Abstract
For establishing the true effect of different response categories in patients with multiple myeloma (MM) treated with autologous stem cell transplantation, we evaluated, after a median follow-up of 153 months, 344 patients with MM who received a transplant between 1989 and 1998. Overall survival (OS) at 12 years was 35% in complete response (CR) patients, 22% in near complete response (nCR), 16% in very good partial response (VGPR), and 16% in partial response (PR) groups. Significant differences in OS and progression-free survival were found between CR and nCR groups (P = .01 and P = .002, respectively), between CR and VGPR groups (P = .0001 and P = .003), or between CR and PR groups (P = .003 and P = < 10−5); no differences were observed between the nCR and VGPR groups (P = .2 and P = .9) or between these groups and the PR group (P = .1 and P = .8). A landmark study found a plateau phase in OS after 11 years; 35% patients in the CR group and 11% in the nCR+VGPR+PR group are alive at 17 years; 2 cases had relapsed in the nCR+VGPR+PR group. In conclusion, MM achieving CR after autologous stem cell transplantation is a central prognostic factor. The relapse rate is low in patients with > 11 years of follow-up, possibly signifying a cure for patients in CR.
Introduction
Despite that the multiple myeloma (MM) is considered an incurable disease, treatment with high doses of chemotherapy with autologous stem cell transplantation (HDT/ASCT) could cure a small fraction of cases. The more recent studies have reported a survival rate of ∼ 15% at 10-15 years after transplantation, with more favorable prognosis in younger patients.1-5
The beneficial effect of HDT/SCT over previous treatments is because of its greater capacity to reduce the tumoral burden. In fact, the concept of complete response (CR) was introduced with HDT/SCT.6 Although the value of this parameter was cause of great debate for a long time, the prognostic significance of achieving CR in MM has finally been accepted. More than 25 studies have shown this, including several randomized trials and prospective trials7-10 ; moreover, it has been confirmed in a meta-analysis and a systematic review of evidence.3,11 In addition, when new drugs are used, obtaining CR is an indicator of good prognosis.12 However, the existing studies have been based on series with a median follow-up of ∼ 5 years, and only studies with 10 years of follow-up has assessed the concept of response.13 In fact, given the current life expectancy for patients with MM, the 5-year follow-up period is insufficient.
Some studies, including randomized trials, have failed to find a correlation between strength of the response and outcome.14-17 The response categories near CR (nCR) or CR by electrophoresis (EP) and very good partial response (VGPR), both used by Intergrupe Françoise du Myelome (IFM),7,19 although included in the recent criteria from International Myeloma Working Group for response assessment of MM as VGPR, have not been validated for prognostic purposes.7,19,20 There is no consensus about whether CR, nCR, and VGPR represent distinct prognostic categories or include a uniform group of patients. In this regard, 3 studies, 2 from the Spanish MM group, did not find differences between the partial response (PR), VGPR, and nCR response categories.9,21,22 If this is so, grouping several response categories under the sign ≥, as ≥ VGPR or ≥ nCR or reporting them as a combination response category, would not be appropriate.
In 2000 our group published a retrospective analysis showing that CR by immunofixation (IF) was an important goal of HDT/SCT therapy in MM and confirming the validity of the classification from the European Study Group for Blood and Marrow Transplantation/International Bone Marrow Transplant Registry to assess the response in MM.21 The aim of the present study is to establish the actual prognosis for the different response categories in the same original cohort of patients with MM treated with HDT/SCT after long-term follow-up.
Methods
Patients and therapeutic procedures
A total of 344 patients with MM from 17 different institutions who received an autologous transplant between 1989 and 1998 have been analyzed. Only first (n = 298) or tandem transplantations (n = 46) were considered; 202 patients received a transplant after a single previous chemotherapy combination, whereas 142 patients had received > 1 transplant. By the time of the cutoff of this study, in February 2010, of 344 cases included in the first study realized in the year 2000 (21), it was possible to update the follow-up of 322 patients. We found that 99 patients were alive with a median follow-up times for the entire series of 12.5 years (range, 1-24 years) from diagnosis and 12 years (range, 1-17 years) from HDT/ASCT. All patient procedures were approved by the Hospital Universitario 12 de Octubre Institutional Review Board.
The stem cell source was peripheral blood stem cells in 335 cases; BM and peripheral blood stem cells in 6 cases, and CD34+-selected cells in 3 cases. The main conditioning high-dose regimens were 200 mg/m2 melphalan in 143 cases, 140 mg/m2 melphalan plus total body irradiation of 8 or 12 Gy in 75 cases, 12 mg/m2 busulphan plus 140 mg/m2 melphalan in 55 cases, and tandem ASCT with 200 mg/m2 melphalan followed by the protocol of 300 mg/m2 bis-chloroethyl-nitroso-urea with 750 mg/m2 etoposide, and 6 g/m2 cyclophosphamide in 46 cases. The remaining 25 patients received other high-dose regimens. After HDT/SCT, 179 patients (56%) received α-IFN maintenance for a maximum of 2 years.
M-component determination, response criteria, and end points
Information about response status after transplantation, evaluated simultaneously by EP and IF for serum and urinary M-protein, was available for all patients. All patients were also monitored by both EP and IF in serum and urine throughout follow-up. No IF data were available in the response evaluations before transplantation.
Disease response was assessed after induction and 90 days after transplantation with the use of criteria from the European Group for Blood and Marrow Transplantation6 modified to include nCR and VGPR. Briefly, CR required the absence of detectable M-component in serum and urine by IF on ≥ 2 measurements during ≥ 6 weeks, together with < 5% plasma cells in the BM. If the EP was negative but the IF was positive, the response was classified as nCR. VGPR was defined by a ≥ 90%-99% reduction in M-component levels with positive EP, whereas decreases between 50% and 90% were considered as PR. The remaining patients were considered as nonresponders, and a distinction was made between cases with progressive disease (PD) and patients with stable disease (SD). In patients with VGPR, PR, or SD an increase of > 50% for the lowest M-component level previously achieved was required to consider progression. For patients in nCR a positive EP was required to constitute relapse; in patients with CR relapse was defined by recurrence of detectable M-component on IF, even if EP remained negative, excluding oligoclonal immune reconstitution. For all therapeutic steps, the reference M-protein level was that at onset of therapy.
Progression-free survival (PFS) was measured from start of treatment to date of progression, relapse, or death. Patients who had not progressed or relapsed were censored on the last date they were known to be alive and progression free. Overall survival (OS) was calculated from start of treatment to date of death or last visit.
Statistical analysis
All data were included in an Access 2003 database (Microsoft Corporation). The statistical analysis was performed with the SPSS program (Version 15.0; Statistical Package for Social Sciences Inc) for Windows. For univariate analysis, survival curves were calculated according to the Kaplan-Meier method, and differences between curves were evaluated with the log-rank test. P values < .05 were considered to reflect statistical significance. PFS and OS were also analyzed by multivariate Cox proportional hazards models, including response as an ordered category together with the baseline variables included in Table 1. The χ2 and Fisher exact 2-sided tests were used for comparisons between categorical variables, and the Wilcoxon rank sum test or t test was used for continuous variables. A landmark study according to the different response categories was performed at 5, 8, 10, and 11 years.
Main patient characteristics at diagnosis according to response group
| . | CR . | nCR . | VGPR . | PR . | SD . | PD . |
|---|---|---|---|---|---|---|
| No. of patients | 84 | 66 | 54 | 114 | 12 | 14 |
| Male sex, % | 60 | 62 | 52 | 67 | 75 | 64 |
| Age, y, mean ± SD | 53 ± 8 | 55 ± 7 | 54 ± 8 | 53 ± 10 | 51 ± 7 | 53 ± 9 |
| M-protein, IgG/IgA/IgD/IgM/light chain, % | 45/27/4/1/23 | 45/27/0/2/26 | 69/21/2/0/8 | 69/18/1/0/12 | 58/16/0/0/25 | 64/21/0/0/14 |
| PS ≥ 2 (ECOG), % | 56 | 54 | 49 | 54 | 50 | 57 |
| Durie-Salmon stage, I/II/III, % | 1/27/72 | 7/14/79 | 6/33/61 | 8/20/72 | 16/33/50 | 0/43/57 |
| Serum creatinine level, μmol/L, mean ± SD | 141 ± 106 | 132 ± 79 | 141 ± 106 | 106 ± 62 | 134 ± 27 | 136 ± 79 |
| Serum albumin level, g/L, mean ± SD | 38 ± 6 | 37 ± 6 | 39 ± 7 | 38 ± 6 | 38 ± 8 | 39 ± 7 |
| Serum calcium level, μmol/L, mean ± SD | 255 ± 46 | 247 ± 35 | 237 ± 33 | 240 ± 30 | 236 ± 20 | 253 ± 23 |
| Hemoglobin level, g/dL, mean ± SD | 10.7 ± 2.5 | 10.7 ± 2.6 | 10 ± 2.4 | 11.1 ± 2 | 10.9 ± 2.1 | 9.5 ± 2.3 |
| β2 microglobulin ≥ 2.5 mg/L, % | 73 | 82 | 70 | 65 | 58 | 71 |
| LDH > normal limit, % | 18 | 13 | 9 | 4 | 10 | 20 |
| . | CR . | nCR . | VGPR . | PR . | SD . | PD . |
|---|---|---|---|---|---|---|
| No. of patients | 84 | 66 | 54 | 114 | 12 | 14 |
| Male sex, % | 60 | 62 | 52 | 67 | 75 | 64 |
| Age, y, mean ± SD | 53 ± 8 | 55 ± 7 | 54 ± 8 | 53 ± 10 | 51 ± 7 | 53 ± 9 |
| M-protein, IgG/IgA/IgD/IgM/light chain, % | 45/27/4/1/23 | 45/27/0/2/26 | 69/21/2/0/8 | 69/18/1/0/12 | 58/16/0/0/25 | 64/21/0/0/14 |
| PS ≥ 2 (ECOG), % | 56 | 54 | 49 | 54 | 50 | 57 |
| Durie-Salmon stage, I/II/III, % | 1/27/72 | 7/14/79 | 6/33/61 | 8/20/72 | 16/33/50 | 0/43/57 |
| Serum creatinine level, μmol/L, mean ± SD | 141 ± 106 | 132 ± 79 | 141 ± 106 | 106 ± 62 | 134 ± 27 | 136 ± 79 |
| Serum albumin level, g/L, mean ± SD | 38 ± 6 | 37 ± 6 | 39 ± 7 | 38 ± 6 | 38 ± 8 | 39 ± 7 |
| Serum calcium level, μmol/L, mean ± SD | 255 ± 46 | 247 ± 35 | 237 ± 33 | 240 ± 30 | 236 ± 20 | 253 ± 23 |
| Hemoglobin level, g/dL, mean ± SD | 10.7 ± 2.5 | 10.7 ± 2.6 | 10 ± 2.4 | 11.1 ± 2 | 10.9 ± 2.1 | 9.5 ± 2.3 |
| β2 microglobulin ≥ 2.5 mg/L, % | 73 | 82 | 70 | 65 | 58 | 71 |
| LDH > normal limit, % | 18 | 13 | 9 | 4 | 10 | 20 |
PS indicates performance status; ECOG, Eastern Cooperative Oncology Group; and LDH, lactate dehydrogenase.
Results
Patient characteristics
Tables 1 and 2 show the patient's characteristics at diagnosis and at transplantation according to response group. The response distribution after HDT/SCT on the series was CR of 84 cases, nCR of 66 cases, VGPR of 54 cases, PR of 114 cases, SD of 12 cases, and PD of 14 cases. Overall characteristics were similar except (statistical differences P > .05) for myeloma isotype distribution and the number of cases with high lactate dehydrogenase in the PR group. The nonresponding patients show a worst prognostic profile before HDT/SCT, longer time between diagnosis and HDT/SCT, worse performance status, as well as higher β2 microglobulin or low hemoglobin. In contrast, the CR group was characterized by more cases treated with just one scheme of chemotherapy before HDT/SCT, more cases in pre-HDT/SCT CR or nCR, and low β2 microglobulin diagnostic values than the remaining response groups.
Patient characteristics at transplantation
| . | CR . | nCR . | VGPR . | PR . | SD . | PD . |
|---|---|---|---|---|---|---|
| Hemoglobin level, g/dL, mean ± SD | 11.8 ± 1.5 | 11.6 ± 1.7 | 11.3 ± 1.7 | 11.2 ± 1.7 | 9.7 ± 1.2 | 11.1 ± 1.2 |
| Serum creatinine level, μmol/Lm mean ± SD | 106 ± 132 | 88 ± 26 | 80 ± 26 | 110 ± 61 | 102 ± 46 | 82 ± 21 |
| Serum calcium level, μmol/Lm mean ± SD | 232 ± 22 | 230 ± 20 | 227 ± 15 | 227 ± 27 | 232 ± 12 | 225 ± 24 |
| Serum albumin level, g/Lm mean ± SD | 30 ± 16 | 29 ± 15 | 30 ± 15 | 31 ± 13 | 30 ± 7 | 34 ± 7 |
| β2 microglobulin ≥ 2.5 mg/L, % | 29 | 52 | 42 | 43 | 42 | 60 |
| LDH > normal limit, % | 9 | 11 | 6 | 13 | 17 | 35 |
| PS ≥ 2 (ECOG), % | 9 | 21 | 22 | 14 | 41 | 50 |
| Pre-HDT/SCT different chemotherapy combinations ≥ 2, % | 24 | 36 | 42 | 50 | 62 | 78 |
| Time from diagnosis to HDT/SCT, mo, mean ± SD | 15 ± 22 | 16 ± 19 | 16 ± 16 | 24 ± 31 | 42 | 60 |
| Pre-HDT/SCT response status: CR or nCR*/PR/SD/PD, % | 23/61/2/14 | 18/58/6/18 | 2/72/6/20 | 0/73/6/21 | 0/33//37/30 | 0/42/42/14 |
| . | CR . | nCR . | VGPR . | PR . | SD . | PD . |
|---|---|---|---|---|---|---|
| Hemoglobin level, g/dL, mean ± SD | 11.8 ± 1.5 | 11.6 ± 1.7 | 11.3 ± 1.7 | 11.2 ± 1.7 | 9.7 ± 1.2 | 11.1 ± 1.2 |
| Serum creatinine level, μmol/Lm mean ± SD | 106 ± 132 | 88 ± 26 | 80 ± 26 | 110 ± 61 | 102 ± 46 | 82 ± 21 |
| Serum calcium level, μmol/Lm mean ± SD | 232 ± 22 | 230 ± 20 | 227 ± 15 | 227 ± 27 | 232 ± 12 | 225 ± 24 |
| Serum albumin level, g/Lm mean ± SD | 30 ± 16 | 29 ± 15 | 30 ± 15 | 31 ± 13 | 30 ± 7 | 34 ± 7 |
| β2 microglobulin ≥ 2.5 mg/L, % | 29 | 52 | 42 | 43 | 42 | 60 |
| LDH > normal limit, % | 9 | 11 | 6 | 13 | 17 | 35 |
| PS ≥ 2 (ECOG), % | 9 | 21 | 22 | 14 | 41 | 50 |
| Pre-HDT/SCT different chemotherapy combinations ≥ 2, % | 24 | 36 | 42 | 50 | 62 | 78 |
| Time from diagnosis to HDT/SCT, mo, mean ± SD | 15 ± 22 | 16 ± 19 | 16 ± 16 | 24 ± 31 | 42 | 60 |
| Pre-HDT/SCT response status: CR or nCR*/PR/SD/PD, % | 23/61/2/14 | 18/58/6/18 | 2/72/6/20 | 0/73/6/21 | 0/33//37/30 | 0/42/42/14 |
LDH indicates lactate dehydrogenase; PS, performance status; and ECOG, Eastern Cooperative Oncology Group.
Electrophoresis was negative. No immunofixation data were available.
Effect of posttransplantation response on PFS/OS
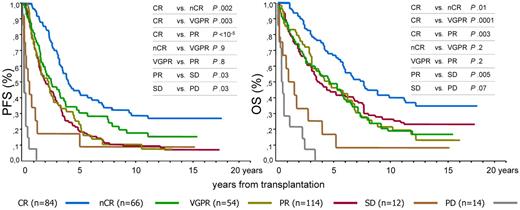
The respective median and 12-year PFS according to each response category of response were 47 months and 28% for CR, 30 months and 19% for nCR, 27 months and 10% for VGPR, 23 months and 11% for PR, 5 months and 8% for SD, and 1 month and 0% for PD. OS values were 91 months and 35% for CR, 56 months and 22% for nCR, 55 months and 16% for VGPR, 43 months and 16% for PR, 8 months and 8% for SD, and 6 months and 0% for PD (Table 3; Figure 1).
Survival values for different response categories at 12 years
| Categories . | No. . | PFS . | OS . | ||
|---|---|---|---|---|---|
| % . | Median, mo (95% CI) . | % . | Median, mo (95% CI) . | ||
| CR | 84 | 28 | 47 (36-59) | 35 | 91 (68-114) |
| PR + nCR + VGPR | 234 | 13 | 26 (21-32) | 20 | 51 (31-64) |
| nCR | 66 | 19 | 30 (16-44) | 22 | 56 (32-81) |
| VGPR | 54 | 10 | 27 (16-31) | 16 | 55 (33-78) |
| PR | 114 | 11 | 23 (16-31) | 16 | 43 (32-55) |
| No response | 26 | 4 | 5 (0-6) | 4 | 8 (4-13) |
| SD | 12 | 8 | 5 (1-11) | 8 | 8 (0-27) |
| PD | 14 | 0 | 1 (0.9-1.1) | 0 | 6 (4-9) |
| Categories . | No. . | PFS . | OS . | ||
|---|---|---|---|---|---|
| % . | Median, mo (95% CI) . | % . | Median, mo (95% CI) . | ||
| CR | 84 | 28 | 47 (36-59) | 35 | 91 (68-114) |
| PR + nCR + VGPR | 234 | 13 | 26 (21-32) | 20 | 51 (31-64) |
| nCR | 66 | 19 | 30 (16-44) | 22 | 56 (32-81) |
| VGPR | 54 | 10 | 27 (16-31) | 16 | 55 (33-78) |
| PR | 114 | 11 | 23 (16-31) | 16 | 43 (32-55) |
| No response | 26 | 4 | 5 (0-6) | 4 | 8 (4-13) |
| SD | 12 | 8 | 5 (1-11) | 8 | 8 (0-27) |
| PD | 14 | 0 | 1 (0.9-1.1) | 0 | 6 (4-9) |
CI indicates confidence interval.
Prognostic influence of 6 response categories obtained after HDC/ASCT. CR indicates complete response; nCR, near complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; and PD, progressive disease.
Prognostic influence of 6 response categories obtained after HDC/ASCT. CR indicates complete response; nCR, near complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; and PD, progressive disease.
Significant differences in PFS and OS were found between the CR and nCR groups (P = .002 and P = .01, respectively), between the CR and VGPR groups (P = .003 and P = .0001, respectively), or between the CR and PR groups (P < 10−5 and P = .003, respectively); no differences were observed between the nCR and VGPR groups (P = .9 and P = .2, respectively) or between these groups and the PR group (P = .8 to P = .1). OS and PFS of patients with SD or PD were lower than the rest of the groups (Figure 1).
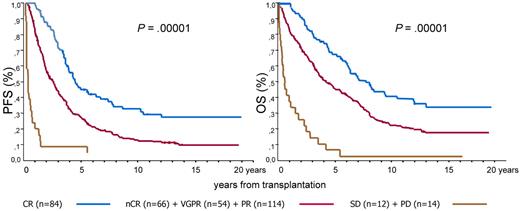
According to the above-mentioned results, the responses were grouped in 3 categories with similar survival performance and then survival was analyzed again. These 3 categories were (1) CR, (2) nCR+VGPR+PR, and (3) SD+PD. With the use of these response aggregation the respective 12-year PFS and OS values were 28% and 35% for CR, 13% and 20% for nCR+VGPR+PR, and 4% and 4% for SD+PD. The medians for PFS and OS were 47 and 91 months for CR, 26 and 51 months for nCR+VGPR+PR, and 4 and 6 months for SD+PD. Important differences were found between the 3 groups in PFS and OS (P = .00001; Figure 2).
Prognostic effect of CR patients versus those in nCR or VGPR or PR versus patients with SD or PD after HDT/ASCT.
Prognostic effect of CR patients versus those in nCR or VGPR or PR versus patients with SD or PD after HDT/ASCT.
In multivariate analysis, the PFS hazard ratio (HR) for each level of response was 1 for CR, 1.7 for nCR, 2.1 for VGPR, 2.7 for PR, 4.7 for SD, and 10.7 for PD (P < .0001); other significant variables were albumin and lactate dehydrogenase. However, if only patients achieving nCR, VGPR, and PR were compared, response had no significant influence on PFS (model, P = .08; nCR vs VGPR vs PR, P = .2). For OS, we obtained the following results: HR = 1 for RC, HR = 1.8 fro nCR, HR = 1.8 for VGPR, HR = 2.2 for PR, HR = 3.5 for SD, and HR = 14.2 for PD (P < .0001). Other significant variables were calcium, albumin, and treatment with α-IFN, whereas the survival influence of nCR was no longer statistically different from that of VGPR or PR (model, P = .005; nCR vs VGPR vs PR, P = .4).
In the CR category the landmark study found an absolute plateau phase in OS and PFS after 11 years, which represents 35% of the patients in CR. Considering together the nCR, VGPR, and PR groups, 11% of patients are still alive with stable status and without additional therapy between 11 and 17.5 years, and only 2 patients had relapsed in this non-CR group. It should be noted that the OS and PFS status of all patients in the plateau phase were reconfirmed at the time of the current analysis. Moreover, after the cutoff date one patient in nCR has died of pancreatic cancer, and another patient, previously in PR, has progressed.
Discussion
In patients with cancer in general and with MM in particular, only the quality of life and survival reflect the true effect of the therapeutic advances. However, at present the complex structure of MM treatment schedules, with many different options at the time of relapse, limits the value of OS in the assessment of the effect of a particular up-front therapeutic plan. As a whole, the true effect of therapeutic advances is only evident after a long follow-up. For these purposes, analyses of long follow-up as population-based studies5,23 or those recently made by cooperative groups2,4 are needed. However, to the best of our knowledge these studies have not examined the long-term effect of the quality of the antitumoral response (depth of response) to initial therapy.
Now, an extended body of evidence that is based principally in the clinical results of HDT/SCT3,11,24 in MM has shown that the depth of the response to treatment in this disease, particularly in CR defined by an absence of the M-component and absence (< 5%) of plasma cells in the BM, is clearly related to a better OS and PFS.24 Besides, most studies do not have sufficient follow-up to know the median OS yet; in our study the median OS for the CR category was 7.6 years. More recent studies in a different number of scenarios, such as nontransplantation settings or treatment with conventional chemotherapy22,25,26 or with regimens based on new drugs,27-29 confirm the importance of an intense cytoreduction in the prognosis of MM.28,30
Furthermore, the limitations of the previously established response criteria, the clinical behavior inherent to the complex biology of MM,31 and fragility of some patients with MM, combined with the adverse events of the treatments used, contrast with the benefit of the tumoral cytoreduction32 and continue to confuse the interpretation and assessment or appraisal of the concept of CR maximum response in this disease.
In MM, some recent studies have confirmed the beneficial effect on long-term survival of HDT/SCT with respect to conventional chemotherapy. These population-based studies23,33,34 or studies with large cohorts of patients treated by cooperative groups4 have made it possible to observe a long survival of between 30% and 45% in younger patients with MM; it could be considered a cure in some cases. It is logical to deduce that this benefit is the consequence of a major antitumoral effect from HDT/SCT; however, only a few studies have analyzed the significance of long-term response.
In 2000 we reported that patients achieving CR by IF after ASCT had a longer survival than patients with other types of response.21 Ten years later we have confirmed these data after a long-term follow-up, although it should be noted that these findings are based on conventional therapies that did not include novel agents such as proteasome inhibitors and immunomodulatory drugs of thalidomide. Patients in CR by IF after HDT/SCT have better OS and PFS than patients with other types of response. In the CR group, 35% of patients are alive and 28% are free of disease at 12 years, proportions that are statistically higher than those achieved in the cases in nCR, VGPR or PR, whose OS (22%, 16%, and 16%, respectively) and PFS (19%, 10%, and 11%, respectively) are not significantly different. Regarding the prognostic significance of achieving CR, our results are similar to the retrospective study by the M.D. Anderson Group22 in which, after a follow-up of 15 years, 40% of the cases in CR continue to be alive in contrast with a 15% in the case of patients with PR. Similarly, a Spanish study reported that 34% of the patients remain in CR from 8 to > 14 years after HDT/SCT with an PFS and OS plateau, suggesting that some patients with MM can be cured with HDT/SCT.35 Unfortunately, an analysis by genetic risk was not possible because these patients were diagnosed between 1989 and 1998, and at that time genetic studies were not part of the standard myeloma workup.
In agreement with our previous study, patients in the nCR category and VGPR have a similar survival to that of PR patients but worse than patients in CR by IF. These results are close to those in the already mentioned M. D. Anderson study,22,36 in which the median survival for patients with a disappearance of myeloma protein on the standard EP but with persistent monoclonal component in IM was similar to that of patients in PR. In addition, another recent prospective study by the Spanish Myeloma Group9 reported that patients achieving nCR had somewhat better outcomes than patients achieving PR. However, in nCR patients, outcomes were significantly worse among patients who achieved nCR after induction and continued in nCR after transplantation compared with outcomes of patients who improved to nCR after transplantation after a lower postinduction response. This indicates that the improved prognosis for patients with a similar or better response of VGPR or nCR is because of a subset of patients with CR by IF also embedded in these categories, frequently hidden in the report of results with the sign ≥ grouping.37 In 2006 Durie et al20 of the International Melanoma Working Group reviewed the categories of response and established a new classification that included new response categories such as VGPR and stringent complete response. Like the previous classification of the European Study Group for Blood and Marrow Transplantation, these categories were not previously validated.6 The VGPR category has been validated by the IFM group in several prospective studies and recently by other groups11,18,19,38,39 ; however, the IFM studies did not use IF in patients' evaluation, and they never compared the VGPR with patients in CR strictly defined by IF.28 As in our study, although with a lower follow-up of 29 months, in a recent European study considering elderly patients included in 3 phase 3 clinical trials treated as initial therapy with melphalan-prednisone or melphalan-prednisone and thalidomide ± bortezomib, only CR was related with improved PFS and OS (79% and 88% at 3 years, respectively), whereas the prognosis of the patients who achieved VGPR or PR was similar (PFS: 24% vs 23%, OS: 65% vs 57%).27
Interestingly, in our series none of the patients remaining in CR after 11 years from HDT/SCT has relapsed with a medium follow-up of 5 years since this 11-year landmark. This could mean a cure for this group of patients in prolonged CR. Even the relapse rate is low in patients with > 11 years of follow-up; at this evolution phase the patients in non-CR presented a similar profile to the gammopathy of uncertain significance. This emphasizes the importance of response stability in MM13,27 and reinforces the need to use maintenance treatments to avoid recurrence of the disease and to increase the response rate.40-43
Nevertheless, IF has well-known limitations; it is a subjective and sometimes difficult to interpret method. In addition, CR does not always have the same meaning and sometimes can depend on the type of treatment used, as suggested by some studies.11 These discrepancies could be in part because of the inclusion of different types of patients in the category of CR by IF, the residual disease-negative patients by more sensitive techniques (as molecular or immunophenotyping), and the residual disease-positive patients.44-46 In the near future the introduction of more objective and sensitive methods, including cytometric, molecular, and imaging techniques to assay response, could replace IF,47,48 but at present it should be considered the goal standard for CR definition in MM, even with its limitations.
As a final conclusion of this study, achieving CR after HDT/SCT is the most important prognostic factor in MM, even after long-term follow-up. The relapse rate is low in patients who remained in CR after > 11 years of follow-up, so that we could consider them cured.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in abstract form at 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Fondo de Investigación Sanitaria (FIS), the Instituto Carlos III, Ministerio de Sanidad (grant PI0089-01/2); the Cooperative Research Thematic Network (G03/136 and RD06/0020/0006); and the CRIS foundation.
Authorship
Contribution: J.M.-L. and J.J.L. developed the concept and design; J.M.-L. and M.C.G.d.C. provided administrative support; J.J.L., J. Blade, M.-V.M., C.G., J.G.-L., A.S., J.d.l.R., E.C., R.M., F.d.l.A., J.L.G.-Z., J. Besalduch, R.C., J.D.G.-S.M., M.C.V., J.C.G.-A., J. S.M., A.A. and J.M.M. provided study material and patients; J.M.-L., J.J.L., and M.C.G.d.C. collected and assembled data; J.M.-L., J.J.L., J. Blade, and J.S.M. analyzed and interpreted data; J.M.-L. and J.J.L. wrote the manuscript; and J.M.-L., J.J.L., J. Blade, and J.S.M. gave final approval of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of study participants, see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Joaquin Martinez-Lopez, Servicio de Hematología, Hospital Universitario 12 de Octubre, Av De Córdoba s/n, 28041 Madrid, Spain; e-mail: jmartinezlo@hematologia12octubre.com.