Abstract
To definitively determine whether the neonatal Fc receptor (FcRn) is required for the acute amelioration of immune thrombocytopenia (ITP) by IVIg, we used FcRn-deficient mice in a murine ITP model. Mice injected with antiplatelet antibody in the presence or absence of IVIg displayed no difference in platelet-associated IgG between FcRn deficient versus C57BL/6 mice. FcRn-deficient mice treated with high-dose (2 g/kg) IVIg or a low–dose (2 mg/kg) of an IVIg-mimetic CD44 antibody were, however, protected from thrombocytopenia to an equivalent extent as wild-type mice. To verify and substantiate the results found with FcRn-deficient mice, we used β2-microglobulin–deficient mice (which do not express functional FcRn) and found that IVIg or CD44 antibody also protected them from thrombocytopenia. These data suggest that for both high-dose IVIg as well as low-dose CD44 antibody treatment in an acute ITP model, FcRn expression is neither necessary nor required.
Introduction
A prominent theory as to the mechanism of action of IVIg in the treatment of immune thrombocytopenia (ITP) and other autoantibody- mediated inflammatory conditions involves the function of the neonatal Fc receptor (FcRn).1-11 FcRn in adults is an Fcγ receptor involved in protecting IgG from rapid catabolism within cells, thereby increasing its half-life.12 In the treatment of ITP, it has been specifically theorized that high-dose IVIg treatment simply saturates FcRn by competition, which results in a more rapid clearance of all other IgG antibodies, including pathogenic antiplatelet IgG.1,3-5,9,13-15
A shortfall with the FcRn hypothesis is that antiplatelet antibody causes severe thrombocytopenia within 1 hour of its injection16 ; conversely, IVIg, the therapeutic that gives one of the most rapid increases in platelet counts in ITP patients, is able to increase platelet counts noticeable within 1 to 2 days of its administration.17 In addition, deglycosylated IVIg and desialylated IVIg, which both retain the ability to functionally bind FcRn, appear to exhibit no anti-inflammatory activity in other models of autoimmunity,18 suggesting that IVIg may not function via an FcRn-dependent pathway. Thus, the potential role of FcRn in IVIg function needs to be definitively answered.
To directly address the hypothesis of the contribution of FcRn activity to IVIg effects in ITP, we used FcRn-deficient mice and clearly show that IVIg ameliorates antiplatelet antibody-mediated thrombocytopenia in the absence of FcRn. These results demonstrate that IVIg is not dependent on FcRn expression for its ameliorative effect in acute murine ITP.
Methods
Reagents
C57BL/6 mice, FcRn-deficient mice (B6.129 × 1-Fcgrttm1Dcr/DcrJ), β2M-deficient mice (B6.129P2-B2mtm1Unc/J), and human FcRn transgenic mice (B6.Cg-Fcgrttm1Dcr Tg(CAG-FCGRT)276Dcr/DcrJ) were from The Jackson Laboratory. IVIg (Gamunex 10%) was from Talecris Biotherapeutics. The anti-CD41 (MWReg30) and anti-CD44 (KM114) antibodies were from BD Biosciences. Rabbit polyclonal antiplatelet antibody was from Intercell.
ITP
Thrombocytopenia was induced and platelets counted as described.19 In separate experiments, thrombocytopenia was induced by injection of 1 μL rabbit polyclonal antiplatelet serum. C57BL/6 and FcRn-deficient mice were treated with 50 μg anti-CD44, 50 mg/mouse of IVIg, or no treatment 30 minutes before induction of thrombocytopenia. Mice were bled 24 hours after antiplatelet antibody injection except as noted. All animal protocols were approved by the St Michael's Hospital Animal Care Committee.
PA-IgG
Murine platelets from appropriate mice were incubated with an F(ab′)2 goat anti–rat IgG-FITC for 30 minutes, washed, resuspended, and analyzed by flow cytometry.
Results and discussion
One major theory as to the mechanism of action of IVIg in the treatment of ITP involves saturation of FcRn by IVIg, which then competes with the ability of antiplatelet antibodies to bind FcRn.1,3-5,9,13-15 This purportedly increases their catabolism, subsequently decreasing their ability to induce platelet clearance. It has also been previously demonstrated that IVIg administration can enhance the clearance of exogenous IgG,4 indicating that IVIg saturation of FcRn can increase the catabolism of exogenously administered antibodies.
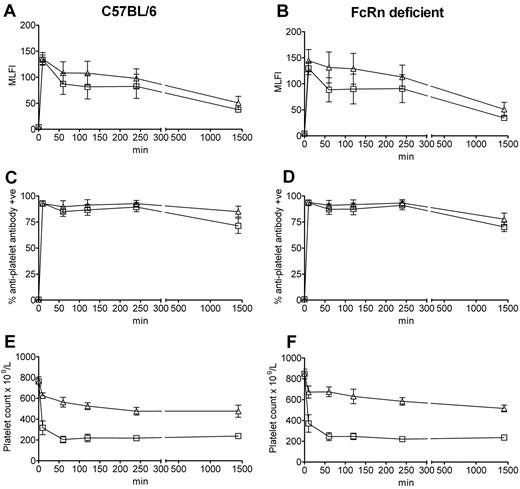
Using a murine model of ITP,19 we first undertook a time course assay to analyze the clearance characteristics of antiplatelet antibody in the presence versus absence of IVIg in C57BL/6 and FcRn-deficient mice. In all mice, all of the antiplatelet IgG was bound to platelets within 10 minutes after antibody administration (assessed by ELISA and flow cytometry, not shown), suggesting that all antiplatelet IgG was quickly bound to the platelets. This is similar to the observation that ITP patients are approximately 83% PA-IgG positive but are significantly less positive for antiplatelet IgG in the indirect assay (∼ 50%).20 This result of rapid binding to platelets is also reminiscent of the Harrington et al demonstration of thrombocytopenia at 1 hour after the administration of plasma from ITP patients into normal volunteers (platelet counts at earlier time points were not tested).16 Analysis of platelets isolated from the C57BL/6 (Figure 1A) and FcRn-deficient (Figure 1B) mice injected with antiplatelet IgG demonstrated that they expressed the same level of platelet-associated IgG in the absence versus presence of IVIg at all time points, suggesting that IVIg did not affect antiplatelet antibody binding to platelets, despite the fact that the IVIg was administered to the mice 30 minutes before the antiplatelet IgG. This observation was also true for the percentage of platelets expressing antiplatelet antibody: platelets from C57BL/6 (Figure 1C) and FcRn-deficient (Figure 1D) mice were all positive for antiplatelet antibody at 10 minutes, which declined slightly by 24 hours. Further platelet analysis at 48 hours and 72 hours showed that antiplatelet antibody binding as assessed by mean log channel fluorescence intensity or percentage positive platelets was barely detectable in both FcRn-deficient and C57BL/6 mice at these time points (data not shown). These observations were also not at all affected by the presence of IVIg at any time point. Platelet counts revealed that administration of antiplatelet antibody to both C57BL/6 (Figure 1E) and FcRn-deficient (Figure 1F) mice induced the equivalent degree of thrombocytopenia in both mouse strains and that IVIg was equally successful at inhibiting platelet clearance at all observed time points in the presence or absence of FcRn. Thus, at both early (10 minutes) and late (24 hours) time points after antiplatelet antibody administration, IVIg can successfully inhibit thrombocytopenia in the absence of FcRn expression.
The absence of FcRn does not result in reduced in vivo antiplatelet antibody binding to platelets or affect the ability of IVIg to inhibit ITP. C57BL/6 wild-type mice and FcRn-deficient mice were injected with antiplatelet antibody alone or antiplatelet antibody 30 minutes after IVIg treatment. Mice were bled at the indicated times for antiplatelet antibody staining or platelet enumeration. Platelets obtained from C57BL/6 mice (A,C) injected with antiplatelet antibody alone (□) or IVIg + antiplatelet antibody (▵) and FcRn-deficient mice (B,D) injected with antiplatelet antibody alone (□) or IVIg + antiplatelet antibody (▵) were stained with a fluorescent anti–rat IgG antibody and analyzed by flow cytometry for mean log channel fluorescence intensity (MLFI; A-B) or percentage positive platelets (C-D) of antiplatelet antibody binding. The x-axis indicates the time of bleeding after antiplatelet antibody injection; and y-axis, the MLFI of antiplatelet antibody binding (A-B) or percentage positive platelets (C-D). n = 8 mice per group from 4 independent experiments. Data are mean ± SEM. Platelet-rich plasma from the C57BL/6 mice (A,C) and FcRn-deficient mice (B,D) were used to enumerate platelets by a Z2 Coulter Counter in panels E and F, respectively.19 The x-axis indicates the time of bleeding after antiplatelet antibody injection; and y-axis, platelet count. n = 8 mice per group from 4 independent experiments. Data are mean ± SEM.
The absence of FcRn does not result in reduced in vivo antiplatelet antibody binding to platelets or affect the ability of IVIg to inhibit ITP. C57BL/6 wild-type mice and FcRn-deficient mice were injected with antiplatelet antibody alone or antiplatelet antibody 30 minutes after IVIg treatment. Mice were bled at the indicated times for antiplatelet antibody staining or platelet enumeration. Platelets obtained from C57BL/6 mice (A,C) injected with antiplatelet antibody alone (□) or IVIg + antiplatelet antibody (▵) and FcRn-deficient mice (B,D) injected with antiplatelet antibody alone (□) or IVIg + antiplatelet antibody (▵) were stained with a fluorescent anti–rat IgG antibody and analyzed by flow cytometry for mean log channel fluorescence intensity (MLFI; A-B) or percentage positive platelets (C-D) of antiplatelet antibody binding. The x-axis indicates the time of bleeding after antiplatelet antibody injection; and y-axis, the MLFI of antiplatelet antibody binding (A-B) or percentage positive platelets (C-D). n = 8 mice per group from 4 independent experiments. Data are mean ± SEM. Platelet-rich plasma from the C57BL/6 mice (A,C) and FcRn-deficient mice (B,D) were used to enumerate platelets by a Z2 Coulter Counter in panels E and F, respectively.19 The x-axis indicates the time of bleeding after antiplatelet antibody injection; and y-axis, platelet count. n = 8 mice per group from 4 independent experiments. Data are mean ± SEM.
We have recently shown that antibodies to the CD44 antigen at low dose can successfully ameliorate murine thrombocytopenia.19 We next compared the ability of one of these IVIg-mimetic anti-CD44 antibodies (KM114) to ameliorate thrombocytopenia in FcRn-deficient versus C57BL/6 mice. Low-dose KM114 (50 μg/mouse, ∼ 2 mg/kg) was able to effectively ameliorate thrombocytopenia in FcRn-deficient mice to the same extent as control C57BL/6 mice (Figure 2A). One of the attributes of the FcRn is that it has an absolute requirement for the protein β2-microglobulin (β2M) to be functionally expressed.21,22 To verify and substantiate the results obtained with FcRn-deficient mice, we used β2M-deficient mice in the murine ITP model and found that β2M-deficient mice treated with high-dose IVIg or low-dose anti-CD44 were also protected from thrombocytopenia to the same extent as C57BL/6 mice (Figure 2B). Thus, using 2 different mouse strains of FcRn deficiency, we demonstrate that both IVIg and an IVIg-mimetic anti-CD44 antibody can alleviate ITP in the absence of FcRn.
IVIg and anti-CD44 antibody inhibit thrombocytopenia in FcRn- and β2M-deficient mice. (A) C57BL/6 or FcRn-deficient mice were pretreated with nothing (“Nil,” “anti-PLT”) or with 50 mg IVIg or 50 μg anti-CD44 antibody KM114. Thirty minutes later, all mice except Nil received antiplatelet antibody. Twenty-four hours later, all mice were bled and platelets enumerated. The x-axis indicates treatment groups; and y-axis, platelet count. n = 10 mice per group from 5 independent experiments. Data are mean ± SEM. (B) C57BL/6 or β2M-deficient mice were treated as in panel A. n = 6 mice per group from 3 independent experiments. Data are mean ± SEM. (C) C57BL/6 or human FcRn transgenic mice were treated as in panel A, except that 1 μL of a rabbit polyclonal antiplatelet antibody was used to induce thrombocytopenia. Twenty-four hours later, all mice were bled and platelets enumerated. n = 4 mice per group from 2 independent experiments. Data are mean ± SEM.
IVIg and anti-CD44 antibody inhibit thrombocytopenia in FcRn- and β2M-deficient mice. (A) C57BL/6 or FcRn-deficient mice were pretreated with nothing (“Nil,” “anti-PLT”) or with 50 mg IVIg or 50 μg anti-CD44 antibody KM114. Thirty minutes later, all mice except Nil received antiplatelet antibody. Twenty-four hours later, all mice were bled and platelets enumerated. The x-axis indicates treatment groups; and y-axis, platelet count. n = 10 mice per group from 5 independent experiments. Data are mean ± SEM. (B) C57BL/6 or β2M-deficient mice were treated as in panel A. n = 6 mice per group from 3 independent experiments. Data are mean ± SEM. (C) C57BL/6 or human FcRn transgenic mice were treated as in panel A, except that 1 μL of a rabbit polyclonal antiplatelet antibody was used to induce thrombocytopenia. Twenty-four hours later, all mice were bled and platelets enumerated. n = 4 mice per group from 2 independent experiments. Data are mean ± SEM.
It has been shown that, in passive antibody-mediated nonhematologic diseases, the degree of disease severity is drastically reduced in FcRn-deficient mice.6,23,24 Here, however, we found that both FcRn-deficient and β2M-deficient mice developed a similar degree of thrombocytopenia compared with C57BL/6 mice. To help understand whether this effect also holds true for a model of autoimmune hemolytic anemia, we examined anemia in FcRn-deficient mice versus C57BL/6 mice and found that antibody-induced anemia in FcRn-deficient mice was also not less severe than C57BL/6 mice (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Antibody binding to erythrocytes was rapid and essentially identical at both early (supplemental Figure 1B) and late (supplemental Figure 1C) time points. These data and the data found in Figure 1A-D suggest that, in these cytopenias, rapid binding of pathogenic antibodies to their target antigen may bypass any requirements for FcRn to prolong their half-life.
FcRn-deficient mice are known to be to be resistant to K/BxN serum-induced inflammatory arthritis.23 To verify the validity of our observations in these cytopenia models, we therefore examined whether disease severity was decreased in the K/BxN model in our laboratory. Indeed, K/BxN serum-induced inflammatory arthritis was significantly less severe in FcRn-deficient mice compared with C57BL/6 mice (supplemental Figure 2A-B).
Humans and mice express different Fc receptors, and FcRn-IgG interactions may differ between species. Thus, we endeavored to determine whether this model system would function in murine FcRn-deficient mice expressing human FcRn (hFcRn), thus making it more translational to a human setting. Using a rabbit polyclonal antiplatelet antibody (which binds hFcRn with high affinity, unlike rat IgG25 ) in the murine ITP model, we found that IVIg works equally well in human FcRn-expressing mice and wild-type mice (Figure 2C), demonstrating that IVIg can successfully prevent murine thrombocytopenia in the presence of human FcRn as well as in its absence (Figure 2A).
Taken together, these data demonstrate that, in this model of acute ITP, the absence of FcRn does not affect the ability of high-dose IVIg or a low-dose anti-CD44 antibody to inhibit platelet clearance in a significant manner. We therefore conclude that this receptor is unlikely to play a major role in the mechanism of action of these 2 therapeutics in ITP.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Joan Legarda, Dr Honghui Yu, Dr Alaa Amash, Dr Lidice Bernardo Reyes, and the St Michael's Research Vivarium staff.
This work was supported by the Canadian Blood Services–Canadian Institutes of Health Research Request For Proposals program.
Authorship
Contribution: A.R.C. designed research, performed experiments, analyzed data, and wrote the manuscript; S.J.S., X.C., and P.J.M. performed experiments and analyzed data; and A.H.L. designed research, analyzed data, obtained grant funding, and wrote the manuscript.
Conflict-of-interest disclosure: A.H.L. received honoraria from Baxter and CSL. The remaining authors declare no competing financial interests.
Correspondence: Alan H. Lazarus, Transfusion Medicine Research, St Michael's Hospital, 30 Bond Street, Toronto, ON, Canada M5B 1W8; e-mail: lazarusa@smh.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal