Abstract
Common variable immunodeficiency disorder (CVID) is the most prevalent form of primary idiopathic hypogammaglobulinemia. Identification of genetic defects in CVID is hampered by clinical and immunologic heterogeneity. By flow cytometric immunophenotyping and cell sorting of peripheral B-cell subsets of 37 CVID patients, we studied the B-cell compartment at the B-cell subset level using the κ-deleting recombination excision circle assay to determine the replication history and the Igκ-restriction enzyme hot-spot mutation assay to assess the somatic hypermutation status. Using this approach, 5 B-cell patterns were identified, which delineated groups with unique replication and somatic hypermutation characteristics. Each B-cell pattern reflected an immunologically homogenous patient group for which we proposed a different pathophysiology: (1) a B-cell production defect (n = 8, 18%), (2) an early peripheral B-cell maturation or survival defect (n = 4, 11%), (3) a B-cell activation and proliferation defect (n = 12, 32%), (4) a germinal center defect (n = 7, 19%), and (5) a postgerminal center defect (n = 6, 16%). The results of the present study provide for the first time insight into the underlying pathophysiologic background in 5 immunologically homogenous groups of CVID patients. Moreover, this study forms the basis for larger cohort studies with the defined homogenous patient groups and will facilitate the identification of underlying genetic defects in CVID.
Introduction
Common variable immunodeficiency disorder (CVID) is the most prevalent form of primary idiopathic hypogammaglobulinemia, frequently leading to clinical complications.1-3 CVID is defined by serum IgG levels below 2 SD of healthy controls in the presence of decreased IgA and/or IgM levels, recurrent infections, impaired response to immunization, exclusion of other defined causes of hypogammaglobulinemia, and age above 2 years (European Society for Immunodeficiencies (ESID)–Pan American Group for Immunodeficiency (PAGID) criteria, available from: www.esid.org). CVID patients suffer from sinopulmonary infections, which eventually result in bronchiectasis in more than 30% of cases. In addition, they may develop complications such as autoimmune disease, granulomatous disease, and malignancies.2-8
Over the past years, deficiencies of ICOS,9,10 TACI,11,12 CD19,13,14 BAFF-R,15 CD20,16 and CD8117 have been identified in patients with CVID or CVID-like conditions. However, less than 10% of CVID patients have a positive family history,2 and a genetic defect has only been identified in less than 10% of the patients who have been reported to the ESID primary immunodeficiency database.1,18 The immunologic and clinical heterogeneity of CVID hampers the discovery of underlying disease-causing mechanisms, genetic defects, and clinically relevant prognostic factors in the majority of patients.
CVID patients fail to produce sufficient amounts of Ag-specific Abs, which can be caused by defects in any critical stage of B-cell differentiation and maturation.7,19 B cells are continuously produced in the BM and then migrate to peripheral lymphoid organs, where they mediate Ag-specific responses. Multiple B-cell subsets circulate in the peripheral blood. Transitional B cells are early BM emigrants and constitute only a small part of the peripheral B-cell pool. In healthy controls, transitional B cells do not proliferate, but instead differentiate into naive mature B cells, which do undergo homeostatic proliferation of 1-2 cell cycles, thereby expanding the naive B-cell pool.20 Activation of the BCR complex by Ag stimulates further B-cell differentiation and maturation. B cells can be activated with T-cell help in a germinal center in lymphoid tissue or independently of T-cell help, such as in the marginal zone of the spleen. Activated B cells generate activation-induced cytidine deaminase–dependent somatic hypermutations (SHMs) in the variable region of the Ig heavy and light chains. Subsequent class-switch recombination changes the IgH constant region to form Ig isotypes with different effector functions. Finally, memory B cells and plasma cells are formed, which are responsible for long-lasting immunologic memory and the production of large numbers of Ig molecules. T cell–independent B-cell responses in the splenic marginal zone are thought to generate a substantial fraction of circulating marginal zone–like B cells.20-22
Recently, CD21lowCD38low B cells have been described as a distinct subpopulation. Whereas their origin and specific function are disputed, they contain mostly autoreactive unresponsive clones and might represent anergic or innate-like B cells.23,24 CD21lowCD38low B cells are very infrequent in healthy individuals, but expansions have been found in several autoimmune diseases and in a subgroup of CVID patients.25,26
In the past decade, the Freiburg and Paris CVID classifications have been developed based on the composition of the peripheral B-cell compartment.27,28 The main aim of these classifications was to predict clinical complications. In the recent EUROclass consensus classification of CVID, a relative decrease of switched-memory B cells was associated with splenomegaly, granulomatous disease, and autoimmunity.5 The other reported associations in this study were an increased proportion of transitional B cells with lymphadenopathy and a decreased proportion of CD21lowCD38low B cells with splenomegaly. In addition, decreased proportions of marginal zone–like B cells29 and an abnormal T-cell phenotype30 have been found to be associated with clinical complications. Because abnormalities in different immune pathways may account for the immune defects in CVID, a classification independent of immune parameters has been proposed by Chapel et al, which groups patients into clinically homogenous categories with a different prognosis.31 Despite multiple attempts at classifying CVID patients, understanding the heterogeneity in terms of immunologic and genetic defects as well as clinical prognosis still imposes a major challenge.
The aim of the present study was to identify immunologically homogenous subgroups of CVID patients based on B-cell subset abnormalities. Using a combined flow cytometric and molecular approach, we provide a link between the composition of the peripheral B-cell compartment and in vivo B-cell replication and SHM status. This resulted in a model that describes 5 different pathophysiologic backgrounds in immunologically homogenous CVID subgroups. Defining these immunologically homogenous groups of CVID patients will facilitate the identification of prognostic factors and the underlying genetic defects.
Methods
Patients
Peripheral blood samples and clinical data were collected from 37 patients with CVID. In addition, we collected blood from 86 healthy, age-matched controls. The research was approved by the medical ethics committee of Erasmus MC, and all participants provided written informed consent in accordance with the Declaration of Helsinki.
Flow cytometric analysis
Six-color flow cytometric immunophenotyping of peripheral blood was performed on a LSRII (BD Biosciences) and data were analyzed using FACSDiva Version 6.1.2 software (BD Biosciences). The following mAbs were used: CD19-PerCP-Cy5.5, CD19-PE-Cy7 (SJ25C1), CD5-APC (L17F12), CD45-PerCP (2D1), CD19-APC (SJ25C1), CD38-PE, CD38-APC and CD38-PE-Cy7 (HB7), CD27-APC (L128), CD3-PerCP-Cy5.5 (SK7), and CD8-APC-Cy7 (SK1) all from BD Biosciences; polyclonal IgD-FITC, IgD-PE, and IgM-PE (Southern Biotechnologies); polyclonal IgG-FITC (Kallestad), IgA-FITC, and IgA-PE (IS11-8E10; Miltenyi Biotec); CD24-FITC (gran-B-ly-1; Sanquin); CD21-PE (LB21; Serotech); CD45RO-FITC (UCHL1; DAKO); and CD4-PC7 (SFCI12T4D11) and CD45-RA-RD1 (2H4; both from Beckman Coulter). The absolute sizes of the peripheral B-cell subsets (transitional B cells, naive mature B cells, marginal zone–like B cells, and memory B cells) were determined by flow cytometric immunophenotyping and compared with healthy, age-matched controls. The gating strategy is depicted in Figure 1. Considering the gating of transitional B cells, the lower border of the transitional B-cell gate, which separates transitional B cells from the naive mature B-cell population, was set in a standardized way to ensure homogenous analysis. A decrease or increase of a B-cell subset was defined as a value below the 5th or above the 95th percentile of 86 healthy, age-matched controls. Analysis of the precursor B-cell compartment was performed as described previously.32
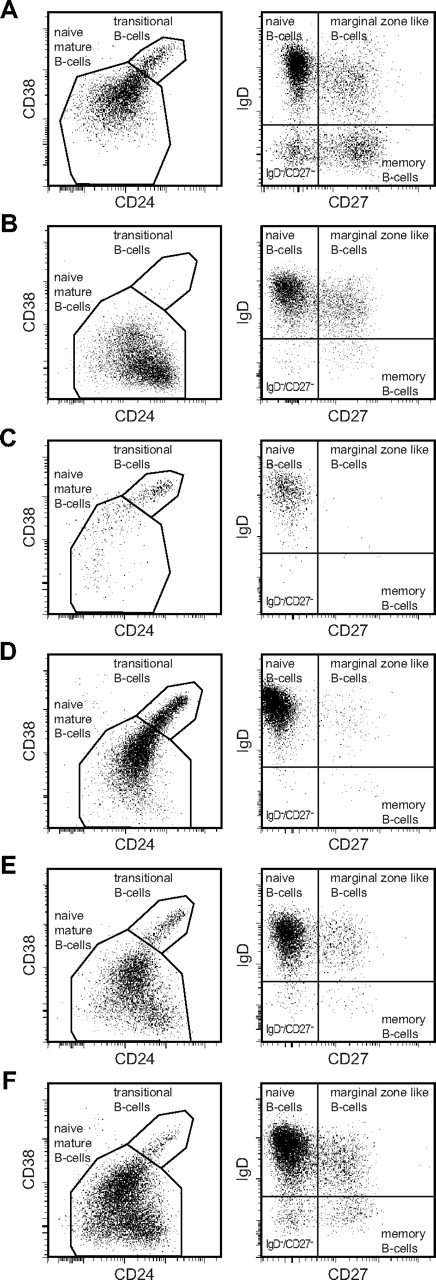
Flow cytometric analysis of blood B-cell subsets in healthy controls and CVID patients. All B-cell subsets are determined within the CD19+ lymphogate. Naive B-cell subsets (transitional B cells and naive mature B cells) are defined within the CD27−IgD+ lymphogate based on expression of CD24 and CD38. (A) Healthy controls. (B-F) B-cell patterns observed in CVID patients. (B) B-cell pattern 1: low transitional and memory B cells. (C) B-cell pattern 2: low naive mature, marginal zone–like, and memory B cells. (D) B-cell pattern 3: low marginal zone–like and memory B cells. (E) B-cell pattern 4: low memory B cells. (F) B-cell pattern 5: normal marginal zone–like and memory B cells. Naive mature B cells cells in CVID patients (B-F) were more often CD38low compared with controls (A) and represent, at least in part, the CD21lowCD38low B-cell population within the naive B-cell compartment.
Flow cytometric analysis of blood B-cell subsets in healthy controls and CVID patients. All B-cell subsets are determined within the CD19+ lymphogate. Naive B-cell subsets (transitional B cells and naive mature B cells) are defined within the CD27−IgD+ lymphogate based on expression of CD24 and CD38. (A) Healthy controls. (B-F) B-cell patterns observed in CVID patients. (B) B-cell pattern 1: low transitional and memory B cells. (C) B-cell pattern 2: low naive mature, marginal zone–like, and memory B cells. (D) B-cell pattern 3: low marginal zone–like and memory B cells. (E) B-cell pattern 4: low memory B cells. (F) B-cell pattern 5: normal marginal zone–like and memory B cells. Naive mature B cells cells in CVID patients (B-F) were more often CD38low compared with controls (A) and represent, at least in part, the CD21lowCD38low B-cell population within the naive B-cell compartment.
High-speed cell sorting of B-cell subsets from peripheral blood
Four B-cell subsets were purified from blood samples of all 37 patients and the 20 healthy controls using a FACSDiva cell sorter (BD Biosciences) after staining of post-Ficoll mononuclear cells with CD24-FITC (1B5), IgD-PE, ITK diagnostics, CD19-PerCP-Cy5.5 (SJ25C1), CD27-APC (L128), and CD38 PE-Cy7 (HB7). CD3 APC-Cy7 (SK7) was used as an exclusion marker (55). The following CD19+ populations were sorted: transitional B cells (CD27−CD24highCD38high), naive mature B cells (CD27−CD24dimCD38dim), marginal zone–like (CD27+IgD+) B cells, and memory B cells (CD27+IgD−). DNA was extracted from the sorted cell fractions using a direct lysis method.33,34
KREC assay to determine the replication history of B cells
The replication history of B cells was determined using the κ-deleting recombination excision circle (KREC) assay (Invivoscribe), which is based on a quantification of coding joints and signal joints of an Igκ-deleting rearrangement (intron RSS-Kde) by real-time quantitative PCR (RQ-PCR),20 The ΔCT between the signal joint and the coding joint exactly represents the number of cell divisions a B cell has undergone. The RQ-PCR mixture of 25μL contained TaqMan Universal MasterMix (Applied Biosystems), 900nM concentrations of each primer, 100nM FAM-TAMRA-labeled probe, 25ng of DNA, and 0.4 ng of BSA, and was run on an ABI PRISM 7700 detection system (Applied Biosystems).20
SHM analysis using a Vκ3-20–specific IgκREHMA on genomic DNA
To investigate the occurrence of SHM in the B-cell subsets, the Igκ-restriction enzyme hot-spot mutation assay (Igκ-REHMA) for genomic DNA was used.20,35 In short, a PCR reaction was performed with a HEX-coupled Vκ3-20 intron forward primer and 2 FAM-coupled Jκ reverse primers recognizing all 5 Jκ gene segments. The PCR products (500 bp) were digested with KpnI and Fnu4HI and run on an ABI3130XL capillary sequencer (Applied Biosystems). Unmutated gene products could be visualized as 244- or 247-bp HEX-coupled fragments and mutated gene products as 262-bp HEX-coupled fragments.20
Statistics
Statistical analysis was performed with Prism Version 5.0 software (GraphPad). The Mann-Whitney test was used to compare 2 groups with continuous outcomes. The nonparametric Kruskal-Wallis rank-sum test was used to compare multiple groups with continuous outcomes, followed by pairwise Mann-Whitney tests if the former indicated significant differences. Spearman correlation coefficients are given. The χ2 or the Fisher exact test (if required) was used for categorical variables. Statistical significance was set at 2-sided P < .05.
Results
Patients
Thirty-seven CVID patients (19 male and 18 female) were included in this study. The age range was 6-76 years, with 22 adults and 15 children. All patients fulfilled the ESID-PAGID criteria for CVID. All patients received Ig-replacement therapy. Excluded from the study were males with decreased peripheral B cells and mutations in the BTK gene, males with CD40L deficiency, patients with other genetic defects known to cause hypogammaglobulinemia (such as uracil-DNA glycosylase and activation-induced cytidine deaminase deficiency), patients with a secondary hypogammaglobulinemia, and patients under immunosuppressive therapy.
The mean age of onset of symptoms was 15.6 years (range, 0.3-64) and the mean age of diagnosis was 24.3 years (range, 2.5-71.0), resulting in a mean diagnostic delay of 8.7 years. The mean age of inclusion in the study was 31.0 years (range, 6.0-76.0).
Composition of the peripheral B-cell compartment in CVID
Flow cytometric analysis of blood B-cell subsets was performed in 37 CVID patients and 86 healthy, age-matched controls. The following peripheral CD19+ B-cell subsets were defined: transitional B cells as CD27−IgM+IgD+CD24highCD38high and naive mature B cells as CD27−IgM+IgD+CD24dimCD38dim. In addition to these 2 naive B-cell subsets, 2 CD38dimCD27+ B-cell subsets were identified: CD27+IgD+IgM+ marginal zone–like B cells and CD27+IgD− memory B cells (Figure 1A). Finally, plasmablasts were defined as CD24−CD38hi. The B-cell subset sizes were calculated as cells per microliter of blood, because in contrast to relative sizes, the absolute size of a specific B-cell subset is not influenced by an increase or decrease of the other B-cell subsets.
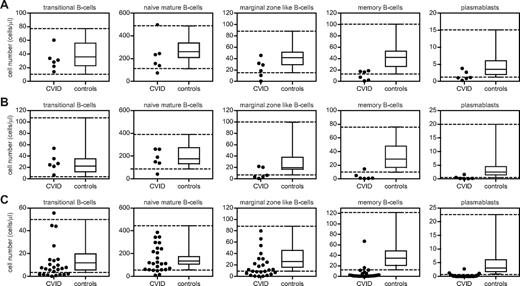
The CVID cohort was divided into 3 age groups (5-10, 10-16, and > 16 years of age) at the time of inclusion in the study to compare the B-cell subset counts of the individual patients with the 5th and 95th percentiles of age-matched controls (Figure 2A-C and Table 1). A peripheral B-cell subset size was considered reduced when the value was below the 5th percentile. Twenty-two percent of CVID patients had reduced numbers of transitional B cells, 14% had reduced numbers of naive mature B cells, and 48% and 84% had reductions in marginal zone–like B cells or memory B cells, respectively. Most patients (81%) also showed a reduction of plasmablasts compared with age-matched controls.
Absolute numbers of B cells per B-cell subset of 37 CVID patients and 86 healthy, age-matched controls. Patients and controls are divided into 3 age groups: 5-10 years (n = 30; A), 10-16 years (n = 28; B), and > 16 years (n = 28; C). The 3 age groups contained 6, 6, and 25 CVID patients, respectively. Boxes depict median values and 25th and 75th percentiles; whiskers, extended by interrupted lines, depict 5th and 95th percentiles for healthy, age-matched controls in the 3 age groups.
Absolute numbers of B cells per B-cell subset of 37 CVID patients and 86 healthy, age-matched controls. Patients and controls are divided into 3 age groups: 5-10 years (n = 30; A), 10-16 years (n = 28; B), and > 16 years (n = 28; C). The 3 age groups contained 6, 6, and 25 CVID patients, respectively. Boxes depict median values and 25th and 75th percentiles; whiskers, extended by interrupted lines, depict 5th and 95th percentiles for healthy, age-matched controls in the 3 age groups.
Age-related normal values of B-cell subset absolute counts
| B-cell subset age, y* . | Transitional . | Naive mature . | Marginal zone–like . | Memory . | Plasmablast . |
|---|---|---|---|---|---|
| 5-10 (n = 30) | 11-77 | 111-486 | 15-88 | 13-100 | 1-15 |
| 10-16 (n = 28) | 4-108 | 87-390 | 7-90 | 10-76 | 0.5-20 |
| >16 (n = 28) | 3-50 | 57-447 | 9-88 | 13-122 | 1-23 |
| B-cell subset age, y* . | Transitional . | Naive mature . | Marginal zone–like . | Memory . | Plasmablast . |
|---|---|---|---|---|---|
| 5-10 (n = 30) | 11-77 | 111-486 | 15-88 | 13-100 | 1-15 |
| 10-16 (n = 28) | 4-108 | 87-390 | 7-90 | 10-76 | 0.5-20 |
| >16 (n = 28) | 3-50 | 57-447 | 9-88 | 13-122 | 1-23 |
Depicted values are 5th and 95th percentiles of normal controls in cells per microliter.
n indicates the number of normal controls per age group.
Subsequently, we divided the CVID patients into groups with a specific composition of the peripheral B-cell compartment (B-cell patterns) based on absolute reductions of transitional, naive mature, marginal zone–like, or memory B cells. We identified 5 main B-cell patterns (Figure 1B-F) consisting of at least 3 patients, a prerequisite for statistical analysis. Eight patients (22%) showed decreased numbers of transitional B cells in combination with a reduction of memory B cells (B-cell pattern 1). Of the patients with normal transitional B cells, 4 patients (11%) showed a reduction of naive mature, marginal zone–like and memory B cells (B-cell pattern 2); 12 patients (32%) showed a reduction of both marginal zone–like and memory B cells (B-cell pattern 3); and 7 (19%) an isolated reduction of switched-memory B cells (B-cell pattern 4). Six patients (16%) did not have a reduction in marginal zone–like or memory B cells (B-cell pattern 5). Remarkably, none of the CVID patients showed an isolated reduction of marginal zone–like B cells. Therefore, using absolute numbers of B-cell subsets, 5 main B-cell patterns could be identified.
Comparison of B-cell patterns with the EUROclass CVID classification
B-cell patterns that have been described previously in CVID classification systems are based on relative B-cell subset sizes and include reductions of marginal zone–like and memory B cells and an expansion of transitional B cells (for comparison with the EUROclass CVID classification, see supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). B-cell pattern 1 (ie, reduced numbers of transitional B cells) has not been described so far as a separate B-cell phenotype, which also applies for B-cell pattern 2 (ie, reduced naive B cells, marginal zone–like B cells, and memory B cells). Furthermore, we showed that 6 patients who are classified as smB+ (switched-memory B cells > 2% of lymphocytes) in EUROclass actually have decreased age-matched memory B-cell counts. Using absolute cell counts, we also noted that only one patient showed a minimal increase of transitional B cells (Figure 2C), whereas 15 patients could be classified as Trhi according to EUROclass. Therefore, a relative expansion of transitional B cells in CVID is the result of a reduction of the other B-cell subsets. In conclusion, the B-cell patterns defined herein only show a limited overlap with the EUROclass CVID classification.
Abnormalities in B-cell proliferation and SHM
To determine whether reductions of peripheral B-cell subsets in the 5 B-cell patterns were associated with aberrant B-cell proliferation, the in vivo B-cell replication history was determined for sorted B-cell subsets of patients and controls by calculating the ratio between genomic coding joints and corresponding signal joints on KRECs of the IGK-deleting rearrangement.20 In addition, SHM levels were determined by measuring the frequency of a mutated hot spot in rearranged Vκ3-20 gene segments with an IgκREHMA.20,35
Transitional B cells are recent BM emigrants that have not undergone proliferation in healthy individuals.20 In virtually all CVID patients, the replication history of transitional B cells was normal, including patients with reduced transitional B cells (Figure 3A). Only in 1 patient with low transitional B cells had the transitional B cells undergone 7 cell divisions. These findings indicate that the absolute number of transitional B cells in CVID patients is not influenced by deregulated proliferation, but rather reflects reduced BM output and/or increased cell death. In 2 patients with low transitional B cells, we examined the precursor B-cell compartment in BM. These patients had a reduced proportion of immature B cells, supporting the hypothesis of a decreased BM output of B cells (Figure 4).
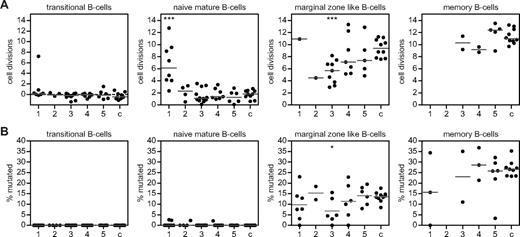
B-cell replication history and SHM levels in 5 different B-cell patterns compared with controls. (A) The in vivo replication history of B-cell subsets as determined by KREC assay in sorted peripheral B-cell subsets of patients and controls (c; n = 10) is given in number of cell divisions. In patients with B-cell pattern 1, proliferation of marginal zone–like and memory B cells was above the detection limit of the KREC assay in 7 patients. (B) The SHM frequency given in percentage mutated hot spot in a rearranged Vk3-20 gene segment was determined by the IgκREHMA assay and compared with 10 healthy controls (c). The 5 B-cell patterns are: (1) low transitional and memory B cells; (2) low naive mature, marginal zone–like, and memory B cells; (3) low marginal zone–like and memory B cells; (4) low memory B cells; and (5) normal marginal zone–like and memory B cells. Individual data points are displayed and bars indicate medians. Groups are compared with controls using the Mann-Whitney test. Significant values compared with healthy controls are indicated. ***P < .0005; **P < .005; *P < .05.
B-cell replication history and SHM levels in 5 different B-cell patterns compared with controls. (A) The in vivo replication history of B-cell subsets as determined by KREC assay in sorted peripheral B-cell subsets of patients and controls (c; n = 10) is given in number of cell divisions. In patients with B-cell pattern 1, proliferation of marginal zone–like and memory B cells was above the detection limit of the KREC assay in 7 patients. (B) The SHM frequency given in percentage mutated hot spot in a rearranged Vk3-20 gene segment was determined by the IgκREHMA assay and compared with 10 healthy controls (c). The 5 B-cell patterns are: (1) low transitional and memory B cells; (2) low naive mature, marginal zone–like, and memory B cells; (3) low marginal zone–like and memory B cells; (4) low memory B cells; and (5) normal marginal zone–like and memory B cells. Individual data points are displayed and bars indicate medians. Groups are compared with controls using the Mann-Whitney test. Significant values compared with healthy controls are indicated. ***P < .0005; **P < .005; *P < .05.
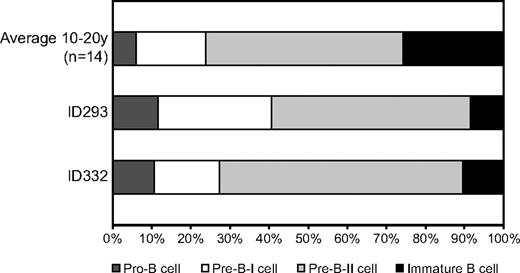
Composition of the BM precursor B-cell compartment of 2 patients with low transitional and memory B cells (B-cell pattern 1). Using flow cytometric immunophenotyping, 4 major precursor B-cell subsets can be identified (pro-B, pre-B-I, pre–B-II, and immature B). In healthy donors, the immature B-cell fraction comprises 25% of the total precursor B-cell compartment. The proportion of immature B cells in CVID patients with pattern 1 was decreased compared with controls.
Composition of the BM precursor B-cell compartment of 2 patients with low transitional and memory B cells (B-cell pattern 1). Using flow cytometric immunophenotyping, 4 major precursor B-cell subsets can be identified (pro-B, pre-B-I, pre–B-II, and immature B). In healthy donors, the immature B-cell fraction comprises 25% of the total precursor B-cell compartment. The proportion of immature B cells in CVID patients with pattern 1 was decreased compared with controls.
Naive mature B cells of controls had undergone a median of 1.8 (range, 0.7-2.7) cell divisions in the absence of SHM (Figure 3A-B), which is known as Ag-independent homeostatic proliferation.20 Naive mature B cells of CVID patients with reduced absolute numbers of transitional B cells (B-cell pattern 1) showed significantly increased proliferation, which did not result in increased naive B-cell numbers. These naive mature B cells were not clonal or oligoclonal based on a normal Igκ/Igλ ratio and a polyclonal pattern on IgH-CDR3 spectratyping (data not shown). Based on the absence of SHM, antigenic stimulation was also excluded as cause of increased proliferation (Figure 3B). Increased proliferation was not observed in any of the other CVID patients. In summary, patients with a combined decrease of transitional and memory B cells showed increased proliferation of naive mature B cells, which did not result in an increased subset size.
Patients with decreased naive mature B cells, marginal zone B cells, and memory B cells (B-cell pattern 2) showed a normal replication of transitional and naive mature B cells. The few naive mature B cells did not show an increase in homeostatic proliferation to compensate for low naive mature B-cell numbers. Because the majority of B cells did not survive beyond the transitional B-cell stage, we propose that these patients suffer from an early defect in peripheral B-cell maturation or survival. The replication history and SHM status of the marginal zone and memory B-cell subsets could not be determined in most of these patients because of very low cell numbers. Therefore, B-cell pattern 2 seems to be the result of an early defect in peripheral B-cell maturation or survival.
Marginal zone–like B cells of patients with a combined reduction of marginal zone–like and memory B cells (B-cell pattern 3) showed a significantly decreased number of cell divisions. In marginal zone–like B cells of controls, the median number of cell divisions was 9.4 (range, 7.6-11.3). This proliferation is Ag-driven, as reflected by the presence of SHM (median, 14%; range, 8%-18%; Figure 3B). Decreased proliferation in B-cell pattern 3 was accompanied by reduced SHM levels (Figure 3B), which is indicative of impaired response to Ag. Therefore, we propose that the reduction in marginal zone B cells is caused by reduced (Ag-driven) proliferation.
In healthy controls, the memory B cells showed the highest number of cell divisions (median, 11.0; range, 9.7-13.3) and SHM levels (median, 27%; range, 23%-35%; Figure 3A-B). Because of limited memory B-cell numbers, the KREC assay could only be performed in 16 of 37 patients. In most CVID patients, replication of memory B cells showed at least 9 cell divisions, which was in the normal range (Figure 3A). Apparently, memory B-cell subset reductions cannot be explained solely in terms of a B-cell proliferation defect.
In patients with a normal marginal zone–like and memory B cells, no significant abnormalities in B-cell replication and SHM could be detected (B-cell pattern 5). Therefore, patients with normal absolute numbers of peripheral B-cell subsets did not show aberrancies in B-cell proliferation and SHM. The seemingly normal B-cell subsets suggest that the immunodeficiency is likely the result of impaired Ab production by plasma cells rather than a B-cell differentiation defect.
CD21lowCD38low B cells and B-cell proliferation
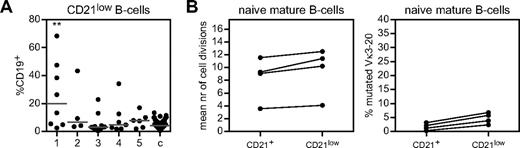
A subgroup of CVID patients show increased frequencies of CD21lowCD38low B cells.5,25 Therefore, we studied the frequency of these aberrant cells that occupy the B-cell compartment in our patient groups. The proportions of CD21lowCD38low B cells were significantly increased in patients with low transitional B cells and memory B cells (B-cell pattern 1; Figure 5A). Because the naive mature B cells of these patients showed increased proliferation, and because most CD21lowCD38low B cells have a naive mature B-cell phenotype, we questioned whether the frequency of these cells was related to the number of cell divisions of naive mature B cells. We sorted CD21+ and CD21lowCD38lowCD27−IgM+IgD+ naive B cells from 4 patients with increased B-cell proliferation (> 4 cell divisions) and > 20% CD21lowCD38low B cells within the total B-cell compartment. In these 4 patients, CD21+ and CD21lowCD38low naive mature B cells showed similar increased levels of proliferation (Figure 5B). Because neither fraction showed a significant increase of mutated IGK alleles (Figure 5B), it is unlikely that the hyperproliferation was Ag driven. Therefore, CD21lowCD38low B cells were significantly increased in patients with low transitional and memory B cells, but did not show more proliferation compared with their CD21+ counterparts and lacked clear signs of antigenic stimulation.
Frequency and proliferation history of CD21low B cells in CVID patients. (A) CD21low B cells are depicted as proportions of CD19+ B cells and compared with healthy controls (c) according to their B-cell subset pattern. Individual data points are displayed and bars indicate medians. B-cell patterns were compared with controls using the Mann-Whitney test. (B) Number of cell divisions and frequency of SHMs of sorted CD21lowCD27−IgM+IgD+ naive B cells compared with sorted CD21+CD27−IgM+IgD+ naive B cells in patients with > 20% CD21low B cells and a naive B-cell replication history of > 4 cell divisions.
Frequency and proliferation history of CD21low B cells in CVID patients. (A) CD21low B cells are depicted as proportions of CD19+ B cells and compared with healthy controls (c) according to their B-cell subset pattern. Individual data points are displayed and bars indicate medians. B-cell patterns were compared with controls using the Mann-Whitney test. (B) Number of cell divisions and frequency of SHMs of sorted CD21lowCD27−IgM+IgD+ naive B cells compared with sorted CD21+CD27−IgM+IgD+ naive B cells in patients with > 20% CD21low B cells and a naive B-cell replication history of > 4 cell divisions.
Naive CD4+ T cells
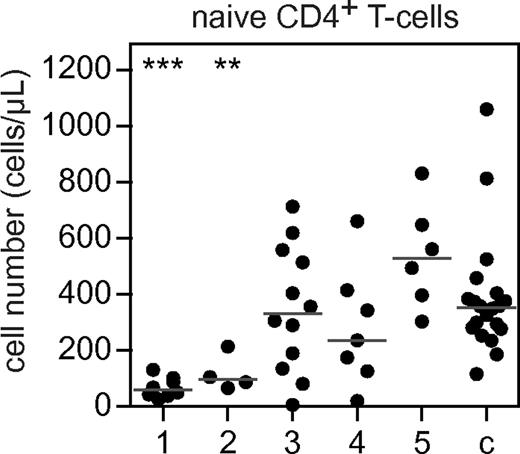
The composition of the B-cell compartment has been associated with abnormalities in naive CD4+ T-cell numbers,30 and low naive CD4+ T cells are associated with clinical complications.36 Therefore, we determined the number of naive CD4+ T cells in CVID patients with the different B-cell subset patterns. B-cell patterns 1 and 2 were associated with a decrease of naive CD4+ T cells compared with healthy controls (Figure 6). A decrease of naive CD4+ T cells in addition to the severe disturbance of peripheral B-cell development suggests that the immunologic defect in these groups is not limited to the B-cell lineage.
CD4+ naive T cells in 5 different B-cell patterns compared with controls. Absolute counts (in cells per microliter) of CD3+CD4+CD27+RA+RO− naive T cells in patients with 5 different B-cell patterns compared with controls. The 5 B-cell patterns are: (1) low transitional and memory B cells; (2) low naive mature, marginal zone–like, and memory B cells; (3) low marginal zone–like and memory B cells; (4) low memory B cells; and (5) healthy marginal zone–like and memory B cells. Individual data points are displayed and bars indicate medians. Groups are compared with controls using the Mann-Whitney test. Significant values compared with healthy controls are indicated. ***P < .0005; **P < .005; *P < .05.
CD4+ naive T cells in 5 different B-cell patterns compared with controls. Absolute counts (in cells per microliter) of CD3+CD4+CD27+RA+RO− naive T cells in patients with 5 different B-cell patterns compared with controls. The 5 B-cell patterns are: (1) low transitional and memory B cells; (2) low naive mature, marginal zone–like, and memory B cells; (3) low marginal zone–like and memory B cells; (4) low memory B cells; and (5) healthy marginal zone–like and memory B cells. Individual data points are displayed and bars indicate medians. Groups are compared with controls using the Mann-Whitney test. Significant values compared with healthy controls are indicated. ***P < .0005; **P < .005; *P < .05.
Clinical complications
Having defined the B-cell replication and SHM characteristics of our CVID patients, we aimed to relate these to the clinical complications (Table 2). The age of inclusion in the study did not differ significantly between patients with different B-cell patterns. However, the mean age of initial symptoms and diagnosis was higher in patients with B-cell pattern 1 (mean, 30 years; range, 4.0-59.0 and mean, 38.0 years; range, 19.0-63.0, respectively) compared with patients with B-cell pattern 2 (mean 11 years; range, 4.0-19.0 and mean, 17.8 years; range, 12.0-28.0, respectively); B-cell pattern 3 (mean, 9.8 years; range, 0.3-64.0 and mean 16.0 years; range, 4.0-71.0) and B-cell pattern 5 (mean, 3.9 years; range, 0.5-9.0 and mean, 11 years; range, 2.5-29.0, respectively). The differences in age of onset of symptoms and diagnosis of patients with B-cell pattern 1 support the hypothesis of a different pathophysiologic background.
Clinical complications in CVID patients
| Complication . | B-cell pattern . | Total (n = 37) . | ||||
|---|---|---|---|---|---|---|
| 1 (n = 8) . | 2 (n = 4) . | 3 (n = 12) . | 4 (n = 7) . | 5 (n = 6) . | ||
| Recurrent RTI and/or ENT infections | 8 (100%) | 4 (100%) | 12 (100%) | 7 (100%) | 6 (100%) | 37 (100%) |
| Recurrent severe pneumonia* | 4 (50%) | 2 (50%) | 4 (33%) | 0 (0%) | 0 (0%) | 10 (27%) |
| Bronchiectasis | 3 (38%) | 2 (50%) | 4 (33%) | 1 (14%) | 0 (0%) | 10 (27%) |
| Autoimmune disease | 4 (50%) | 2 (50%) | 1 (8%) | 1 (14%) | 1 (17%) | 9 (24%) |
| Granulomatous inflammation | 1 (13%) | 1 (25%) | 2 (17%) | 0 (0%) | 0 (0%) | 4 (11%) |
| Splenomegaly | 6 (75%) | 1 (25%) | 3 (25%) | 0 (0%) | 0 (0%) | 10 (27%) |
| Recurrent herpes zoster | 3 (38%) | 1 (25%) | 3 (25%) | 1 (14%) | 0 (0%) | 8 (22%) |
| Recurrent lymphadenopathy | 3 (38%) | 2 (50%) | 1 (8%) | 2 (29%) | 1 (17%) | 12 (32%) |
| Complication . | B-cell pattern . | Total (n = 37) . | ||||
|---|---|---|---|---|---|---|
| 1 (n = 8) . | 2 (n = 4) . | 3 (n = 12) . | 4 (n = 7) . | 5 (n = 6) . | ||
| Recurrent RTI and/or ENT infections | 8 (100%) | 4 (100%) | 12 (100%) | 7 (100%) | 6 (100%) | 37 (100%) |
| Recurrent severe pneumonia* | 4 (50%) | 2 (50%) | 4 (33%) | 0 (0%) | 0 (0%) | 10 (27%) |
| Bronchiectasis | 3 (38%) | 2 (50%) | 4 (33%) | 1 (14%) | 0 (0%) | 10 (27%) |
| Autoimmune disease | 4 (50%) | 2 (50%) | 1 (8%) | 1 (14%) | 1 (17%) | 9 (24%) |
| Granulomatous inflammation | 1 (13%) | 1 (25%) | 2 (17%) | 0 (0%) | 0 (0%) | 4 (11%) |
| Splenomegaly | 6 (75%) | 1 (25%) | 3 (25%) | 0 (0%) | 0 (0%) | 10 (27%) |
| Recurrent herpes zoster | 3 (38%) | 1 (25%) | 3 (25%) | 1 (14%) | 0 (0%) | 8 (22%) |
| Recurrent lymphadenopathy | 3 (38%) | 2 (50%) | 1 (8%) | 2 (29%) | 1 (17%) | 12 (32%) |
RTI indicates respiratory tract infection; and ENT, ear-nose-throat.
Recurrent severe pneumonia is > 1 episode of infiltrate on the chest x-ray, hospitalization, and IV antibiotics.
The occurrence of bronchiectasis was not associated with age of onset of symptoms, diagnostic delay, IgG level at diagnosis (as reported previously by others31 ), or a specific B-cell pattern. However, patients with bronchiectasis experienced more episodes of severe pneumonia, defined as an infiltrate on the chest X-ray, hospitalization, and the need for IV antibiotics (supplemental Figure 1A). The occurrence of splenomegaly, defined by ultrasound or by clinical examination (n = 10), was associated with decreased numbers of transitional B cells and increased proliferation of naive mature B cells (supplemental Figure 1B). Splenomegaly significantly clustered in patients with B-cell pattern 1 (P = .007). Lymphadenopathy, autoimmunity, and granulomatous disease were not significantly correlated with any of the 5 B-cell patterns. However, autoimmunity was associated with an increased proportion of CD21lowCD38low B cells (supplemental Figure 1C). Therefore, only splenomegaly was associated with a specific B-cell pattern, although we cannot draw firm conclusions about the association between B-cell patterns and clinical complications because of the limited number of patients.
Discussion
CVID represents a heterogeneous group of disease entities that are expected to result from various underlying immunopathologic mechanisms. The level of immunologic heterogeneity has been mainly described in terms of abnormalities in the relative size of B-cell subsets in CVID patients,5,27,28 and studies unraveling the immunologic causes are limited. Using a combined flow cytometric and molecular approach, we identified in our CVID cohort 5 unique B-cell patterns based on reductions in absolute numbers of specific B-cell subsets, and linked these 5 B-cell patterns to abnormalities in B-cell replication and SHM. These results provide new insight into our understanding of the different pathophysiologic backgrounds of CVID.
In the present study, we used healthy, age-matched controls and absolute B-cell subset numbers to define reductions in the various B-cell subsets. This has the advantage over relative frequencies because the absolute size of a specific B-cell subset is not influenced by an increase or decrease of the other B-cell subsets. In this way, we could demonstrate that a relative increase of transitional B cells in CVID patients, as has been reported in the EUROclass classification,5 is the result of reductions in the other B-cell subsets rather than an expansion of transitional B cells.
CVID patients with low numbers of transitional B cells and memory B cells (n = 8) showed increased proliferation of naive mature B cells without an increase in the naive mature B-cell subset. Furthermore, 2 of these patients had a reduced frequency of immature B cells in the BM, which might reflect a reduced production of B cells in the BM (Figure 7B). Therefore, the increased number of cell divisions likely compensates for decreased BM output or for increased cell death of immature or naive mature B cells. In addition, these patients had decreased memory B cells, which is indicative of a germinal center defect (Figure 7B). A partial defect in precursor B-cell development at the pre–B-I to pre–B-II stage was recently reported by Ochtrop et al in 9 of 25 CVID patients and was associated with low transitional B cells.37 This subgroup of CVID patients probably shows overlap with B-cell pattern 1, and supports the hypothesis that these patient have a different pathophysiology. We identified a similar immunophenotype with increased B-cell proliferation of naive mature B cells in patients with the Nijmegen Breakage Syndrome (NBS).38 NBS patients have a DNA repair defect that leads to a quantitative V(D)J recombination defect, and consequently a defect of precursor B-lymphopoiesis, which is compensated for by increased proliferation of naive mature B cells.38 In addition, NBS patients have a germinal center defect defined by defective SHM and class-switch recombination.39 The observed B-cell pattern in CVID patients with decreased transitional and memory B cells might be compatible with a DNA-repair defect. Several studies have shown that increased radiosensitivity of lymphocytes and aberrancies in DNA-repair genes can be found in some CVID patients.40,41 Therefore, we are currently investigating DNA-repair defects in CVID patients with this B-cell pattern. Other defects that affect precursor B-cell development are potentially involved in the pathophysiology of B-cell pattern 1.
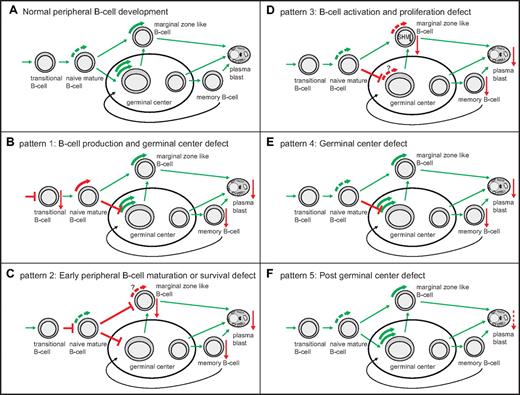
Model of the pathophysiological background of 5 B-cell patterns in CVID patients based on proliferation history and SHM levels. (A) Healthy peripheral B-cell development. Green curved arrows depict healthy B-cell proliferation. (B-F) abnormal peripheral B-cell development in the 5 B-cell patterns. Top left side: proposed pathophysiology and corresponding B-cell pattern. Red straight arrows depict decreased subset size (arrow pointing downward). T-shaped red bars depict a proposed block in B-cell development. (B) Red curved arrow depicts increased proliferation of naive B cells. (C) Red interrupted curved arrows depict decreased proliferation of marginal zone–like B cells.
Model of the pathophysiological background of 5 B-cell patterns in CVID patients based on proliferation history and SHM levels. (A) Healthy peripheral B-cell development. Green curved arrows depict healthy B-cell proliferation. (B-F) abnormal peripheral B-cell development in the 5 B-cell patterns. Top left side: proposed pathophysiology and corresponding B-cell pattern. Red straight arrows depict decreased subset size (arrow pointing downward). T-shaped red bars depict a proposed block in B-cell development. (B) Red curved arrow depicts increased proliferation of naive B cells. (C) Red interrupted curved arrows depict decreased proliferation of marginal zone–like B cells.
Naive CD4+ T cells were also reduced in patients with low transitional and memory B cells. Although this finding is compatible with a defect in DNA repair,42 the decrease of naive CD4+ T cells and the increased incidence of splenomegaly also show similarities to the new CVID subset with “late onset combined immunodeficiency,” as proposed by Malphettes et al,43 although our patients did not suffer from opportunistic infections. In agreement with our observations in naive mature B cells, the reduction of naive CD4+ T cells in CVID patients has been associated with decreased thymic output44 and increased proliferation and apoptosis of naive T cells.30 The compensatory hyperproliferation does not seem to be limited to the B-cell lineage. Serana et al reported that a subgroup of CVID patients showed decreased thymic output, as measured with TRECs, in combination with an increased proliferation of total B cells, as measured with the KREC assay.44 The observed increase of B-cell proliferation in a subgroup of CVID patients supports our observation of increased naive mature B-cell proliferation in patients with B-cell pattern 1. However, B-cell pattern 1 does not fully correspond to the findings of Serana et al, who reported a normal proportion of memory B cells in patients with increased B-cell proliferation. Because we studied B-cell replication at the B-cell subset level, our analysis gives more accurate information on the impact of B-cell subset proliferation on the composition of the peripheral B-cell compartment. Increased naive mature B-cell proliferation was associated with increased CD21low B cells. Rakhmanov et al reported a more extensive proliferative history of CD21low B cells in CVID patients compared with naive B cells of controls.23 We showed that in patients with increased naive mature B-cell proliferation, both the CD21low naive B cells and the CD21+ naive B cells hyperproliferated; therefore, the aberrant proliferation was present in all naive mature B cells irrespective of CD21 expression. Because CD21low B cells contain mostly autoreactive, unresponsive clones,24 we hypothesize that down-regulation of CD21 expression on hyperproliferating naive mature B cells could be a mechanism to silence them.
Patients with reduced numbers of naive mature, marginal zone–like, and memory B cells (B-cell pattern 2) suffer from an early block in peripheral B-cell development affecting B-cell maturation and survival after the transitional B-cell stage (Figure 7C). As a result, marginal zone–like B cells and memory B cells are also severely decreased. Subsequent analysis of the CD27+ B-cell subsets for replication history and SHM was therefore not possible. In addition, in patients with BAFF-R deficiency, B-cell development is arrested at the transitional B-cell stage.15 However, naive CD4+ T cells were also severely decreased in association with B-cell pattern 2, so in these patients a combined B- and T-cell defect is more likely.
The existence of a combined decrease of marginal zone–like and memory B cells has been described previously in CVID patients, but the pathophysiology remains unclear.5,28 We showed that a decrease of marginal zone–like B cells in 12 CVID patients was associated with decreased proliferation. Furthermore, the frequency of SHMs was decreased, which is indicative for an impaired response to Ag. Therefore, these data suggest that impaired activation and subsequently impaired proliferation is implicated in the pathophysiology of this group of CVID patients (Figure 7D). The number of memory B cells was also severely reduced, but the replication history of the few generated memory B cells could not be reliably established because of the extremely low cell numbers. In agreement with our hypothesis of decreased B-cell activation and subsequent proliferation, a combined decrease of marginal zone and memory B cells has been observed in patients with CD19 and CD81 deficiency.13,17 Thus far, only in vitro B-cell proliferation defects have been reported in CVID patients with defective B-cell TLR9 signaling.45,46 Mutations in the TLR9 gene were absent in these patients, suggesting that decreased TLR9 signaling was a secondary phenomenon. Information about the immunophenotype of these patients is scarce, but also points toward a combined decrease of marginal zone and memory B cells.46 Based on our own data and on the observations in CD19- and CD81-deficient patients, we hypothesize that a combined decrease of marginal zone–like and memory B cells could best be explained by impaired response to Ag, although more detailed studies are necessary to define the defect more precisely.
In the present study, an isolated reduction of memory B cells was associated with a proliferation of this subset in the lower normal range. Because most CVID patients with decreased memory B cells showed at least 9 cell divisions, this number apparently is a prerequisite for memory B-cell development. An isolated reduction of memory B cells, which we identified in 7 CVID patients, is compatible with defects that predominantly affect the generation of switched-memory B cells in the germinal center (Figure 7E).9,47,48 As-yet-unidentified costimulation or class-switch recombination defects could underlie this B-cell phenotype.
CVID patients with normal memory B cells and marginal zone–like B cells (n = 6) represent a group without B-cell proliferation and SHM abnormalities and have fewer clinical complications compared with patients with low memory B cells. We hypothesize that this B-cell pattern is compatible with a predominantly post–germinal center defect, most likely a terminal plasma-cell maturation or homing defect (Figure 7F). Taubenheim et al showed that B cells could only reach the initial stage of plasma-cell differentiation in the lymph nodes of 3 reported CVID patients. Analysis of terminal plasma-cell development in lymphoid tissues has the potential to unravel the pathophysiology of this B-cell pattern.49
In conclusion, our combined flow cytometric and molecular approach resulted in the identification of 5 main B-cell patterns in CVID, delineating 5 immunologic homogenous patient groups for which different pathophysiologic backgrounds are proposed. Detailed studies in these defined homogenous groups of patients are needed to further unravel the defects at a molecular level. Furthermore, this approach might also be applicable to “CVID-like” disorders. Recently, progress has been made by uncovering multiple novel susceptibility loci for CVID using genome-wide analysis of single nucleotide polymorphisms and copy number variations.50 Integration of (high-throughput) genomic analysis, detailed flow cytometric immunophenotyping, functional molecular assays, and clinical data collection in a large cohort of CVID patients is important for the identification of the clinical correlates, prognostic factors, and underlying genetic defects in CVID patients with different B-cell patterns.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to E. F. E. de Haas, S. Postumus-van Sluijs, and B. Van Turnhout for technical assistance; to S. De Bruin-Versteeg for technical support and assistance with preparing the figures; and to P. van Jaarsveld-Bakker and M. W. van der Ent van for assistance with collecting blood samples and clinical data.
Authorship
Contribution: G.J.D., P.M.v.H., N.G.H., A.W., E.d.V., B.H.B., M.T., and I.P. performed the research; W.H. assisted in the statistical analysis of the data; M.C.v.Z. and M.v.d.B. designed the research; and G.J.D., M.C.v.Z., J.J.M.v.D., and M.v.d.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mirjam van der Burg, PhD, Erasmus MC, Department of Immunology, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands; e-mail: m.vanderburg@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal