In this issue of Blood, Elks et al describe a novel role for activated hypoxia inducible factor (HIF)–1α in sustaining inflammation by delaying neutrophilic retrograde emigration and preventing neutrophil apoptosis through the inhibition of prolyl hydroxylase (PHD) activity. This property is a novel function for HIFs, the master regulators of our body's response to hypoxia.
HIFs are hypoxia-driven transcription factors that are transcribed and translated constitutively. In oxygen-rich environments, the half-life of HIF-α is limited by prolyl-hydroxylation, ubiquitination, and proteosomal degradation of the translated protein. At normoxia, prolyl hydroxylases (PHDs) hydroxylate the HIF-α subunits on critical proline residues and the hydroxylated proteins are recognized by ubiquitin ligases like von Hippel-Lindau proteins, targeting the HIF-α subunits for degradation. In the absence of oxygen, hydroxylation of the HIF-α protein is blocked, resulting in HIF-α pairing with HIF-β (ARNT), translocating to the nucleus and binding to hypoxic response elements (HREs) to induce gene transcription. The article by Elks et al1 targets HIF-1α, the master regulator of vascular endothelial growth factor (VEGF), as the focused isoform. HIF-1α activation is not only associated with tissue angiogenesis, an adaptive response to tissue hypoxia, but also vascular development, metabolism, inflammation, and cellular processes such as differentiation, survival, and autophagy. For example, HIF-1α is directly implicated in epithelial-to-mesenchymal transition (EMT), an important process in cancer and in repair/remodeling disease and a putative mechanism to produce collagen-producing cells in an affected compartment. HIF-1α expression is critical developmentally, as deletion of this gene results in fetal loss from the lack of vasculature in the animal. Interestingly, the absence of another HIF-α isoform, HIF-2α, does not mirror the developmental problems of HIF-1α, suggesting nonoverlapping roles for HIF-1α and HIF-2α in health and disease.
The first published observation connecting inflammation and the HIF pathway was by Hellwig-Bürgel et al Blood in 1999, illustrating that IL-1β and TNF-α augment cellular DNA binding of HIF-1α at normal oxygen in human hepatoma cells. Moreover, combining IL-1β and TNF-α with hypoxia to stimulate cells creates a synergistic effect on DNA binding and cellular activation. The initial model proposed that HIF-1α directly modulates gene expression during inflammation.2 By incorporating a zebrafish model, Elks and colleagues are the first to report that HIF-1α directs inflammation by interrupting the migratory behavior of neutrophils during the resolution phase of inflammation in a whole body organism. By generating dominant-active and dominant-negative variants of the 2 zebrafish homologues of HIF-1α (HIF-1αa and HIF-1αb), they show that temporal resolution of neutrophil-mediated inflammation and neutrophil survival is dependent on prolyl hydroxylation. Their report in this issue of Blood demonstrates further detail about the complex activities of HIF-1α in orchestrating the acute inflammatory response. Further, they show neutrophil-specific expression of a dominant-active isoform HIF-1αb is sufficient to modulate the resolution of neutrophilic inflammation while dominant-negative HIF-1αb abrogates the effects of the hypoxia-mimic, DMOG, a pan-prolyl hydroxylase inhibitor.
Why is the HIF pathway so important in acute and chronic inflammatory diseases? Several well-known HIF-1α–regulatable genes expressed during hypoxia include the potent angiogenic factor, VEGF, which induces tissue remodeling, enhances vascular permeability, and enhances TH2-mediated sensitization and lung inflammation3 ; erythropoietin, a glycoprotein that stimulates red blood cell production from the bone marrow; Glut-1, or glucose transporter 1, which transports glucose across endothelial membranes during episodes of increased glycolysis such as hypoxia; several MMPs that continuously remodel inflamed tissue releasing matrix-bound proinflammatory proteins; and CXCR4 and other chemokines that recruit lymphocytes and other cells that augment the inflammatory response.
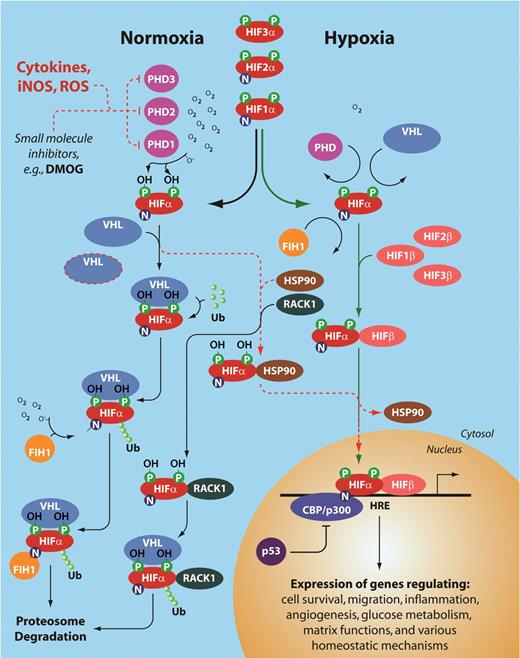
The complexity of HIF activation. This schema illustrates the numerous levels of regulation of the HIF pathway at both normoxia and hypoxia. The red dotted line indicates regulatory pathways for HIF stabilization and transcription of hypoxia-regulatable genes even at normal tissue oxygen. Note the 3 different prolyl hydroxylases, and 3 different HIF-α and HIF-β subunits. PHD indicates prolyl hydroxylase; HIF-α, hypoxia inducible factor α subunit; VHL, von Hipple Lindau protein; FIH, factor inhibiting HIFs; HIF-β, hypoxia inducible factor β subunit, or ARNT (aryl hydrocarbon receptor nuclear translocator); HSP90, heat shock protein 90; RACK1, receptor of activated kinase 1; p53, tumor suppressor protein 53; CBP/p300, CREB binding protein; and HRE, hypoxia regulatory element.
The complexity of HIF activation. This schema illustrates the numerous levels of regulation of the HIF pathway at both normoxia and hypoxia. The red dotted line indicates regulatory pathways for HIF stabilization and transcription of hypoxia-regulatable genes even at normal tissue oxygen. Note the 3 different prolyl hydroxylases, and 3 different HIF-α and HIF-β subunits. PHD indicates prolyl hydroxylase; HIF-α, hypoxia inducible factor α subunit; VHL, von Hipple Lindau protein; FIH, factor inhibiting HIFs; HIF-β, hypoxia inducible factor β subunit, or ARNT (aryl hydrocarbon receptor nuclear translocator); HSP90, heat shock protein 90; RACK1, receptor of activated kinase 1; p53, tumor suppressor protein 53; CBP/p300, CREB binding protein; and HRE, hypoxia regulatory element.
As the master regulator of the body's response to tissue hypoxia, the HIFs are central regulators of angiogenic pathways. However, recent evidence suggests that the HIFs also have central roles in cell survival, innate immune responses, and inflammatory diseases such as asthma, atherosclerosis, rheumatoid arthritis, and cancer. Normally, neutrophilic inflammation resolution is initiated in the first few hours after the acute inflammatory response begins as myeloid cells arrive and release prostaglandins and leukotrienes to initiate termination.4 No longer required, neutrophils undergo apoptosis and are phagocytized by infiltrating macrophages. Postponement of this process leads to sustained acute inflammation and adjacent tissue injury. What Elks et al have elegantly illustrated in this report is that PHD-mediated degradation of HIF-1α is responsible for inflammation resolution and neutrophil survival rather than recruitment of neutrophils to the wound site. Further, they reveal that retrograde chemotaxis is reduced during the resolution phase of inflammation, an effect abrogated by dominant-negative HIF subunits. This result suggests that HIF signaling regulates neutrophil retention at sites of acute inflammation. Coincidentally, this phenomenon is also observed in tumor-associated macrophages that stall in hypoxic patches in the tumor microenvironment. Interestingly, several HIF-1α–mediated pathways have been shown to be activated in the absence of hypoxia. For example, respiratory syncytial virus (RSV) infection leads to oxygen-independent stabilization of HIF-1α.5 Also, inflammation-mediated HIF-1α accumulation occurs in response to IL-1β and PGE2 in breast cancer cells at normal oxygen.6 These reports highlight the critical and complex role of HIF proteins as potential therapeutic targets for inflammatory diseases as we recognize their role in normal oxygen environments.
Interestingly, in some cell types it seems that another HIF-α isoform, HIF-2α, may play an unexpected role in alleviating a proinflammatory microenvironment. For example, we found that in macrophages, HIF-1α and HIF-2α, even though competing for the same promoter hypoxia regulatory element, act in a contradictory manner in the tumor environment. While HIF-1α drives VEGF production and angiogenesis in hypoxic tumor environments, HIF-2α production blocks angiogenesis by inducing the expression of soluble VEGF receptor-1, inactivating biologically active free VEGF.7 It is possible that HIF-2α evolved subsequent to HIF-1α to regulate VEGF in response to hypoxia to regulate the host vascular network.
What is the significance of this report by Elks and colleagues? This study is an extension of the sentinel paper in Cell in 2003 that first described the role of HIF-1α in inflammation by myeloid cells, specifically macrophages.8 The most significant features of the current study include the correlation of the HIF pathway with neutrophil behavior during whole organism inflammatory responses and their role in inflammation resolution; the generation of stable myeloid cell–specific transgenic cell lines expressing dominant active HIF-1α subunits in which the hydroxyl-modifiable prolines are removed, leaving the PHDs ineffective in HIF-1α degradation; and the creation of a truncated dominant-negative HIF-1α isoform that suppresses HIF-1α activity and increases the understanding of neutrophil-regulated inflammation. However, questions still exist about the HIF pathway and acute inflammation and application to more complex systems. What is the mechanistic link between HIF-1α activation and neutrophil migration and apoptosis and is intervention against these pathways sufficient to jumpstart a stalled inflammatory resolution phase in humans?
In summary, the HIF proteins were initially thought to be relevant only in tissue environments suffering from oxygen deprivation, resulting in VEGF expression and angiogenesis. But the more we learn about the complexity of the HIF system, the more we understand its link with the regulation of homeostatic mechanisms, even outside hypoxia. The fact that, in humans, there are 3 HIF-α subunits (HIF-1α, -2α, and -3α), each with their own binding nuclear translocation partners (HIF-1β, -2β, and -3β) emphasizes an evolutionary need that was addressed by the development of these different isoforms, to solve not only tissue-specific problems as they arose during hypoxia, but also to maintain homeostasis not regulated by oxygen tension. To add to the complexity of this system, there are 3 prolyl hydroxylases (PHD-1, -2, and -3) that regulate HIF activity as well as the interaction of proteins that either inhibit or enhance proteosomal degradation of the α-subunits: VHL, FIH-1, RACK1, and HSP90. Today, the list of mechanisms regulated by the HIF system is growing, including anemia, a myriad of inflammatory diseases, and cancer. Our understanding of the complex HIF regulatory system (see figure) and its function offers tremendous opportunity for understanding human health and advancing treatment of disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal