Abstract
SCID resulting from mutations in IL2RG or JAK3 is characterized by lack of T and natural killer cells; B cells are present in normal number, but antibody responses are defective. Hematopoietic cell transplantation (HCT) is curative for SCID. However, B-cell dysfunction persists in a substantial proportion of patients. We hypothesized that impaired B-cell responses after HCT in IL2RG/JAK3 deficiency results from poor donor B-cell engraftment and defective γc-dependent cytokine signaling in host B cells. To test this, and to identify which γc cytokine(s) is critical for humoral immunity, we studied 28 transplanted patients with IL2RG/JAK3 deficiency. Lack of donor B-cell engraftment associated with persistent humoral dysfunction and significantly reduced memory B cells. B-cell proliferation induced by CD40L alone or together with CpG, anti-Ig, IL-4, IL-10, or IL-13 was comparable in healthy controls and in post-HCT SCID patients, irrespective of their chimerism status. However, in vitro stimulation with CD40L/IL-21 induced B-cell proliferation, plasmablast differentiation, and antibody secretion in patients with donor B cells, but not in patients with autologous B cells. These data imply that IL-21–mediated signaling is critical for long-lived humoral immunity and to restore antibody responses in IL2RG/JAK3-deficient patients after HCT. Furthermore, in vitro stimulation with CD40L/IL-21 can predict in vivo B-cell immunity in IL2RG/JAK3 SCID after transplantation.
Introduction
After encountering foreign antigen (Ag), naive B cells can differentiate into numerous fates depending on the nature of the signals received within the lymphoid microenvironment. T-independent and T-dependent (TD) Ags induce naive B cells to become short-lived antibody (Ab)–secreting plasmablasts that localize to extrafollicular regions of lymphoid tissues. TD Ag also induce naive B cells to form germinal centers (GCs) that yield high-affinity, long-lived memory and plasma cells (PCs), the effector B cells responsible for long-term humoral immunity and serologic memory.1,2 The contribution of CD4+ T cells to these events is mediated by T-follicular helper (TFH) cells that undergo cognate interactions with Ag-specific B cells to provide cues for their growth, survival, and differentiation into these distinct effector subsets.3,4
Elucidation of some of the molecular requirements for generating long-lived memory cells and PCs has come from the study of gene-targeted mice and patients with primary immunodeficiencies caused by loss-of-function mutations in key genes. Thus, mutations in CD40LG (encoding CD40 ligand [CD40L]), CD40, IKBKG (encoding NFκB essential modulator [NEMO]), ICOS, SH2D1A (encoding SAP), or STAT3 compromise GC function, thereby reducing or abolishing the generation of memory B cells and PCs.2 Remarkably, many of these molecules—CD40L, ICOS, SAP—are highly expressed by TFH cells,3-5 underscoring the importance of this helper cell subset in establishing robust humoral immune responses.
Another key feature of TFH cells is their production of the pleiotropic cytokine IL-21,3-5 a potent inducer of proliferation, immunoglobulin (Ig) isotype switching, PC generation, and Ab secretion by human B cells.6-10 IL-21 exerts its effects by binding a heterodimeric receptor comprising the IL-21 receptor (R) and the common γ chain (γc) that is also a component of receptors for IL-2, IL-4, IL-7, IL-9, and IL-15.11 After binding to its receptor, IL-21 activates JAK/STAT signaling pathways.11 STAT3 mediates IL-21–induced differentiation of human naive B cells into plasmablasts in vitro by up-regulating PRDM1 (encoding BLIMP-1) and XBP1,12,13 both of which are required for PC generation.1,2 Recent analyses of gene-targeted mice have provided evidence for an important role for B-cell intrinsic IL-21R signaling in GC formation and humoral immune responses to TD Ag.14,15 However, it remains to be determined whether IL-21/IL-21R plays a similar role in human B cells.
Mutations in IL2RG encoding γc causes X-linked severe combined immunodeficiency (X-SCID), whereas biallelic mutations in JAK3, which initiates signaling downstream of γc-containing receptors, manifest as autosomal recessive SCID.16 SCID resulting from IL2RG or JAK3 mutations is characterized by a lack of T and natural killer (NK) cells, because of a requirement for IL-7 and IL-15, respectively, in their development, but normal or increased numbers of B cells.16 Despite intact development, B-cell responses are impaired in X-SCID or JAK3-SCID because of a paucity of T-cell help. By allowing T-cell reconstitution, hematopoietic cell transplantation (HCT) is life-saving for patients with SCID.17 However, despite normalization of T-cell numbers, B-cell dysfunction persists in a substantial proportion of transplanted patients who subsequently require Ig replacement therapy (IgRT).17-20 This impaired reconstitution of humoral immunity is more often associated with split chimerism, that is, the presence of donor-derived T cells and the persistence of autologous, genetically defective B lymphocytes.17-20 Robust in vivo B-cell function is often observed in patients with IL7R, ADA, or CD3 deficiency who also develop split chimerism after HCT,17,19 indicating that the specific nature of the genetic defect may affect the quality of humoral immune reconstitution among SCID patients who retain autologous B cells post-HCT. Indeed, only 27% of patients with IL-7R deficiency required IgRT after HCT, whereas such treatment was necessary in 66% and 50% of patients with SCID because of mutations in IL2RG and JAK3, respectively.21
We have taken advantage of the chimeric state of SCID patients after HCT to examine the B-cell compartment of individuals who have donor-derived, genetically intact CD4+ T cells and either normal or IL2RG/JAK3-mutant B cells. Our results demonstrate that IL2RG/JAK3-mutant B cells respond as well as normal B cells to a diverse array of costimulatory signals, including B-cell receptor (BCR) engagement; Toll-like receptor (TLR) ligands; and cytokines, including IL-4, IL-10 and IL-13. However, IL2RG/JAK3-mutant B cells were completely unresponsive to the stimulatory effects of IL-21. In vitro responsiveness to CD40L/IL-21 correlated with development of humoral immunity in vivo as evidenced by development of memory B cells, protective Ab responses to TD antigens, and lack of a requirement for IgRT. These findings indicate that IL-21 is the key γc-binding/JAK3–activating cytokine necessary for the initiation of humoral immunity in humans.
Methods
Patients, blood samples, and EBV-transformed B-cell lines
Thirty-six patients with molecularly defined SCID were studied (Table 1). Twenty-six of these patients had X-SCID because of mutations in IL2RG. Ten patients had autosomal recessive SCID because of mutations in JAK3 (n = 5), IL7RA (n = 2), RAG1 (n = 2), or CD3δ (n = 1). Thirty-three of the 36 patients received HCT from matched sibling donors (MSDs; n = 4), 5/6-antigen MSDs (n = 2), phenotypically identical related donors (n = 2), mismatched related donors (MMRDs; n = 22), unrelated cord blood (n = 2), or a matched unrelated donor (n = 1; Table 2). Sixteen patients received HCT without any conditioning regimen (CR). Eight patients received myeloablative CR with busulfan (16 mg/kg), treosulfan, or Thio-Tepa plus cyclophosphamide (200 mg/kg), with or without fludarabine (150 mg/m2) and anti-thymocyte globulin. Submyeloablative CR with lower doses of busulfan (8 mg/kg) and immunosuppressive regimens with various combinations of cyclophosphamide/fludarabine/anti-thymocyte globulin or anti-CD20 Ab alone were given to 9 patients. IgRT was discontinued in patients according to the following criteria: (1) presence of normal levels of serum IgA; (2) presence of isohemagglutinins; (3) lack of recurrent infections, severe infections, or both; and (4) 6 months or more of follow-up after HCT. Ab response to tetanus toxoid was tested at 3 months or longer after discontinuation of IgRT. Patients with protective titers were considered to have achieved humoral immune reconstitution. All experiments were approved by jurisdictional ethics committees in Sydney, Melbourne, Adelaide, Brisbane, and the Institutional Review Board of Children's Hospital Boston. EBV-transformed lymphoblastoid B-cell lines were established as described previously.12
Molecular features of 36 patients with SCID
| Patient no.* . | Mutated gene . | Gene mutation† . | Protein mutation . |
|---|---|---|---|
| 1a | IL2RG | c.441-444delACTG | K147fs |
| 1b | IL2RG | c.441-444delACTG | K147fs |
| 2a | IL2RG | c.677G > A | R226H |
| 2b | IL2RG | c.677G > A | R226H |
| 3 | IL2RG | c.803G > A | G268D |
| 4 | IL2RG | c.545G > A | C182Y |
| 5 | IL2RG | c.854G > A | R285Q |
| 6 | IL2RG | c.1A > G | M1V |
| 7 | IL2RG | c.664C > T | R222C |
| 8 | IL2RG | c.865C > T | R289X |
| 9 | IL2RG | c.676C > T | R226C |
| 10 | IL2RG | c.695G > T | G232V |
| 11a | IL2RG | c.17T > A | L6X |
| 11b | IL2RG | c.17T > A | L6X |
| 12 | IL2RG | c.676C > T | R226C |
| 13 | IL2RG | c.823delA | S275fs |
| 14 | IL2RG | c.709T > C | W237R |
| 15 | IL2RG | c.115 + 1G > T | Splicing defect |
| 16 | IL2RG | c.809T > G | M270S |
| 17 | IL2RG | c.273C > A | Y91X |
| 18 | IL2RG | c.insA729 | I244fs |
| 19 | IL2RG | c.670C > T | R224W |
| 20 | IL2RG | c.816_820delGATT | I273fs |
| 21a | JAK3 | c.[2351 + 1g > t]; c.[2351 + 1 g > t] | Splicing defect |
| 21b | JAK3 | c.[2351 + 1g > t]; c.[2351 + 1 g > t] | Splicing defect |
| 22 | JAK3 | c.[1428C > T]; c.[1428C > T] | R445X |
| 23 | IL2RG | c.insA18 | P7fs |
| 24 | IL2RG | c.677G > A | R226H |
| 25 | IL7R | c.644G > T | G215V |
| 26 | IL7R | c.[898_902delCCTGA];c.[539A > C] | P300fs;H180P |
| 27 | CD3D | c.202C > T | R68X |
| 28 | RAG1 | c.[1303A > G];c.[3016A > G] | M435V;M1006V |
| 29 | RAG1 | g.[9.5Mb del];c.[1303A > G] | M435V |
| 30 | IL2RG | c.266A > G | Y89C |
| 31 | JAK3 | c.[578G > A];c.[1786 + 3G > T] | C193Y; splicing defect |
| 32 | JAK3 | c.[578G > A];c.[1786 + 3G > T] | C193Y; splicing defect |
| Patient no.* . | Mutated gene . | Gene mutation† . | Protein mutation . |
|---|---|---|---|
| 1a | IL2RG | c.441-444delACTG | K147fs |
| 1b | IL2RG | c.441-444delACTG | K147fs |
| 2a | IL2RG | c.677G > A | R226H |
| 2b | IL2RG | c.677G > A | R226H |
| 3 | IL2RG | c.803G > A | G268D |
| 4 | IL2RG | c.545G > A | C182Y |
| 5 | IL2RG | c.854G > A | R285Q |
| 6 | IL2RG | c.1A > G | M1V |
| 7 | IL2RG | c.664C > T | R222C |
| 8 | IL2RG | c.865C > T | R289X |
| 9 | IL2RG | c.676C > T | R226C |
| 10 | IL2RG | c.695G > T | G232V |
| 11a | IL2RG | c.17T > A | L6X |
| 11b | IL2RG | c.17T > A | L6X |
| 12 | IL2RG | c.676C > T | R226C |
| 13 | IL2RG | c.823delA | S275fs |
| 14 | IL2RG | c.709T > C | W237R |
| 15 | IL2RG | c.115 + 1G > T | Splicing defect |
| 16 | IL2RG | c.809T > G | M270S |
| 17 | IL2RG | c.273C > A | Y91X |
| 18 | IL2RG | c.insA729 | I244fs |
| 19 | IL2RG | c.670C > T | R224W |
| 20 | IL2RG | c.816_820delGATT | I273fs |
| 21a | JAK3 | c.[2351 + 1g > t]; c.[2351 + 1 g > t] | Splicing defect |
| 21b | JAK3 | c.[2351 + 1g > t]; c.[2351 + 1 g > t] | Splicing defect |
| 22 | JAK3 | c.[1428C > T]; c.[1428C > T] | R445X |
| 23 | IL2RG | c.insA18 | P7fs |
| 24 | IL2RG | c.677G > A | R226H |
| 25 | IL7R | c.644G > T | G215V |
| 26 | IL7R | c.[898_902delCCTGA];c.[539A > C] | P300fs;H180P |
| 27 | CD3D | c.202C > T | R68X |
| 28 | RAG1 | c.[1303A > G];c.[3016A > G] | M435V;M1006V |
| 29 | RAG1 | g.[9.5Mb del];c.[1303A > G] | M435V |
| 30 | IL2RG | c.266A > G | Y89C |
| 31 | JAK3 | c.[578G > A];c.[1786 + 3G > T] | C193Y; splicing defect |
| 32 | JAK3 | c.[578G > A];c.[1786 + 3G > T] | C193Y; splicing defect |
Patients 1a and 1b, 2a and 2b, 11a and 11b, and 20a and 20b, respectively, are siblings.
The position of the mutation is relative to the first nucleotide corresponding to the “A” in the initiating ATG codon that encodes the first amino acid (ie, methionine) of the protein.
Characteristics of hematopoietic cell transplantation, lymphoid chimerism, and humoral immune reconstitution in 33 SCID patients
| Patient no. . | HCT . | Time post-HCT, y . | Chimerism, % . | Humoral reconstitution . | Group . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor . | CR . | T . | B . | B cells (× 109/L) . | Serum IgM, mg/mL . | Serum IgA, mg/mL . | Ig RT . | Ab response* . | |||
| 1a | MSD | None | 17.0 | 100 | 0 | 0.13 | 0.66 | 0.18 | No | Poor | 2 |
| 1b | MSD | None | 12.0 | 100 | 0 | 0.45 | 1.07 | 0.95 | No | − | 2 |
| 2a | 5/6-Ag MSD | Flu ATG | 12.0 | 100 | 100 | 0.86 | 3.16 | 0.88 | No | + | 1 |
| 2b | 5/6-Ag MSD | None | 10.0 | 100 | 21 | 0.3 | 2.63 | 1.25 | No | + | 1 |
| 3 | PIRD | Treo Flu Camp | 0.5 | 100 | 100 | 0.38 | 1.6 | 0.46 | Yes | n.a. | 1 |
| 4 | PIRD | Cy Flu ATG | 8.0 | 100 | 100 | 0.33 | n.a | No | + | 1 | |
| 5 | MMRD | Bu Cy ATG | 8.0 | 100 | 0 | 0.24 | 0.73 | < 0.06 | Yes | n.a. | 2 |
| 6 | MMRD | None | 15.0 | 100 | 0 | 0.14 | 0.18 | < 0.07 | Yes | n.a. | 2 |
| 7 | MMRD | Flu ATG | 18.0 | 100 | 100 | 0.21 | 1.33 | 4.12 | No | + | 1 |
| 8 | MMRD | Flu | 12.0 | 100 | 0 | 0.2 | 1.4 | 0.6 | Yes | n.a. | 2 |
| 9 | MMRD | None | 10.0 | 98 | 5 | 0.44 | 0.99 | < 0.07 | Yes | n.a. | 2 |
| 10 | MMRD | None | 4.1 | 95 | 1 | 0.81 | 0.72 | < 0.07 | Yes | − | 2 |
| 11a | MMRD | None | 5.5 | 97 | 1 | 0.10 | 0.04 | < 0.07 | Yes | − | 2 |
| 11b | MMRD | None | 5.5 | 97 | 1 | 0.11 | 0.18 | 0.12 | Yes | − | 2 |
| 12 | MMRD | Bu Cy | 23.0 | 100 | 98 | 0.13 | 1.85 | 2.19 | No | + | 1 |
| 13 | MMRD | None | 3.1 | 97 | 0 | 0.90 | 0.25 | < 0.07 | Yes | − | 2 |
| 14 | MMRD | Bu8 Cy | 9.8 | 100 | 34 | 0.21 | 3.58 | < 0.3 | Yes | − | 1 |
| 15 | MMRD | Flu | 4.0 | 100 | 100 | 0.59 | 1.43 | 0.46 | No | + | 1 |
| 16 | MMRD | None | 22.3 | 97 | 0 | 0.09 | 0.78 | < 0.07 | Yes | n.a. | 2 |
| 17 | MMRD | Bu Cy ATG | 6.3 | 100 | 100 | 0.22 | 1.09 | 1.27 | No | + | 1 |
| 18 | MMRD | None | 18.5 | 100 | 0 | 0.05 | < 0.04 | < 0.06 | Yes | − (isohemagglutinins) | 2 |
| 19 | MMRD | None | 11.25 | 100 | 0 | 0.42 | 1.9 | < 0.06 | Yes | n.a. | 2 |
| 20 | MMRD | None | 15.7 | 100 | 0 | 0.29 | 0.38 | < 0.1 | Yes | n.a. | 2 |
| 21a | MMRD | Bu 8 Cy | 8.2 | 100 | 15 | 3.2 | 2.9 | No | + | 1 | |
| 21b | MMRD (no TCD) | None | 1.7 | 100 | 0 | 0.76 | < 0.3 | Yes | n.a. | 2 | |
| 22 | MMRD | TT, Cy, ATG | 12.3 | 100 | 100 | 0.42 | 2.18 | 0.92 | No | + | 1 |
| 23 | UCB | Bu Cy Flu ATG | 7.0 | 100 | 100 | 1.78 | 0.89 | 0.32 | No | + | 1 |
| 24 | UCB | Bu Cy ATG | 2.0 | 100 | 100 | 2.69 | 2.39 | 0.97 | No | + | 1 |
| 25 | MSD | None | 12.2 | 97 | 11 | na | 1.18 | 1.50 | No | + | n.a. |
| 26 | MMRD | Anti-CD20 | 2.4 | 94 | 2 | na | 1.05 | < 0.07 | Yes | − | n.a. |
| 27 | MMRD | Cy ATG | 17.0 | 95 | 1 | na | 0.83 | 2.47 | No | + | n.a. |
| 28 | MSD | None | 3.5 | 90 | 50 | na | 0.2 | 0.35 | Yes | n.a. | n.a. |
| 29 | MUD | Bu Cy ATG | 4.5 | 99 | 98 | 0.03 | 0.89 | 0.97 | No | + | n.a. |
| Patient no. . | HCT . | Time post-HCT, y . | Chimerism, % . | Humoral reconstitution . | Group . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor . | CR . | T . | B . | B cells (× 109/L) . | Serum IgM, mg/mL . | Serum IgA, mg/mL . | Ig RT . | Ab response* . | |||
| 1a | MSD | None | 17.0 | 100 | 0 | 0.13 | 0.66 | 0.18 | No | Poor | 2 |
| 1b | MSD | None | 12.0 | 100 | 0 | 0.45 | 1.07 | 0.95 | No | − | 2 |
| 2a | 5/6-Ag MSD | Flu ATG | 12.0 | 100 | 100 | 0.86 | 3.16 | 0.88 | No | + | 1 |
| 2b | 5/6-Ag MSD | None | 10.0 | 100 | 21 | 0.3 | 2.63 | 1.25 | No | + | 1 |
| 3 | PIRD | Treo Flu Camp | 0.5 | 100 | 100 | 0.38 | 1.6 | 0.46 | Yes | n.a. | 1 |
| 4 | PIRD | Cy Flu ATG | 8.0 | 100 | 100 | 0.33 | n.a | No | + | 1 | |
| 5 | MMRD | Bu Cy ATG | 8.0 | 100 | 0 | 0.24 | 0.73 | < 0.06 | Yes | n.a. | 2 |
| 6 | MMRD | None | 15.0 | 100 | 0 | 0.14 | 0.18 | < 0.07 | Yes | n.a. | 2 |
| 7 | MMRD | Flu ATG | 18.0 | 100 | 100 | 0.21 | 1.33 | 4.12 | No | + | 1 |
| 8 | MMRD | Flu | 12.0 | 100 | 0 | 0.2 | 1.4 | 0.6 | Yes | n.a. | 2 |
| 9 | MMRD | None | 10.0 | 98 | 5 | 0.44 | 0.99 | < 0.07 | Yes | n.a. | 2 |
| 10 | MMRD | None | 4.1 | 95 | 1 | 0.81 | 0.72 | < 0.07 | Yes | − | 2 |
| 11a | MMRD | None | 5.5 | 97 | 1 | 0.10 | 0.04 | < 0.07 | Yes | − | 2 |
| 11b | MMRD | None | 5.5 | 97 | 1 | 0.11 | 0.18 | 0.12 | Yes | − | 2 |
| 12 | MMRD | Bu Cy | 23.0 | 100 | 98 | 0.13 | 1.85 | 2.19 | No | + | 1 |
| 13 | MMRD | None | 3.1 | 97 | 0 | 0.90 | 0.25 | < 0.07 | Yes | − | 2 |
| 14 | MMRD | Bu8 Cy | 9.8 | 100 | 34 | 0.21 | 3.58 | < 0.3 | Yes | − | 1 |
| 15 | MMRD | Flu | 4.0 | 100 | 100 | 0.59 | 1.43 | 0.46 | No | + | 1 |
| 16 | MMRD | None | 22.3 | 97 | 0 | 0.09 | 0.78 | < 0.07 | Yes | n.a. | 2 |
| 17 | MMRD | Bu Cy ATG | 6.3 | 100 | 100 | 0.22 | 1.09 | 1.27 | No | + | 1 |
| 18 | MMRD | None | 18.5 | 100 | 0 | 0.05 | < 0.04 | < 0.06 | Yes | − (isohemagglutinins) | 2 |
| 19 | MMRD | None | 11.25 | 100 | 0 | 0.42 | 1.9 | < 0.06 | Yes | n.a. | 2 |
| 20 | MMRD | None | 15.7 | 100 | 0 | 0.29 | 0.38 | < 0.1 | Yes | n.a. | 2 |
| 21a | MMRD | Bu 8 Cy | 8.2 | 100 | 15 | 3.2 | 2.9 | No | + | 1 | |
| 21b | MMRD (no TCD) | None | 1.7 | 100 | 0 | 0.76 | < 0.3 | Yes | n.a. | 2 | |
| 22 | MMRD | TT, Cy, ATG | 12.3 | 100 | 100 | 0.42 | 2.18 | 0.92 | No | + | 1 |
| 23 | UCB | Bu Cy Flu ATG | 7.0 | 100 | 100 | 1.78 | 0.89 | 0.32 | No | + | 1 |
| 24 | UCB | Bu Cy ATG | 2.0 | 100 | 100 | 2.69 | 2.39 | 0.97 | No | + | 1 |
| 25 | MSD | None | 12.2 | 97 | 11 | na | 1.18 | 1.50 | No | + | n.a. |
| 26 | MMRD | Anti-CD20 | 2.4 | 94 | 2 | na | 1.05 | < 0.07 | Yes | − | n.a. |
| 27 | MMRD | Cy ATG | 17.0 | 95 | 1 | na | 0.83 | 2.47 | No | + | n.a. |
| 28 | MSD | None | 3.5 | 90 | 50 | na | 0.2 | 0.35 | Yes | n.a. | n.a. |
| 29 | MUD | Bu Cy ATG | 4.5 | 99 | 98 | 0.03 | 0.89 | 0.97 | No | + | n.a. |
Flu indicates fludarabine; ATG, anti-thymocyte globulin; PIRD, phenotypically identical related donor; Treo, treosulfan; Camp, Compath (anti-CD52); n.a., not available; Bu, busulfan; Cy, cyclophsophamide; TCD, T-cell depletion; TT, thiotepa; UCB, umbilical cord blood; and MUD, matched unrelated donor.
− indicates undetectable Ag-specific Ab response; Poor, induced but not protective Ag-specific Ab response; and +, protective Ag-specific Ab responses. For P18, no isohemagglutinins were detected.
mAbs and reagents
The following mAbs were used: FITC–anti-CD20, PE–anti-CD19, allophycocyanin (APC)–anti-CD10; biotinylated anti–human IgM, IgG, and IgA; PE–anti-CD27; APC–anti-CD27, APC–anti-IgG and PE-Cy7 anti-CD38; Streptavidin-PerCp (BD Biosciences); and biotin and APC–anti-CD27 (eBioscience and BD Biosciences).
Chimerism analysis
Lineage-specific chimerism was assessed by molecular analysis at highly polymorphic loci. Genomic DNA was isolated from T, B, and myeloid cells purified by positive selection using magnetic beads coated with anti-CD3, anti-CD19, and anti-CD14 mAb, respectively (Miltenyi Biotec). Molecular genotyping at the IL2RG and JAK3 loci in transplanted patients was performed to detect autologous (mutant) versus donor-derived (wild-type [WT]) alleles by extracting genomic DNA from sort-purified T and B lymphocytes and amplifying the region encompassing the mutation by PCR using Pfu polymerase and appropriate primers.22 Sequencing was performed by the Australian Genomic Research Facility (Westmead, New South Wales, Australia).
B-cell phenotyping and isolation
PBMCs were incubated with mAb to CD19 or CD20, CD10, and CD27. The frequencies of naive (CD20+CD10−CD27−) and memory (CD20+CD10−CD27+) B cells were then determined.10,12 Expression of Ig isotypes on memory B cells also was determined.12,23 Naive B cells were purified by labeling total B cells with mAb against CD20, CD27, and IgG and sorting CD20+CD27−IgG− cells (FACSAria; BD Immunohistochemistry Systems). The purity of the recovered naive population was typically > 98%.
In vitro activation of naive B cells and B-cell lines
PBMCs were labeled with 5μM CFSE and then cultured (3 × 105/200 μL) in 96-well round-bottomed plates in the absence or presence of CD40L/IL-21 or CpG 2006 (1.25μM). After 4 days, B-cell proliferation (ie, CFSE dilution) and the frequency of CD19+CD27++CD38++ plasmablasts24 were determined by flow cytometry. Secretion of IgG or IgM was measured by ELISA (Zeptometrix). Purified naive B cells were cultured with CD40L alone or together with IL-4 (100 U/mL), IL-10 (100 U/mL), IL-21 (50 ng/mL; PeproTech), CpG 2006 (1 μg/mL; Proligo), or F(ab′)2 fragments of goat anti-human Ig (Jackson ImmunoResearch Laboratories). Ig secretion was determined by ELISA after culture of naive B cells (∼ 10-20 × 103/200 μL/well; BD Biosciences Discovery Labware) for 10 to 12 days.8,23 Expression of AICDA (5′, gactttggttatcttcgcaataaga; 3′, aggtcccagtccgagatgta; Integrated DNA Technologies) was determined using the LightCycler 480 Probe Master Mix and System (Roche Diagnostics). All reactions were standardized to GAPDH.12 Induction of phosphorylation of STAT1, STAT3, and STAT5 by IL-21 in, and modulation in expression of CD23 and HLA-DR in response to IL-4 and IL-13 stimulation on, lymphoblastoid B-cell lines was determined as described previously.12,25,26
Results
Patients
We analyzed 33 patients who received HCT because of SCID. Median time since HCT was 9.8 years (range, 0.5-23.0; Table 2). All 28 patients with X-SCID/JAK3 deficiency attained full (≥ 95%) donor T-cell chimerism (Table 2). In contrast, ≥ 95% donor B-cell chimerism was observed in 10 patients, mixed B-cell chimerism (5%-94% donor-derived B cells) was observed in 3 patients, and 15 patients showed absent or negligible (≤ 5%) donor B-cell engraftment (Table 2). Patients with more than 5% donor B-cell chimerism were categorized as group 1 (n = 13), and those with ≤ 5% B-cell chimerism were deemed group 2 (n = 15). Of the 28 transplanted X-SCID/JAK3 patients analyzed, 14 (50%) received unconditioned HCT; the remaining patients received myeloablative CR (n = 7), or reduced intensity CR and/or immunosuppressive regimens (n = 7). Use of CR of any kind was strongly associated with an ability to attain donor B-cell engraftment (χ2 = 14.36; P = .0002). Specifically, only 1 of 14 patients (7%; P2b) with X-SCID/JAK3 deficiency who received unconditioned HCT attained mixed B-cell chimerism; the other 13 unconditioned patients belonged to group 2 (Table 2). Of the 14 patients undergoing conditioning, 10 (71%) developed full B-cell chimerism. Mixed chimerism was observed in 2 patients (14%) receiving submyeloablative doses (8 mg/kg) of busulfan. Thus, 86% of transplanted X-SCID/JAK3 patients undergoing conditioning were classified as group 1. In contrast, 2 of 14 patients with X-SCID/JAK3 deficiency who received CR failed to show donor B-cell engraftment (Table 2).
Serum Ig levels, functional antibody responses, and B-cell chimerism in SCID patients posttransplant
We assessed whether reconstitution of humoral immune reconstitution (defined as lack of requirement for IgRT and the ability to generate protective Ab responses) after HCT correlated with B-cell chimerism. At the time of analysis, IgRT was required in 15/28 patients, including 13/15 patients (87%) with autologous B-cell reconstitution (group 2) but only 2/13 patients with donor B-cell chimerism (group 1; Table 2). Among the 13 patients who were off IgRT, 11 developed protective Ab titers to tetanus toxoid. Of the remaining 2 patients, P1b showed nonprotective Ab responses against tetanus toxoid and his sibling (P1a) showed weak responses to tetanus and pneumococcal Ag (Table 2). The ability of transplanted X-SCID/JAK3-deficient patients to generate a robust humoral immune response in vivo segregated with the presence (> 5%) of donor-derived B cells (χ2 = 17.51; P < .0001); remarkably, none of the 15 patients with ≤ 5% donor B cells attained humoral immune reconstitution. Highly significant differences also were noted for serum IgM and IgA levels between group 1 versus group 2 after HCT X-SCID/JAK3 patients (IgM, 2.1 ± 0.26 vs 0.67 ± 0.13 mg/mL; IgA, 1.3 ± 0.35 vs 0.12 ± 0.07 mg/mL; Table 2; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Two patients failed to attain functional humoral reconstitution despite donor B-cell engraftment. However, 1 patient (P3) was only 6 months post-HCT, and no attempts were made to discontinue IVIG; the other patient (P14) received anti-CD20 mAb after HCT because of lymphoproliferative disease. A positive correlation also was observed between functional humoral immune reconstitution after HCT and use of CR (χ2 = 9.58; P = .002). However, conditioning had a less significant impact than donor B-cell engraftment on humoral immune reconstitution. In particular, 4 patients (including 3 receiving MMRD-HCT) exhibited impaired humoral immunity despite the use of CR. Among 5 patients who received HCT for other forms of SCID (IL7R, CD3D, RAG1 mutations; P25-29; Tables 1 and 2), 3 attained full (P29) or mixed (P25 and P28) donor B-cell chimerism. P25 and P27 displayed protective Ab responses in vivo despite the low number (P25) or the virtual absence (P27) of donor-derived B cells, suggesting B-cell chimerism is more important to achieve functional humoral reconstitution among patients with X-SCID/JAK3 deficiency than in other forms of SCID.
X-linked SCID patients with poor donor B-cell reconstitution and compromised humoral immunity have reduced total and isotype-switched memory B cells
We extended our findings of a correlation between donor B-cell engraftment and restoring humoral immunity in vivo by examining the B-cell compartment of transplanted X-SCID/JAK3 patients (Table 2). The proportion of circulating B cells was similar between age-matched controls (range, 1-17 years old; mean ± SD, 10.5 ± 6.5 years) and SCID patients irrespective of the genotype of the B cells (Figure 1A). In age-matched healthy controls, ∼ 15% of B cells exhibited a memory (ie, CD27+) phenotype (Figure 1B).2 There was no difference in the frequency of memory B cells between controls and group 1 patients (P > .05; Figure 1B). However, there were significantly fewer memory B cells in X-SCID/JAK3–deficient patients with poor donor B-cell chimerism (group 2) compared with both healthy controls and group 1 post-SCT patients (Figure 1B).
Impaired humoral immune responses in X-SCID/JAK3–deficient patients correlates with reduced generation of total and class-switched memory B cells. (A-B) PBMCs from age-matched controls (n = 11 [total B cells]; n = 18 [memory B cells]) or transplanted X-SCID/JAK3–deficient patients with either donor-derived B cells (group 1; n = 13) or autologous host B cells (n = 15 [total B cells]; n = 11 [memory B cells]; group 2) were labeled with mAb against CD19 (or CD20) and CD27. The frequencies of total B cells (A) or memory B cells (B) were then determined by flow cytometry. The plots are from representative donors and patients, with the values representing the mean of each group. The graphs show data points for all donors and patients examined, with the horizontal line representing the mean. ns indicates not significant (*P < .05; **P < .01). (C-D) Memory (CD20+CD27+) B cells from age-matched controls (n = 11) or group 1 (n = 6) or group 2 (n = 8) X-SCID/JAK3–deficient patients were labeled with mAb specific for IgM, IgG, or IgA. The frequency of memory B cells that were IgM+, IgG+, or IgA+ was then determined. (C) Histogram plots show IgG and IgA expression on memory B cells from representative patients corresponding to group 1 (gray-filled histogram) or group 2 (dashed black histogram). (D) Data points for all donors and patients examined, with the horizontal line representing the mean (*P < .05; ***P < .005).
Impaired humoral immune responses in X-SCID/JAK3–deficient patients correlates with reduced generation of total and class-switched memory B cells. (A-B) PBMCs from age-matched controls (n = 11 [total B cells]; n = 18 [memory B cells]) or transplanted X-SCID/JAK3–deficient patients with either donor-derived B cells (group 1; n = 13) or autologous host B cells (n = 15 [total B cells]; n = 11 [memory B cells]; group 2) were labeled with mAb against CD19 (or CD20) and CD27. The frequencies of total B cells (A) or memory B cells (B) were then determined by flow cytometry. The plots are from representative donors and patients, with the values representing the mean of each group. The graphs show data points for all donors and patients examined, with the horizontal line representing the mean. ns indicates not significant (*P < .05; **P < .01). (C-D) Memory (CD20+CD27+) B cells from age-matched controls (n = 11) or group 1 (n = 6) or group 2 (n = 8) X-SCID/JAK3–deficient patients were labeled with mAb specific for IgM, IgG, or IgA. The frequency of memory B cells that were IgM+, IgG+, or IgA+ was then determined. (C) Histogram plots show IgG and IgA expression on memory B cells from representative patients corresponding to group 1 (gray-filled histogram) or group 2 (dashed black histogram). (D) Data points for all donors and patients examined, with the horizontal line representing the mean (*P < .05; ***P < .005).
In healthy donors, ∼ 40% of memory B cells express IgM, whereas ∼ 20% to 25% express either IgG or IgA.12,23 The proportions of memory B cells that had undergone isotype switching to IgG and IgA was significantly reduced in group 2 X-SCID/JAK3–deficient patients compared with healthy controls and group 1 patients (Figure 1C-D), consistent with previous findings.20 Thus, a likely explanation for poor humoral immune responses in vivo, and requirement for IgRT, in group 2 SCID patients is an inability to generate a normal pool of memory B cells.
Plasmablast differentiation and Ig secretion in vitro is defective in X-SCID/JAK3–deficient patients with poor donor B-cell chimerism after HCT
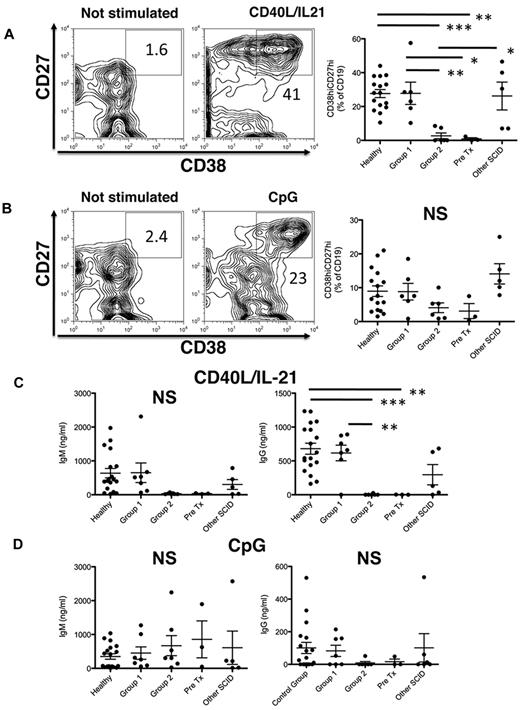
We next determined whether WT IL2RG/JAK3 is required for differentiation of B cells into plasmablasts. Stimulation of total B cells isolated from PBMCs with CD40L alone induces very low levels of secreted Ig; however, this is increased 50- to 100-fold by IL-21 (supplemental Figure 2). Thus, Ig secretion detected in cultures of B cells activated with CD40L/IL-21 primarily reflects IL-21–induced plasmablast formation. Thus, PBMCs from groups 1 or 2 SCID patients were stimulated with either CD40L together with IL-21 as a mimic of TD signals, or with CpG to mimic T-independent signaling.27 Four days after in vitro activation, differentiation into plasmablasts was assessed by determining the frequency of CD19+CD38hiCD27hi cells24 by flow cytometry (Figure 2A-B). Although differentiation of B cells into plasmablasts in response to CD40L/IL-21 was similar (∼ 25%-30%) for healthy controls and X-SCID/JAK3–deficient patients with good donor B-cell chimerism (group 1), plasmablast formation was strikingly reduced in patients with poor donor B-cell chimerism (group 2). In fact, it was comparable with that measured in untransplanted X-SCID or JAK3-SCID patients (Figure 2A). Although there was a trend for reduced plasmablast formation in response to CpG in group 2 and untransplanted X-SCID/JAK3–deficient PBMC compared with controls, this did not reach statistical significance (Figure 2B). The reduced formation of plasmablasts in response to CpG by group 2 patients probably reflects their diminished frequency of memory B cells (Figure 1), coupled with the greater sensitivity of memory B cells to TLR9 stimulation.27 Generation of plasmablasts in patients with other forms of SCID was comparable with group 1 patients on stimulation with either CD40L/IL-21 or CpG (Figure 2A-B).
Diminished plasmablast formation and Ig secretion in response to CD40L/IL-21 in SCID patients with poor donor-derived B-cell reconstitution. PBMCs of the indicated patient-groups were either unstimulated, or cultured with CD40L/IL-21 (A,C) or CpG (B,D). After 4 days, the frequency of B cells that differentiated into plasmablasts, as determined by acquisition of a CD38hiCD27hi phenotype (A-B), and secretion of IgM and IgG (C-D) were determined by flow cytometry and ELISA, respectively. Representative FACS plots derived from healthy donors are presented in panels A and B. Statistical analysis was performed using 1-way ANOVA and Bonferroni posttest analysis (*P < .05; **P < .01; ***P < .001). NS indicates nonsignificant changes.
Diminished plasmablast formation and Ig secretion in response to CD40L/IL-21 in SCID patients with poor donor-derived B-cell reconstitution. PBMCs of the indicated patient-groups were either unstimulated, or cultured with CD40L/IL-21 (A,C) or CpG (B,D). After 4 days, the frequency of B cells that differentiated into plasmablasts, as determined by acquisition of a CD38hiCD27hi phenotype (A-B), and secretion of IgM and IgG (C-D) were determined by flow cytometry and ELISA, respectively. Representative FACS plots derived from healthy donors are presented in panels A and B. Statistical analysis was performed using 1-way ANOVA and Bonferroni posttest analysis (*P < .05; **P < .01; ***P < .001). NS indicates nonsignificant changes.
On stimulation with CD40L/IL-21 PBMCs from healthy controls and group 1 patients secreted large quantities of IgM and IgG (Figure 2C). Similar levels of IgM and IgG also were observed for PBMCs from transplanted patients with SCID resulting from mutations in genes other than JAK3 or IL2RG (Figure 2C). These data are consistent with the detection of abundant numbers of plasmablasts in cultures of these cells (Figure 2A). In contrast, PBMC-derived B cells from group 2 X-SCID/JAK3 patients failed to produce Ig in response to CD40L/IL-21 stimulation, being comparable with that found for untransplanted X-SCID/JAK3 patients (Figure 2C). This mirrors their inability to yield plasmablasts in response to this mimic of TD activation (Figure 2A). On the other hand, IgM and IgG secretion after stimulation with CpG were not significantly different among the different groups tested (Figure 2C-D). The nonsignificant trend to lower IgG responses after CpG stimulation in group 2 versus group 1 and healthy control–derived B cells is probably because of higher memory B-cell numbers in the latter cohorts (Figure 1B).27 Indeed, we have found that naive B cells, which predominate in group 2 patients, produce minimal amounts of IgG after CpG stimulation (data not shown). Collectively, these results imply that IL2RG/JAK3-mutant SCID B cells are intrinsically capable of responding to exogenous stimuli but are nonresponsive to T-helper cell-derived IL-21 signals.
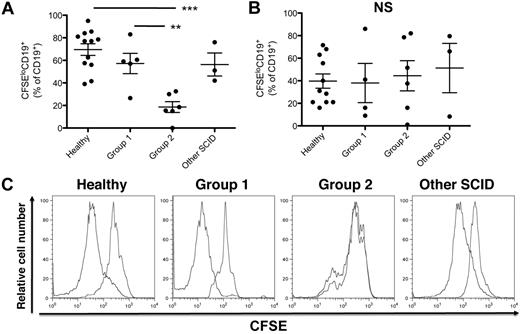
B cells from X-SCID patients with poor donor B-cell reconstitution fail to proliferate on stimulation with CD40L/IL-21
The reduced plasmablast formation and diminished Ig secretion observed in group 2 SCID patients could result from reduced B-cell activation and proliferation or, more selectively, impaired differentiation of activated B cells into plasmablasts. To analyze B-cell proliferation, PBMCs were labeled with CFSE before stimulation with CD40L/IL-21 or CpG. PBMC-derived B cells from group 2 X-SCID/JAK3–deficient patients failed to proliferate, but those from group 1 patients proliferated comparably with B cells from healthy controls (Figure 3A,C). This suggests that expression of γc/JAK3 is involved in both early (proliferation) and late (plasmablast formation) stages of B-cell activation after CD40L/IL-21 stimulation. In contrast, proliferation of B cells from group 1 and group 2 patients to CpG was comparable with that of B cells from normal donors (Figure 3B), demonstrating that γc/JAK3 mutant B cells can proliferate in response to TLR ligands but not IL-21.
B cells from Group 2 X-SCID/JAK3-deficient patients fail to proliferate in response to CD40L/IL-21 stimulation. (A-C) PBMCs were labeled with CFSE and were either unstimulated or stimulated with CD40L/IL-21 or CpG. After 4 days, proliferation of B cells was assessed by incubating the cultured cells with anti-CD19 mAb and determining dilution of CFSE by CD19+ cells by flow cytometric analysis. (A-B) Percentage of CFSElo cells in cultures of PBMCs from the indicated donor groups after stimulation with CD40L/IL-21 (A) or CpG (B). (C) Representative FACS plots of CFSE dilution in PBMCs isolated from the indicated donor groups after stimulation in the absence or presence of CD40L/IL-21. Statistical analysis was performed using 1-way ANOVA and Bonferroni posttest analysis (**P < .01; ***P < .001).
B cells from Group 2 X-SCID/JAK3-deficient patients fail to proliferate in response to CD40L/IL-21 stimulation. (A-C) PBMCs were labeled with CFSE and were either unstimulated or stimulated with CD40L/IL-21 or CpG. After 4 days, proliferation of B cells was assessed by incubating the cultured cells with anti-CD19 mAb and determining dilution of CFSE by CD19+ cells by flow cytometric analysis. (A-B) Percentage of CFSElo cells in cultures of PBMCs from the indicated donor groups after stimulation with CD40L/IL-21 (A) or CpG (B). (C) Representative FACS plots of CFSE dilution in PBMCs isolated from the indicated donor groups after stimulation in the absence or presence of CD40L/IL-21. Statistical analysis was performed using 1-way ANOVA and Bonferroni posttest analysis (**P < .01; ***P < .001).
Analysis of responses of naive B cells expressing WT or mutant IL2RG genes
The experiments performed using total PBMCs suggested selective defects in responses of B cells expressing mutant IL2RG or JAK3 to IL-21. However, a caveat to these results is that the B-cell compartment of group 1 X-SCID/JAK3–deficient patients contained significantly more memory cells than group 2 patients (Figure 1B). Because memory cells undergo significantly greater responses than naive cells,8,28 it was possible that differences in responses of group 1 and group 2 B cells reflected the greater frequency of memory B cells in the former patient cohort. Furthermore, these experiments were performed using total PBMCs; thus, the indirect contribution of activated non-B cells to the B-cell response needs to be considered. For these reasons, we took a reductionist approach and performed detailed analyses of the response of sort-purified naive B cells from transplanted X-SCID patients with donor (group 1; n = 6) versus autologous (group 2; n = 7) B cells relative to that of healthy donors (n = 15).
Mutations in IL2RG abolish IL-21–induced proliferation of naive B cells in vitro
Naive B cells sorted from healthy donors and transplanted X-SCID/JAK3 deficient patients were cultured with CD40L alone or in combination with CpG, anti-Ig, or cytokines that promote B-cell growth and differentiation. Proliferation was determined after 5 days by measuring [3H]thymidine incorporation during the final 18 hours of culture. The results are expressed as fold-change in proliferation relative to that induced by CD40L alone. Addition of CpG, anti-Ig, IL-4, IL-10, or IL-13 augmented B-cell proliferation induced by CD40L by ∼ 2-to 6-fold in all groups, irrespective of their IL2RG genotype, whereas IL-15 had little effect (Figure 4A). Of note, although γc is a component of the IL-4R, IL-4 induced normal proliferation in CD40L-activated naive B cells from group 2 X-SCID patients (Figure 4A). This probably reflects the ability of IL-4 to signal via an alternate receptor composed of the IL-4RA and IL-13R chains11,25,29 thus bypassing the requirement for an intact γc in the IL-4R complex. Indeed, IL-4 could up-regulate CD23 and HLA-DR expression on EBV-lymphoblastoid cell lines in both healthy donors and patients with mutant IL2RG to a similar extent as IL-13 (supplemental Figure 3A). However, although CD40L-stimulated naive B cells from healthy donors and group 1 X-SCID patients responded vigorously to IL-21, corresponding B cells from group 2 patients were completely unresponsive to the stimulatory effect of IL-21 (ie, fold-change compared with CD40L alone, 1.12 ± 0.3 for group 2 B cells vs 4.56 ± 2.2 for normal B cells and 4.0 ± 2.2 for group 1 B cells; Figure 4A). This is consistent with rapid and transient IL-21–induced phosphorylation of STAT1 and STAT3, and weak phosphorylation of STAT5, in EBV-lymphoblastoid cell lines from healthy donors but not in those from X-SCID patients (supplemental Figure 3B).
IL2RG mutant naive B cells fail to proliferate in response to IL-21. (A) Naive B cells from control donors (n = 15) or transplanted X-SCID/JAK3–deficient patients with either donor-derived (group 1; n = 6) or autologous (group 2; n = 7) B cells were stimulated with CD40L alone or together with anti-Ig, CpG, IL-4, IL-10, IL-13, IL-15, or IL-21. Proliferation was determined after 5 days by measuring incorporation of [3H]thymidine during the final 18 hours of culture. The results are expressed as fold-change in proliferation relative to CD40L alone. [3H]Thymidine uptake (mean cpm ± SEM) by naive B cells isolated from healthy donors (n = 15), group 1 (n = 6), or group 2 (n = 7) transplanted X-SCID/JAK3–deficient patients who were stimulated with CD40L alone was 393 ± 63, 419 ± 104, and 442 ± 71, respectively. (B-C) Naive B cells from control donors or transplanted group 1 (B) or group 2 (C) X-SCID/JAK3–deficient patients were labeled with CFSE and then cultured with CD40L alone or together with IL-4 or IL-21. Proliferation, as determined by dilution of CFSE, was measured after 5 days of culture. Data from experiments that examined responses of naive B cells from 3 different group 2 X-SCID patients is presented in panel C.
IL2RG mutant naive B cells fail to proliferate in response to IL-21. (A) Naive B cells from control donors (n = 15) or transplanted X-SCID/JAK3–deficient patients with either donor-derived (group 1; n = 6) or autologous (group 2; n = 7) B cells were stimulated with CD40L alone or together with anti-Ig, CpG, IL-4, IL-10, IL-13, IL-15, or IL-21. Proliferation was determined after 5 days by measuring incorporation of [3H]thymidine during the final 18 hours of culture. The results are expressed as fold-change in proliferation relative to CD40L alone. [3H]Thymidine uptake (mean cpm ± SEM) by naive B cells isolated from healthy donors (n = 15), group 1 (n = 6), or group 2 (n = 7) transplanted X-SCID/JAK3–deficient patients who were stimulated with CD40L alone was 393 ± 63, 419 ± 104, and 442 ± 71, respectively. (B-C) Naive B cells from control donors or transplanted group 1 (B) or group 2 (C) X-SCID/JAK3–deficient patients were labeled with CFSE and then cultured with CD40L alone or together with IL-4 or IL-21. Proliferation, as determined by dilution of CFSE, was measured after 5 days of culture. Data from experiments that examined responses of naive B cells from 3 different group 2 X-SCID patients is presented in panel C.
We also examined the ability of IL-4 and IL-21 to augment proliferation of CD40L-stimulated naive B cells, as revealed by CFSE dilution. Although IL-4 could equally promote proliferation of CD40L-stimulated naive B cells from normal donors and either genotype of X-SCID patients, IL-21 only improved expansion of B cells from healthy controls and group 1 X-SCID patients (Figure 4B-C). Thus, the inability of IL2RG-mutated PBMC-derived B cells to respond to IL-21 was similarly seen in purified naive B cells.
IL2RG mutant naive B cells undergo isotype switching and Ig secretion in response to non–γc-dependent signals, but not to IL-21
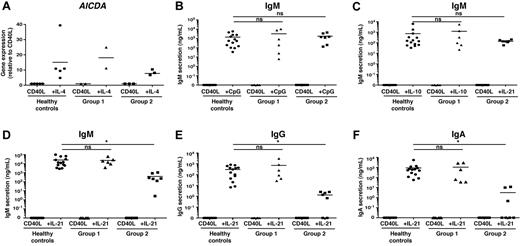
Next, we examined the functional responses of naive B cells with respect to isotype switching and differentiation to plasmablasts and subsequent Ig secretion. IL-4 is a well-characterized switch factor.26 This is mediated by its ability to induce expression of AICDA, encoding activation-induced cytidine deaminase, which is necessary for class switch recombination.30 In keeping with preserved activation and proliferation in response to IL-4 (Figure 4 and supplemental Figure 3), we observed comparable induction of AICDA in naive B cells from healthy controls and from both group 1 and group 2 patients after culture with CD40L/IL-4 relative to that observed for CD40L alone (Figure 5A). We also assessed secretion of IgM in the presence of CD40L/CpG or CD40L/IL-10, cultures that induce much higher levels of secreted IgM than CD40L alone.12 Here, IgM secretion by both donor-derived (group 1) and autologous (group 2) naive B cells was comparable with that by cells isolated from normal donors (Figure 5B-C). Thus, together with the data for proliferation and AICDA expression, X-SCID B cells can exhibit normal responses at least in vitro to activation signals that do not rely on signaling through the γc, that is, BCR, TLR9, IL-4, and IL-10.
IL2RG mutant naive B cells can express AICDA and differentiate into Ig-secreting cells in response to diverse stimuli, but not IL-21. Naive B cells from control donors (A; n = 5; B-E; n = 14) or transplanted X-SCID/JAK3–deficient patients with either donor-derived (group 1; A, n = 2; B-E, n = 6) or autologous (group 2; A, n = 3; B-E, n = 7) B cells were stimulated with CD40L alone or together with IL-4, IL-10, CpG, or IL-21. (A) Expression of AICDA was determined by quantitative PCR after 5 days. The levels of secreted IgM (B-D), IgG (E), and IgA (F) were determined by ELISA after 12 days. Each symbol represents AICDA expression of Ig secretion by naive B cells from an individual normal donor or patient (*P < .05).
IL2RG mutant naive B cells can express AICDA and differentiate into Ig-secreting cells in response to diverse stimuli, but not IL-21. Naive B cells from control donors (A; n = 5; B-E; n = 14) or transplanted X-SCID/JAK3–deficient patients with either donor-derived (group 1; A, n = 2; B-E, n = 6) or autologous (group 2; A, n = 3; B-E, n = 7) B cells were stimulated with CD40L alone or together with IL-4, IL-10, CpG, or IL-21. (A) Expression of AICDA was determined by quantitative PCR after 5 days. The levels of secreted IgM (B-D), IgG (E), and IgA (F) were determined by ELISA after 12 days. Each symbol represents AICDA expression of Ig secretion by naive B cells from an individual normal donor or patient (*P < .05).
Because the primary defect in group 2 patients is impaired humoral immune responses in vivo, we compared the ability of naive B cells from controls and group 1 and group 2 patients to differentiate into plasmablasts in vitro. IL-21 increased IgM secretion by normal (25 388 ± 7 900 ng/mL) and group 1 (22 888 ± 8 600 ng/mL) CD40L-stimulated naive B cells by more than 1000-fold compared with cells stimulated with CD40L alone (2.0 ± 1.3 and 1.15 ± 1.0 ng/mL, respectively), and it induced production of IgG (normal controls, 297 ± 75 ng/mL; group 1: 714 ± 480 ng/mL) and IgA (normal controls, 910 ± 370 ng/mL; group 1, 1070 ± 560 ng/mL). In contrast, the ability of naive B cells with IL2RG mutations to respond to IL-21 was dramatically and significantly reduced (IgM, 393 ± 151 ng/mL [> 50-fold]; IgG, 1.3 ± 0.5 ng/mL [> 200-fold]; IgA, 3.2 ± 2.0 ng/mL [> 300-fold]; Figure 5D-F). This indicates that an intact γc is essential for IL-21–induced Ig secretion by naive B cells.
Discussion
In this study, we have provided mechanistic insight not only to explain the frequent persistence of humoral defects after HCT for X-SCID and JAK3-SCID but also elucidated the role of cytokines during B-cell activation and differentiation in normal individuals. Although human B-cell development is not affected by IL2RG or JAK3 mutations, B cells from patients with these forms of SCID are nonfunctional16,17,19 because of a lack of “help” by CD4+ T cells, which are absent from these patients.16 HCT is the mainstay of treatment for SCID and restores T-cell development and function in the majority of patients. In contrast, lack of humoral immune reconstitution is often observed after HCT,17,19 but the underlying mechanisms have remained elusive. Long-term engraftment of donor-derived B cells has been recognized as an important factor to facilitate functional humoral reconstitution,31 with only donor-derived B cells undergoing class switching in X-SCID patients with mixed chimerism after HCT.32 Here, we have confirmed that engraftment of donor-derived B cells is strongly associated with improved humoral reconstitution, with respect to generating memory B cells and protective Ab responses, after HCT for X-SCID and JAK3 deficiency.
Use of CR is often advocated to promote donor B-cell chimerism. Although some studies showed that CR preceding MMRD-HCT for SCID associates with better donor B-cell chimerism,33 this is not always observed.18,34,35 Indeed, ∼ 30% of X-SCID patients treated by unconditioned MMRD-HCT develop donor B cells.17 Although we found that improved B-cell function correlated with conditioning, this association was less significant than that with donor B-cell engraftment. However, successful humoral reconstitution after unconditioned MSD-HCT, in spite of an apparent lack of donor B-cell engraftment, also has been reported.33 It is possible that some degree of microchimerism is present, but remains undetected, in these patients. Alternatively, HLA matching between donor T and recipient B cells may allow effective cooperation. However, this is not supported by X-SCID patients with somatic reversion restricted to the T-lineage who remain dependent on IgRT.36
By studying X-SCID/JAK3–deficient patients who retained autologous, genetically defective B cells, or attained successful engraftment of donor-derived, genetically intact B lymphocytes, we can better define the molecular mechanisms governing human B-cell differentiation. Many cytokines can activate human B cells and induce their differentiation in vitro. IL-4 and IL-13 mediate class switching to IgE and IgG4,26,37 whereas IL-10 directs switching to IgG1 and IgG3, supports survival of GC B cells, and induces memory B cells to differentiate into PCs.28,37 BAFF, APRIL, IL-15, and IL-6, when combined with IFN-α or IL-12, induce class switching, Ig secretion, or both and support plasmablast survival.37-40 Previous studies revealed similar functions for TNF-α, TGF-β, and TLR ligands.27,37 Based on these observations in vitro, it is surprising that most transplanted SCID patients who retain host (ie, mutant) B cells but have engrafted donor CD4+ T cells continue to exhibit poor humoral immune responses after natural infection or immunization.18,19,41 This points to the strict requirement for B-cell intrinsic, γc-dependent signaling to attain humoral immune reconstitution. Indeed, our findings are consistent with, and significantly extend, those from previous studies that offered similar conclusions but did not provide a mechanism. First, although X-chromosome inactivation in naive B cells from female carriers of X-SCID is random, memory B cells preferentially express the WT allele.42 Second, somatic reversion has been reported in an X-SCID patient and resulted in expression of WT IL2RG in T cells but not B, NK, or myeloid cells.36 Similar to group 2 SCID patients after HCT, this patient lacked memory B cells and was unable to mount Ag-specific Ab responses despite the presence of normal T cells.36 A caveat to this latter interpretation is that the patient with an IL2RG reversion had reduced numbers of CD4+ T cells and a slightly restricted TCR Vβ repertoire.36 By revealing a critical role for IL-21, our data provide an explanation for the B-cell intrinsic requirement of signals through γc for the generation of memory B cells and effective humoral immunity.
Of the 6 currently identified γc cytokines, IL-2, IL-4, IL-15, and IL-21 have been best characterized for B-cell stimulatory function. IL-7 is dispensable for human B-cell development, because B cells are generated in normal numbers in patients with mutations in IL7RA.43 Furthermore, IL-4 can function on X-SCID/JAK3–deficient B cells via its ability to signal via an alternative IL-4R25,29 composed of the IL-4Rα and IL-13R chains.11 Indeed, we confirmed the ability of IL-4 to activate IL2RG mutant B cells. Although IL-2 and IL-15 may be involved in restoring humoral immunity in transplanted X-SCID/JAK3–deficient patients with donor-derived B cells, they are unlikely to have a dominant role because patients with mutations in IL2RA44,45 , and IL-15–deficient mice,46 have normal or elevated levels of serum Ig. Thus, IL-21 clearly seems to be the predominant γc-activating cytokine critical for mediating B-cell differentiation in response to TD Ag.
Cytokines such as IL-4, IL-10, and BAFF, as well as TLR ligands, can cooperate with IL-21 to enhance activation of CD40L-stimulated human B cells.6,8-10,47 Although our data identify IL-21 as the key mediator of humoral immunity in humans in vivo, it is likely that other factors contribute to this process by augmenting the effects of IL-21. However, these factors seem insufficient for the initiation of TD B-cell responses in vivo. Indeed, humoral responses to TD Ags are unaffected by mutations in MYD88 or IRAK4, thereby questioning the role of TLR signaling in protective serologic immunity.48 We propose that the requirement for IL-21 in humoral immunity reflects its pleiotropic and potent effects on human B cells inasmuch that it induces proliferation, isotype switching, and PC generation resulting in secretion of large quantities of all Ig isotypes.6-10,13 The effects of IL-21 on these functions exceed that of other cytokines by up to 50-fold. IL-21 exerts its effect by inducing AICDA expression for class switching, and PRDM1 (encoding Blimp-1) for commitment to the PC lineage.6,12,13,49 IL-21 also induces the GC transcription factor BCL612,49 , which explains the reduced frequency of GCs in lymphoid tissues of IL-21/IL-21R–deficient mice.14,15 Thus, IL-21 simultaneously achieves the individual effects of numerous cytokines (IL-4, IL-10, IL-6, IL-13, BAFF/APRIL) on human B cells.
Our findings also provide insight into mechanisms underlying Ag-specific immune responses in normal individuals as well as defects in other immunodeficiencies. For example, X-SCID/JAK3–deficient patients with autologous B cells after HCT and STAT3-deficient patients both have a paucity of memory B cells and defective immune responses to TD Ag, and their B cells are unresponsive to IL-21.12 However, STAT3-deficient patients have normal serum Ig levels (except IgE), whereas transplanted X-SCID/JAK3–deficient patients with autologous B cells have reduced levels of all serum Igs. This suggests that IL-21R/γc/JAK3/STAT3 are important for Ag-specific humoral immunity in humans, and for restoring humoral immunity in SCID patients post HCT, and this pathway represents an attractive target for improving B-cell function in cases of vaccination or immune deficiency. Our data therefore imply that γc-dependent/STAT3–independent pathways maintain basal serum Ab levels, possibly via IL-21R/γc–mediated activation of STAT1, STAT5, or both. Lastly, and most importantly, our findings have clinical significance. Currently, assessment of in vivo B-cell function in X-SCID/JAK3 SCID patients after HCT requires cessation of IgRT for 3 months, immunization, and analysis of specific Ab responses. This procedure may lead to hypogammaglobulinemia and an increased risk of infections. Although a reduced memory B-cell number and the presence of autologous B cells could be used to predict responsiveness to IL-21, these are not without disadvantages. For example, memory B cells after HCT accumulate over time, reaching adult levels after ∼ 2 to 3 years.50 Thus, these cells will be reduced in HCT patients for a substantial period irrespective of origin of the B cells. Assessing chimerism may not be helpful to predict functionality in vivo in cases of mixed B-cell chimerism. Thus, examining in vitro responses to IL-21 in IL2RG/JAK3 mutant patients after HCT represents a robust procedure that (1) can be performed on total PBMCs; (2) correlates with B-cell function in vivo; and (3) can define if, and when, to discontinue Ig RT and commence immunization programs.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chris Brownlee and Nikki Ailing for cell sorting, Anthony Yee for providing clinical data, Drs Tony Yee, Daman Langguth, and Paul Bauert for coordinating patient sample collection, Dr Rene de Waal Malefyt (DNAX Research Institute, Palo Alto, CA) for providing reagents (IL-4 and IL-10), and the patients and their families for participating in this project.
This work was supported by research grants and fellowships awarded by the National Health and Medical Research Council of Australia (L.J.B. and S.G.T.), the Swiss National Science Foundation (M.R.), U54 AI082973-02 (L.D.N. and M.C.), Fondazione “Angelo Nocivelli” (S.G.), The Manton Foundation (L.D.N.), and the Dubai Harvard Foundation to Medical Research (L.D.N. and W.A.).
Authorship
Contribution: M.R. and L.J.B. designed the research, performed experiments, analyzed and interpreted results, and contributed to the writing of the manuscript; D.T.A., S.G., and F.C. performed experiments; H.E. provided valuable reagents for the experiments; J.M.P. and G.A.T. genotyped some of the X-SCID patients; U.P. generated EBV–transformed B-cell lines; S.G. helped with determining the mutations; M.J.C., A.R.G., S.-Y.P., S.B., W.A., H.O., J.E.W., J.S., J.P., M.W., M.R., and J.B.Z. provided patient samples and clinical details; S.G.T. and L.D.N. designed the research, analyzed and interpreted results, and wrote the manuscript; and all authors commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stuart Tangye, Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst 2010, New South Wales, Australia; e-mail: s.tangye@garvan.org.au; or Luigi Notarangelo, Division of Immunology, Children's Hospital Boston, 1 Blackfan Circle, Boston MA 02115; e-mail: luigi.notarangelo@childrens.harvard.edu.
References
Author notes
M.R. and L.J.B contributed equally to this study.
S.G.T. and L.D.N. contributed equally to this study.

![Figure 1. Impaired humoral immune responses in X-SCID/JAK3–deficient patients correlates with reduced generation of total and class-switched memory B cells. (A-B) PBMCs from age-matched controls (n = 11 [total B cells]; n = 18 [memory B cells]) or transplanted X-SCID/JAK3–deficient patients with either donor-derived B cells (group 1; n = 13) or autologous host B cells (n = 15 [total B cells]; n = 11 [memory B cells]; group 2) were labeled with mAb against CD19 (or CD20) and CD27. The frequencies of total B cells (A) or memory B cells (B) were then determined by flow cytometry. The plots are from representative donors and patients, with the values representing the mean of each group. The graphs show data points for all donors and patients examined, with the horizontal line representing the mean. ns indicates not significant (*P < .05; **P < .01). (C-D) Memory (CD20+CD27+) B cells from age-matched controls (n = 11) or group 1 (n = 6) or group 2 (n = 8) X-SCID/JAK3–deficient patients were labeled with mAb specific for IgM, IgG, or IgA. The frequency of memory B cells that were IgM+, IgG+, or IgA+ was then determined. (C) Histogram plots show IgG and IgA expression on memory B cells from representative patients corresponding to group 1 (gray-filled histogram) or group 2 (dashed black histogram). (D) Data points for all donors and patients examined, with the horizontal line representing the mean (*P < .05; ***P < .005).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2011-06-362533/4/m_zh89991183340001.jpeg?Expires=1767711358&Signature=3qndkJXUay53SfRmZ3jkzBi5ssSnZc4OW2z6OjAIfF97gDWs~iBMCBszTag9UwTIM1NidYjA5yseQql0N01fEl5AFiMDL8IoQC9x8FDxoBxexVowVAM8A6QmSKsMedkE5GfGrZDDN6pKn3k1j1tp~d5f-E1~PoNyzd9lprYzaM8TYkrVaVJL71622T1w9~6fHI4wlVY8Es9LC6ffsUjM6RebedvYFX1ibS5dNnN7oxvmzEIVIi-3kOjE3T3UzquxfI6f7q50i-gfitewz5gqJb4rir9k2~UZUDO79RIm6Ei99apAuvGCewvmeDvLRKAKZB5VFBXWaPOvH9tjXuj8gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. IL2RG mutant naive B cells fail to proliferate in response to IL-21. (A) Naive B cells from control donors (n = 15) or transplanted X-SCID/JAK3–deficient patients with either donor-derived (group 1; n = 6) or autologous (group 2; n = 7) B cells were stimulated with CD40L alone or together with anti-Ig, CpG, IL-4, IL-10, IL-13, IL-15, or IL-21. Proliferation was determined after 5 days by measuring incorporation of [3H]thymidine during the final 18 hours of culture. The results are expressed as fold-change in proliferation relative to CD40L alone. [3H]Thymidine uptake (mean cpm ± SEM) by naive B cells isolated from healthy donors (n = 15), group 1 (n = 6), or group 2 (n = 7) transplanted X-SCID/JAK3–deficient patients who were stimulated with CD40L alone was 393 ± 63, 419 ± 104, and 442 ± 71, respectively. (B-C) Naive B cells from control donors or transplanted group 1 (B) or group 2 (C) X-SCID/JAK3–deficient patients were labeled with CFSE and then cultured with CD40L alone or together with IL-4 or IL-21. Proliferation, as determined by dilution of CFSE, was measured after 5 days of culture. Data from experiments that examined responses of naive B cells from 3 different group 2 X-SCID patients is presented in panel C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2011-06-362533/4/m_zh89991183340004.jpeg?Expires=1767711358&Signature=EROl8bdMJXUJktV4cCga1z1wzkthXVdNsvEHYEKrOgssN6gN~TSt~UXOh8-oz7IMP6h~Vwh4686E2sqHYQIiCZpRWEBQDuGzBqxR6-Q7q5uPKkxAGl1d1JsZ7egwzH6WoEEeBceJDKlV2MmIa586S0A8Irhr4UAHpVFxlYDm76fH6Zo-3qGdYeh98Hu3WFoVfmzr921FyXc5UXWN3ubD1Cq-OI3geY1DICAzKfECucRA6euXRnslw9oz89K8F7H~jHcjAHYSrxMxVzpEGffBTZ9VuArv2nJiN7KXbls9oXUnHZYPu30e3Ntp7l64epJ9u1KX9DSB1zTzGcIZi2pwGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal