Blood vessels bring life to tissues and their absence signal that something is very wrong. In this issue of Blood, Hanjaya-Putra et al report the development of a synthetic matrix that allows the building of functional human microvasculature in vivo, which has great therapeutic potential.1
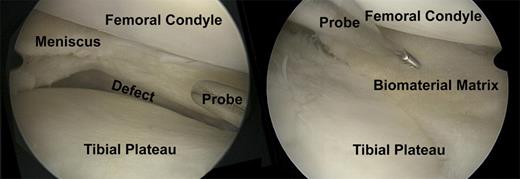
A case of chronic pain postsubtotal meniscectomy after a complex bucket handle tear of the meniscus in a soccer player. (Left) The probe indicates the large size meniscal defect. (Right) This is an arthroscopic view after meniscus transplantation with a cell-free biomaterial. The probe indicates the meniscal defect filling with the cell-free biomaterial. Vascular-forming matrices would improve the integration of the biomaterial and the in-growth of repair cells for regeneration of meniscus tissue.
A case of chronic pain postsubtotal meniscectomy after a complex bucket handle tear of the meniscus in a soccer player. (Left) The probe indicates the large size meniscal defect. (Right) This is an arthroscopic view after meniscus transplantation with a cell-free biomaterial. The probe indicates the meniscal defect filling with the cell-free biomaterial. Vascular-forming matrices would improve the integration of the biomaterial and the in-growth of repair cells for regeneration of meniscus tissue.
Building new tissues and organs is no longer science fiction; but it is a very complex process. Like constructing intricate electronic circuits, we must understand all individual components and their interrelationships so that needed functions can be supplied. Essential vessel components include both cellular and structural elements (see figure). From the cellular perspective, stem cell biology is steadily advancing. By better understanding and purifying the stem cells that remain in reserve to replenish our body systems, we theoretically have the ability to replace those systems. Unfortunately, even with the great capacity that these cells hold, it is not as simple as throwing in some stem cells and watching the new tissue or organ grow. To accomplish organ or tissue rebuilding, we must also provide substructures and signaling molecules to guide the correct functional redevelopment of the damaged system. Because of this obstacle, some groups have attempted, with impressive initial success, to recreate new whole organs using a decellularization reconstitution strategy, whereby the cellular component is seeded into a cell-depleted scaffold.2 Indeed, growing relatively less complex structures (versus whole organs) such as ears, bladder and trachea has been widely publicized.3,4 Growing new complex organs has yet to be accomplished. Perhaps more tangibly, the fields of regenerative medicine and tissue engineering have also opened the new approaches to tissue repair and healing. In the field of orthopedic surgery, autologous chondrocyte transplantation in sponge-like biomaterials can provide long-term regeneration of large chondral defects.5,6 Meniscus transplantation with cell-free biomaterials shows promising results in clinical trials.7 For successful integration of the repair tissue into the surrounding native meniscus, neovascularization is crucial (see figure). Although it will likely be an easier task to repair rather than to completely rebuild a tissue or organ, bringing in new blood vessels is vital to both approaches. The work presented here by Hanjaya-Putra and colleagues is especially important because it offers a first glimpse into how functional vascular networks can be engineered in vivo.1
Hanjaya-Putra et al devised their blood vessel–producing synthetic matrix using a biocompatible tunable hyaluronic acid hydrogel possessing key properties, including a high water content to promote cell viability, and flexible degradability for vessel maturation through a system of peptide cross-linkers that can be broken via matrix metalloproteinases.1 The latter feature is particularly critical to allow for seeded human endothelial colony-forming cells to undergo the process of vacuolization, coalescence, and finally, vascular morphogenesis where endothelial cells branch and sprout to form a nascent vascular network. Important also to their matrix system was the incorporation of cell adhesive (via α5β1 and αVβ3 integrins) RGD peptides that regulate the initial process of endothelial cell vacuolization and lumen formation.8 Therefore, using RGD peptides in the matrix, and through expression of metalloproteinases by endothelial cells, vasculogenesis could be properly initiated and advanced to the point of forming proper interconnecting blood microvessels. Most importantly, the formation of human blood vessels was demonstrated after matrices impregnated with human colony-forming cells were implanted into mice, with evidence that these vessels were carrying blood cells; moreover, the vessels appear to integrate and even anastomose with adjacent mouse tissues, as evidenced by a mixture of human and mouse cells found in these areas. This result suggests that migrating cells supportive of vessel formation and stability (eg, smooth muscle cells, pericytes) could further promote vascular morphogenesis initiated by the synthetic matrix. Indeed, it would be most desirable if an orthotopically placed synthetic matrix could mesh with existing damaged tissue, thus providing a new source of nutrients and immune cells to aid in repair processes; interestingly, macrophages were found in large numbers in the area of the implanted synthetic matrix in this study. Of course, several questions still remain unanswered. Do other cells need to be directly incorporated into the matrix, such as pericytes or smooth muscle cells? Is the vessel formation in vivo well enough organized to be functional? Will allogeneic matrix components be attacked by the immune system? Nonetheless, this study is extraordinary in that it shows in vivo vasculogenesis is possible from a synthetic matrix.
If synthetic matrix products for vascular morphogenesis do become available in the future, one can envision a wide variety of applications that could have a major impact on medicine. For example, limbs where there is peripheral vascular disease (eg, diabetes) could be treated with injections of this material to promote healing as could patients with other wound-repair problems. With regard to growing organs or tissue for replacement therapy, such a vascular-forming matrix would be valuable in bringing this science forward. We envision from our own experience with the use of mesenchymal stem cell therapy for meniscal tissue repair9,10 that a combination therapy with vascular-promoting matrices could be of great benefit to patients with joint disease. In our experiments, stem cells deliver repair cells and chemokines to the biomaterials and could therefore optimize regeneration. However smart biomaterials like the ones presented by Hanjaya-Putra and colleagues could achieve similar results in meniscus regeneration without cost-intensive cell culture procedures and could improve the regeneration process.1
There is no question that blood brings life, and opportunities to restore life. The work presented by Hanjaya-Putra et al is a significant scientific and translational step forward that brings new hope of improving vascular disease treatment and repairing, or even replacing, damaged human tissues.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal