dysregulation have been reported in T-ALL.22 To evaluate the presence of LEF1 mutations in B-lineage ALL, we performed DNA sequencing of LEF1 in 40 B-precursor ALL patients. We restricted our analysis to exons 2 and 3, as mutations in T-ALL were found in these 2 exons of the LEF1 gene.
In 2 patient samples, we found a missense Asp85Asn single nucleotide polymorphism (rs61752607, National Center for Biotechnology Information build 129/132) in exon 2. No further mutations in exon 2 or exon 3 were detected. This may indicate that transcriptional activity of the LEF1 gene rather than truncating mutations of LEF1 play a role in B-precursor ALL.
Discussion
The identification of prognostic markers in B-precursor ALL, particularly in standard-risk patients, is important for the development of new molecular therapies and might also allow to improve risk-adapted treatment stratification for these patients.
In this study, we have identified high LEF1 expression as an independent prognostic factor associated with a high risk of relapse and inferior RFS in adult standard-risk B-precursor ALL patients. Standard-risk patients with high LEF1 expression (Q4) had an approximately 2.5-fold shorter RFS compared to standard-risk patients with low LEF1 expression (Q1-Q3). The impact of LEF1 expression on OS was age-dependent and most pronounced in standard-risk patients older than 35 years. In this small standard-risk subgroup (n = 41), high LEF1 expression was independently predictive for inferior OS, with a 5-year survival rate of only 27% for high LEF1 expressers compared to 63% in the low LEF1 group. In addition, LEF1 expression was of stronger prognostic impact for OS than expression of BAALC, a gene we have previously demonstrated to be of prognostic significance in standard-risk B-precursor ALL patients.30 In younger standard-risk patients, the influence of LEF1 on OS was not statistically significant.
Interestingly, high LEF1 expression was inversely associated with expression of myeloid markers. This finding corresponds to the predominant role of LEF1 in lymphoid development, where LEF1 protein directly promotes cell cycle entry and proliferation of pro-B cells.13,31 We found no association between LEF1 expression and molecular genetic subgroups of B-precursor ALL, suggesting that LEF1 was not activated by BCR-ABL or MLL-AF4. In contrast, induction of LEF1 by AML1-ETO and FLT3-ITD has been described in AML.9,32
Thus far, little is known about the impact of LEF1 expression on outcome of patients with acute leukemias or other malignancies. Gutierrez et al22 analyzed microarray-based expression data of 40 pediatric T-ALL patients and found no association of clinical characteristics with high LEF1 expression levels, whereas in the same study T-ALL patients with inactivating LEF1 mutations showed a trend toward a favorable OS. Conversely, in patients with myelodysplastic syndrome, advanced disease and poor prognosis were associated with down-regulation of LEF1,33 probably reflecting the impaired maturation of myeloid progenitors associated with loss of LEF1 function.15 In leukemic cells, LEF1 enhanced self-renewal properties and survival in vitro9,18 and was shown to confer leukemogenic potential in a mouse model.20 Our clinical findings underscore the prosurvival effect of LEF1 in acute leukemias, revealing an aggressive subtype of B-precursor ALL with inferior outcome associated with high LEF1 expression.
The optimal therapy management for standard-risk patients, particularly the indication for allogeneic SCT in CR1, remains controversial.34-36 In the GMALL studies, postremission therapy for standard-risk patients is guided by determination of MRD.4,37 This approach intends to molecularly detect a poor response or upcoming relapse to start salvage therapy at an early time point, as outcome for ALL patients with full-blown hematologic relapse is very poor.38 Pretreatment markers for standard-risk ALL patients predicting a high relapse risk at the time of diagnosis might therefore add prognostic information to the subsequent MRD assessment. In this regard, determination of LEF1 expression could possibly facilitate to discriminate standard-risk patients who might benefit from early treatment intensification, including allogeneic SCT. However, future studies are needed to confirm the prognostic significance of LEF1 expression in the context of MRD.
Furthermore, patients with high LEF1 expression should be considered for new molecular directed therapies, especially agents targeting the Wnt pathway39 or demethylating drugs.12 Recently, 2 small molecules have been identified that specifically inhibit the LEF1/β-catenin complex and have shown promising in vitro efficacy in AML cell lines.6,39 Of note, most relapses in B-precursor ALL patients with high LEF1 expression occurred early within the first year of remission, underlining the importance of adapted first-line therapies for this particular high-risk group. As Wnt signaling has been implicated in self-renewal of leukemic stem cells,40-42 early relapse of patients with LEF1 overexpression might reflect a survival advantage of leukemic stem cells because of LEF1 expression. Thus, targeting the LEF1/β-catenin protein complex might overcome persistence of residual leukemic cells.
Because inactivating mutations of LEF1 have previously been identified in pediatric T-ALL,22 we performed mutational analysis of the 2 hotspot regions, exons 2 and 3, in 40 B-precursor ALL patients. In 2 patients, we detected a missense single nucleotide polymorphism (rs61752607), which was also found in one of 44 T-ALL patients.22 However, because of the small patient number, the clinical relevance of this finding is not clear. We did not find any other single nucleotide polymorphisms or mutations in our cohort. In the study of Gutierrez et al,22 the frequency of LEF1 mutations was 7% (3 of 44), and array comparative genomic hybridization revealed focal microdeletions in the LEF1 coding sequence in 11% of cases.22 In contrast, in pediatric B-precursor ALL, the frequency of inactivating copy number alterations of the LEF1 gene was only 1.6%.43 Thus, compared with T-ALL, genomic lesions of LEF1 might not play a pathogenetic role in B-precursor ALL but rather transcriptional activation of LEF1 mRNA expression.
In recent years, several genetic and molecular markers with independent prognostic significance in adult B-precursor ALL have been identified, such as cytogenetics,44,45 high BAALC expression,30 or deletion of the IKAROS gene in BCR-ABL–positive ALL.46 This increasingly detailed cytogenetic and molecular classification of adult ALL might help to better predict a patient's individual treatment response and to develop targeted therapies for different subgroups of B-precursor ALL.
In conclusion, we have, for the first time, identified high LEF1 expression as an independent adverse prognostic factor in adult B-precursor ALL. Our data support a pathogenetic role of Wnt signaling in B-lineage ALL. Determination of LEF1 expression might contribute to risk assessment of standard-risk B-precursor ALL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the GMALL study group for their support and the participating centers for enrolling patients and providing the clinical data for the study.
This study was supported by the Deutsche Krebshilfe (Max Eder Nachwuchsförderung; C.D.B.). The GMALL studies were supported by the Deutsche Krebshilfe and the Deutsche José Carreras Leukämiestiftung.
Authorship
Contribution: A.K. designed and performed research, analyzed data, and wrote the manuscript; N.G. coordinated the GMALL study center and reviewed the manuscript; M.K. analyzed data; C.S. performed laboratory work; A.S. performed statistical analyses; T.B. provided diagnostic molecular genetic data and reviewed the manuscript; L.H.M. provided primers and reviewed the manuscript; D.H. supervised the GMALL 06199 and 07103 studies as principle investigator; W.-K.H. reviewed the manuscript; E.T. supervised the central immunophenotyping and molecular genetic laboratory and reviewed the manuscript; and C.D.B. designed the research, analyzed data, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the GMALL study group appears in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Claudia D. Baldus, Charité-Universitätsmedizin Berlin, Campus Benjamin Franklin, Hämatologie und Onkologie, Hindenburgdamm 30, 12203 Berlin, Germany; e-mail: claudia.baldus@charite.de.