Abstract
We used a retroviral integration screen to search for novel genes that regulate HSC function. One of the genes that conferred HSC dominance when overexpressed due to an adjacent retroviral insertion was Musashi 2 (Msi2), an RNA-binding protein that can act as a translational inhibitor. A gene-trap mouse model that inactivates the gene shows that Msi2 is more highly expressed in long-term (LT) and short-term (ST) HSCs, as well as in lymphoid myeloid primed progenitors (LMPPs), but much less in intermediate progenitors and mature cells. Mice lacking Msi2 are fully viable for up to a year or more, but exhibit severe defects in primitive precursors, most significantly a reduction in the number of ST-HSCs and LMPPs and a decrease in leukocyte numbers, effects that are exacerbated with age. Cell-cycle and gene-expression analyses suggest that the main hematopoietic defect in Msi2-defective mice is the decreased proliferation capacity of ST-HSCs and LMPPs. In addition, HSCs lacking Msi2 are severely impaired in competitive repopulation experiments, being overgrown by wild-type cells even when mutant cells were provided in excess. Our data indicate that Msi2 maintains the stem cell compartment mainly by regulating the proliferation of primitive progenitors downstream of LT-HSCs.
Introduction
The genetic pathways that control the balance between self-renewal and differentiation in HSCs are a field of intensive investigation. These 2 processes must be tightly regulated to maintain homeostasis of the hematopoietic system and to prevent the development of blood cell malignancies. The identification of genes that have a role in self-renewal and differentiation is impeded by the low abundance of HSCs, difficulties in isolating them to purity, and the virtual inaccessibility of their niches within the BM.1,2 Alternative strategies consist of in vivo approaches that do not depend on HSC isolation. A classic approach of this type consists of tracking HSC clones marked with stably integrated retroviral vectors.3 Originally, it was assumed that these integrations are randomly distributed over the genome and do not confer a selective advantage to the cell. However, the subsequent demonstration that murine leukemia virus (MLV)–based vectors preferentially target the promoter regions of active genes,4 and the recurrent activation of the LMO2 oncogene after integration of an MLV–based vector in a human gene therapy trial,5 suggested that insertion events can modify the expression of selected target genes. Supporting this hypothesis, it was demonstrated that specific retroviral integrations could cause clonal expansion of HSCs by the up-regulation of adjacent proto-oncogenes involved in the induction of leukemia (eg, Evi-1).6
In another type of in vivo screen for genes involved in HSC function, candidate genes are overexpressed or knocked down in BM cells, the cells transplanted into irradiated hosts, and the mice tested for expansion or inhibition of the stem cell pool. Such screens have recently led to the discovery that Musashi 2 (Msi2), a putative RNA-binding protein and translational inhibitor, regulates HSC function.7,8 The Musashi family, originally described in Drosophila melanogaster, consists of 2 highly conserved genes in mammals, Msi1 and Msi2, which act as RNA-binding proteins.9,10 Msi1 was shown to repress translation by binding to the polyA-binding protein, thus competing for binding of the eukaryotic initiation factor eIF4G.11 It plays an important role in the maintenance of neural stem cells through binding to and translation inhibition of Numb, a powerful repressor of Notch signaling.12 Mice with an ablation of Msi1 exhibit a defect in CNS stem cells and a severe impairment of nervous system development,9,10,13 causing death within 1-2 months after birth. In 3 recent publications, Msi2 was identified as a regulator of the HSC compartment and of leukemic stem cells after transplantation of cells with loss and gain of function of the gene.7,8,14 In addition, the gene was shown to cause the transition from a chronic to an acute form of myeloid leukemia, and this property was attributed to its ability to inactivate Numb.8,14 However, there has as yet been no gene ablation experiments to determine the physiologic role of Msi2.
In the present study, we used a retroviral insertion screen to identify Msi2 as an important player in the regulation of the HSC compartment, confirming previous reports with a completely different approach. Gene ablation experiments of Msi2 show a dramatic reduction in early progenitors and leukocytes under steady-state conditions, effects that become exacerbated with age. In addition, mutant progenitors have a dramatically reduced capacity for competitive repopulation of irradiated hosts. Surprisingly, our data point to a role of the gene primarily in primitive progenitors downstream of long-term repopulating HSCs.
Methods
BM infection and transplantation
The BM of 4-month-old CD45.1 mice (B6.SJL-Ptprca Pep3b/BoyJ; EMBL) treated previously with 5-fluorouracil for 4 days (150 mg/kg body weight) was cultured and the cells were infected with the Moloney MLV retroviral green fluorescent protein (RV-GFP) virus15 at a multiplicity of infection of 1. The retroviral vector uses the enhancer/promoter of the wild-type Moloney MLV. After 3 days of culture, 2 × 105 cells were intravenously transplanted into 12-week-old CD45.2 (C57BL6/J) lethally irradiated (8 Gy) mice. BM from 5 primary recipients was pooled and 1.5 × 107 cells transplanted into 5 secondary lethally irradiated CD45.2 mice. Retroviral insertion sites in HSC clones were identified by ligation-mediated PCR (LM-PCR; for further details, see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This study received Barcelona Biomedical Research Park (PRBB) review board approval for the use of mice.
Generation of Msi2 mutant mice
The Msi2-deficient mouse cell line Msi2Gt/Gt (Mgi allele: Msi2Gt(E035H03)Wrst) was generated by insertional mutagenesis in a large-scale gene-trap screen (German Gene Trap Consortium). A promoterless reporter gene (βgeo) was inserted into the mouse genome by integration of a retroviral gene trap vector (rFlipROSAflgeo +1; http://genetrap.helmholtz-muenchen.de/ggtc/info/protocols/public_vectors/V09_d.php). The insertion site was determined after 5′-rapid amplification of cDNA ends PCR and 5′ and 3′ splinkerette PCR16 (dbGSS submission: 3′SPLK: 63678, 5′SPLK: 60606, rapid amplification of cDNA ends PCR: 42806; supplemental Figure 2A). Embryonic stem cells were derived from strain Sv129P2 Ola/Hsd mice. Mutant mice were generated through blastocyst injection and the resulting chimeras were bred to C57BL/6J females and crossed to C57BL/6J for 4 generations.
Antibody staining and flow cytometry
Cell suspensions were stained with various antibodies from either BD Pharmingen or eBiosciences. Cells were analyzed with an LSR II flow cytometer (BD Biosciences) using Diva v6.1.2 (BD Biosciences) and FlowJo software v7.5.5 (TreeStar). Details are available in the supplemental Methods. Cell-surface marker phenotypes are described in supplemental Table 1.
β-galactosidase staining
The analysis of β-galactosidase activity using di-β-D-galactopyranoside (FDG; Invitrogen) was performed as described previously.17
Apoptosis and cell-cycle analyses
Freshly isolated BM cells stained with the appropriate antibodies were washed, resuspended in binding buffer (10mM HEPES/NaOH, pH 7.4, 150mM NaCl, 5mM KCl, 1mM MgCl2, and 1.8mM CaCl2), and incubated with annexin V–Pacific blue (Invitrogen). To analyze the cell-cycle status, cells were stained with antibodies against Lin+ cells, c-kit and sca-1, fixed and permeabilized with Fix & Perm (Invitrogen), incubated with Ki67 antibody, and counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI).
qRT-PCR experiments
RNA was extracted from GFP+ cells using either TriPure Isolation Reagent (Roche Applied Science) for methylcellulose colonies or the RNeasy Mini Kit (QIAGEN) for LSK cells. cDNA was prepared with the High Capacity RNA-to-cDNA Master Mix (Applied Biosystems). All quantitative RT-PCR (qRT-PCR) reactions were performed in triplicate using the SybrGreen PCR Master Mix (Applied Biosystems) and data were acquired on the AB7900HT detection system (Applied Biosystems). Primer sequences are provided in supplemental Table 2. Ct values were calculated and normalized to Gusb or Hprt, and the relative expression ratio was calculated using the Pfaffl method.18
Microarray and bioinformatics analyses
RNA from 40 000 LSK cells was obtained using the RNeasy Mini Kit (QIAGEN) and processed as described in supplemental Methods. All microarray data have been deposited into the Gene Expression Omnibus (National Center for Biotechnology Information) under accession number GSE29432.
BM transplantation assay
For competitive transplantation, nucleated BM cells from 12-week-old mutant or control CD45.2 (C57BL6/J) mice were mixed at different ratios (1:1 and 7:3) with competitor CD45.1 (B6.SJL-Ptprca Pep3b/BoyJ) BM cells, and 106 cells were transplanted. In the noncompetitive assays, 107 BM cells from Msi2+/+ or Msi2Gt/Gt mice were used as donor cells. For the transplantations to test the niche effect, 107 BM cells from CD45.1 mice were transplanted into Msi2+/+ or Msi2Gt/Gt mice. All transplantations were done by retro-orbital injection into 8- to 12-week-old lethally irradiated (8 Gy) CD45.1/CD45.2 or CD45.2 mice.
Results
A retroviral insertion screen identifies Msi2 as a potential regulator of the HSC compartment
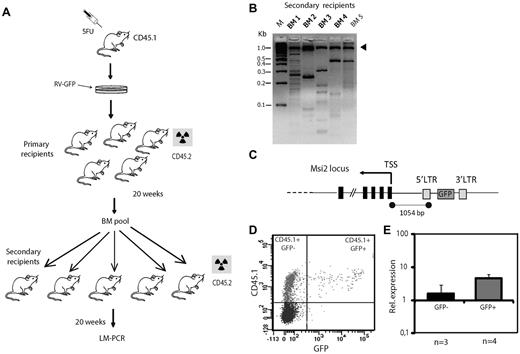
To screen for retroviral integrations that induce an expansion of the stem cell pool, we used the strategy summarized in Figure 1A. Briefly, BM cells from CD45.1 mice treated for 4 days with 5-fluorouracil were infected with the RV-GFP Moloney MLV at a multiplicity of infection of 1 to minimize the occurrence of more than one retroviral insertion per cell.19 Infected cells were transplanted into 5 lethally irradiated CD45.2 mice and after 20 weeks their BM was pooled and 1.5 × 107 cells were transplanted into each of 5 lethally irradiated CD45.2 animals. Five months later, the BM of these secondary recipients was analyzed for retroviral insertion sites using LM-PCR (Figure 1B). The level of engraftment in the BM of primary and secondary recipients is shown in supplemental Figure 1. The sequencing of amplicons yielded 81 and 43 sequences clustering in 30 and 16 loci for the primary and secondary recipients, respectively (Table 1 and supplemental Table 3). Four of the amplicons detected in the secondary recipients were detected in more than one animal: Meis1, Abcc1, Fbxo6, and Msi2. Of these, the Meis1 oncogene had already been identified as a common retrovirus integration site in a murine leukemia model.20 Meis-1 is preferentially expressed in the HSC compartment, and mice lacking the gene display multiple defects in hematopoietic development.21 Abcc1 is a multidrug transporter of the family of ABC transporters, some of which have been found to be important for HSC function.22 A connection between Fbxo6 and HSCs remains to be demonstrated. The insertion most frequently detected (in 3 of the 5 secondary recipients) was 1054 bp upstream of the Msi2 gene in a reverse orientation (Figure 1C), a location and direction frequently associated with the ability of insertions to act as transcription regulatory elements.23 Because Msi2 belongs to the Musashi family of RNA-binding proteins, which had previously been found to be critical for asymmetric division of sensory organ precursors and for the maintenance of germline stem cell identity in D melanogaster,24,25 we decided to study this gene further. To confirm that the insertion activates the expression of Msi2 gene in hematopoietic cells, we sorted CD45.1+GFP+ cells from methylcellulose colonies derived from the BM of secondary recipients (Figure 1D). The analysis of mRNA by qRT-PCR showed that expression of Msi2 was 2.8 times higher in the GFP+ cells than in the GFP− cells (Figure 1E). In light of these results, we decided to examine how the lack of Msi2 influences HSC and progenitor cell function in a physiologic setting.
A retroviral screen for potential regulators of HSC function. (A) Experimental design of BM transplantation assays using donor cells infected with RV-GFP retrovirus. The radioactivity symbol denotes that the mice have been lethally irradiated. (B) Agarose gel showing the results of LM-PCR, with the arrowhead pointing to the internal control band (1 kb). M indicates marker DNA. (C) Schematic illustration of the Msi2 gene showing the provirus insertion site (not to scale) and the distance between the insertion and the transcription start site (TSS). LTR indicates long terminal repeat. (D) FACS plot from granulocyte-macrophage colonies obtained from a secondary recipient. (E) Msi2 expression as determined by qRT-PCR from sorted CD45.1+GFP− and CD45.1+GFP+ donor populations.
A retroviral screen for potential regulators of HSC function. (A) Experimental design of BM transplantation assays using donor cells infected with RV-GFP retrovirus. The radioactivity symbol denotes that the mice have been lethally irradiated. (B) Agarose gel showing the results of LM-PCR, with the arrowhead pointing to the internal control band (1 kb). M indicates marker DNA. (C) Schematic illustration of the Msi2 gene showing the provirus insertion site (not to scale) and the distance between the insertion and the transcription start site (TSS). LTR indicates long terminal repeat. (D) FACS plot from granulocyte-macrophage colonies obtained from a secondary recipient. (E) Msi2 expression as determined by qRT-PCR from sorted CD45.1+GFP− and CD45.1+GFP+ donor populations.
Retroviral insertions found at least twice in the BM of secondary transplantation recipients
| Tagged gene . | No. of hits in primary recipients . | No. of hits in secondary recipients . | Chromosome no. . | Retrovirus orientation . | Distance to transcription start site . |
|---|---|---|---|---|---|
| Abcc1 | 0 | 2 | 16 | F | 35 000 |
| Fbxo6 | 0 | 2 | 4 | F | 4165 (intron 1) |
| Meis1 | 2 | 2 | 11 | F | 149 230 |
| Msi2 | 1 | 3 | 11 | R | 1054 |
| Tagged gene . | No. of hits in primary recipients . | No. of hits in secondary recipients . | Chromosome no. . | Retrovirus orientation . | Distance to transcription start site . |
|---|---|---|---|---|---|
| Abcc1 | 0 | 2 | 16 | F | 35 000 |
| Fbxo6 | 0 | 2 | 4 | F | 4165 (intron 1) |
| Meis1 | 2 | 2 | 11 | F | 149 230 |
| Msi2 | 1 | 3 | 11 | R | 1054 |
The table lists retroviral insertions found at least twice in the BM of 5 secondary transplantation recipients. The orientation of retroviral vector transcription was either forward (F) when oriented in the same sense as the tagged gene or reverse (R) when pointing in the opposite direction. The distance (in bp) between the retroviral insertion site and the transcription start site of the closest gene is given in the last column.
Generation of Msi2 gene-trap mice through insertion of the β-galactosidase neomycin resistance gene
Msi2-deficient mice were generated from mouse embryonic stem cells carrying a retroviral gene-trap vector insertion, introducing a cassette that consists of a fusion between β-galactosidase and the neomycin resistance gene (bgeo26 ; Figure 2A). The insertion site was mapped to intron 5 of Msi2, generating a truncation of the polyA-binding protein domain. Genotyping of mice with Msi2-specific primers flanking the insertion site confirmed vector integration at this locus (supplemental Figure 2A-B). Matings of Msi2Gt/+ mice yielded lower proportions of Msi2Gt/Gt mice than predicted by Mendelian laws (Msi2+/+: Msi2Gt/+: Msi2Gt/Gt = 29:56:15, n = 100), and approximately half of the homozygotic animals died before birth. The Msi2Gt/Gt mice that came to term were viable, fertile, and displayed no apparent gross abnormalities or signs of leukemia, except that they exhibited a 20% decrease in body weight compared with Msi2+/+ littermates (supplemental Figure 2C). The strain had to be kept as heterozygotes, because knockout males and females were fertile when crossed with wild-type mice but not when crossed among themselves.
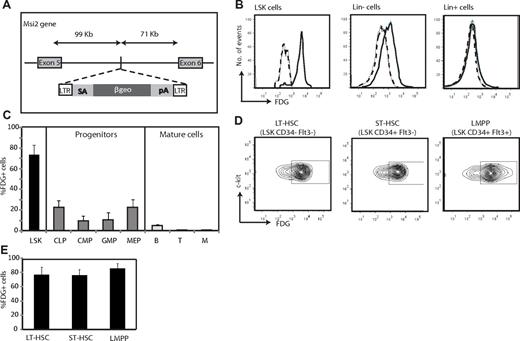
Expression of Msi2 within the hematopoietic system. (A) Diagram of the genetic modification of the Msi2 locus in Msi2Gt/Gt mice. A promoterless reporter gene (βgeo) was inserted into the mouse genome by integration of a retroviral gene-trap vector (rFlipROSAβgeo +1) carrying a splice acceptor sequence (SA) and a polyadenylation signal (pA). It was inserted in intron 5, at 99 465 bp 3′ of exon 5 of Msi2, generating an Msi2/βgeo fusion protein, preserving the N-terminal part of Msi2. (B-E) Expression of Msi2 using staining for β-galactosidase activity with a fluorescent substrate (FDG) as the readout. (B) Expression of Msi2 in LSK cells (Lin−c-Kit+Sca-1+) as well as in Lin− and Lin+ BM subsets of an Msi2+/Gt adult mouse. FDG fluorescence intensity is shown for cells incubated with the reagent (solid lines) or kept untreated (broken lines). (C) Histograms showing the percentage of cells expressing Msi2 in LSK cells, intermediate progenitors, and mature cells. Error bars indicate SEM (n = 5). (D-E) Expression of Msi2 in LT-HSCs, ST-HSCs, and LMPPs (n = 5).
Expression of Msi2 within the hematopoietic system. (A) Diagram of the genetic modification of the Msi2 locus in Msi2Gt/Gt mice. A promoterless reporter gene (βgeo) was inserted into the mouse genome by integration of a retroviral gene-trap vector (rFlipROSAβgeo +1) carrying a splice acceptor sequence (SA) and a polyadenylation signal (pA). It was inserted in intron 5, at 99 465 bp 3′ of exon 5 of Msi2, generating an Msi2/βgeo fusion protein, preserving the N-terminal part of Msi2. (B-E) Expression of Msi2 using staining for β-galactosidase activity with a fluorescent substrate (FDG) as the readout. (B) Expression of Msi2 in LSK cells (Lin−c-Kit+Sca-1+) as well as in Lin− and Lin+ BM subsets of an Msi2+/Gt adult mouse. FDG fluorescence intensity is shown for cells incubated with the reagent (solid lines) or kept untreated (broken lines). (C) Histograms showing the percentage of cells expressing Msi2 in LSK cells, intermediate progenitors, and mature cells. Error bars indicate SEM (n = 5). (D-E) Expression of Msi2 in LT-HSCs, ST-HSCs, and LMPPs (n = 5).
To discard any compensatory role of Msi1, we compared by qRT-PCR the mRNA levels in LSK cells from Msi2+/+ and Msi2Gt/Gt mice. Msi1 was slightly up-regulated in Msi2 mutant animals, although the difference was not significant (supplemental Figure 3).
Msi2 is most highly expressed in primitive progenitors within the hematopoietic system
To determine the expression of Msi2 in hematopoietic cells at various stages of differentiation, BM cells from Msi2Gt/+ animals were incubated with the green fluorescent β-galactosidase substrate FDG. The highest levels of expression of Msi2 were detected in the Lin−Sca-1+c-Kit+ fraction (LSK), a cell population that is highly enriched for HSCs,27,28 and in which > 70% of the cells were FDG+. In contrast, Lin− progenitors showed weak Msi2 expression and Lin+ cells had no detectable expression (Figure 2B-C). An analysis of intermediate progenitors showed that common lymphoid progenitors (CLPs) and megakaryocyte-erythroid progenitors (MEPs) exhibited low levels of expression, whereas common myeloid (CMPs) and granulocyte macrophage progenitors (GMPs) were essentially negative, confirming previous observations8 (Figure 2C and supplemental Figure 4A). Among the more differentiated cells, B-lineage cells were slightly positive, whereas T- and myeloid lineage cells were negative (Figure 2C and supplemental Figure 4B). A more detailed analysis of the LSK population showed that long-term and short-term HSCs (LT-HSCs and ST-HSCs, respectively), as well as lymphoid myeloid primed multipotent progenitors (LMPPs) exhibited 75%-82% FDG+ cells (Figure 2D-E). These results show that the expression of Msi2 is high in primitive progenitors and steeply decreases during differentiation.
Lack of Msi2 reduces the numbers of primitive hematopoietic and intermediate progenitors
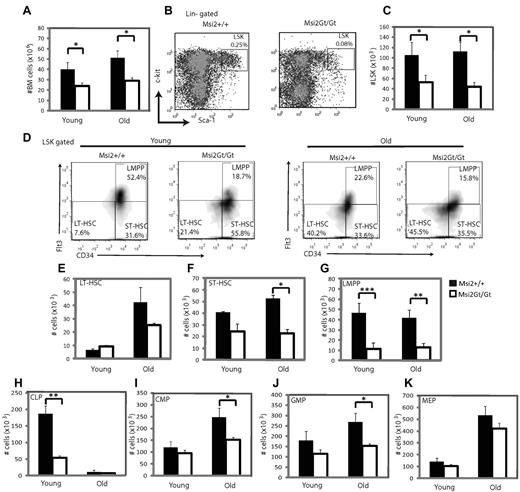
Analysis of Msi2Gt/Gt mice for the abundance of various hematopoietic progenitor cell populations showed a reduction in the total number of BM cells that was more pronounced in “old” (> 12 months) than in “young” mice (2-5 months; Figure 3A). The size of the LSK compartment was reduced > 2 fold, an effect that also became more pronounced with age (Figure 3B-C). Further analysis of the LSK fraction revealed a 5-fold reduction in LMPPs in both young and old mice, a significant decrease in the total number of ST-HSCs in old but not young mice, and a slight reduction of LT-HSCs in old mice only (Figure 3D-G). Among the intermediate progenitors, CLPs were severely reduced in young Msi2Gt/Gt mice, whereas CMPs and GMPs were significantly decreased only in old mice (Figure 3H-J and supplemental Figure 5). Finally, MEPs and erythroblasts were slightly reduced in number, whereas mature cells from the B-cell and myeloid lineages showed significant reductions in both young and adult mice (Figure 3K and supplemental Figures 5-6). These findings show that ablation of Msi2 induces a severe decrease in the proportion of LSK cells (especially LMPPs and ST-HSCs) and their downstream derivatives, and that this effect becomes exacerbated with age.
Flow cytometric analysis of hematopoietic progenitors of Msi2Gt/Gt mice. (A) Histogram showing total number of nucleated cells in hind limbs of wild-type (Msi2+/+) and Msi2Gt/Gt mice for young (2-5 months, n = 6) and old mice (> 12 months, n = 6). (B) FACS profiles of WT and Msi2Gt/Gt LSK subsets in 3.5-month-old animals. (C) Numbers of WT and Msi2Gt/Gt LSK cells in young and old mice (n = 6 for each). (D-K) Frequency of LT-HSCs, ST-HSCs, LMPPs, CLPs, CMPs, GMPs, and MEPs of young and old WT and Msi2Gt/Gt mice. Six mice were studied for the primitive progenitors of each group, 4 for the intermediate progenitors in young mice and 5 in old mice. Black bars show cells derived from WT, and white bars are cells from Msi2Gt/Gt mice. Error bars indicate SEM. *P < .05; **P < .005.
Flow cytometric analysis of hematopoietic progenitors of Msi2Gt/Gt mice. (A) Histogram showing total number of nucleated cells in hind limbs of wild-type (Msi2+/+) and Msi2Gt/Gt mice for young (2-5 months, n = 6) and old mice (> 12 months, n = 6). (B) FACS profiles of WT and Msi2Gt/Gt LSK subsets in 3.5-month-old animals. (C) Numbers of WT and Msi2Gt/Gt LSK cells in young and old mice (n = 6 for each). (D-K) Frequency of LT-HSCs, ST-HSCs, LMPPs, CLPs, CMPs, GMPs, and MEPs of young and old WT and Msi2Gt/Gt mice. Six mice were studied for the primitive progenitors of each group, 4 for the intermediate progenitors in young mice and 5 in old mice. Black bars show cells derived from WT, and white bars are cells from Msi2Gt/Gt mice. Error bars indicate SEM. *P < .05; **P < .005.
Msi2Gt/Gt mice exhibit smaller spleens and thymi and fewer circulating leukocytes
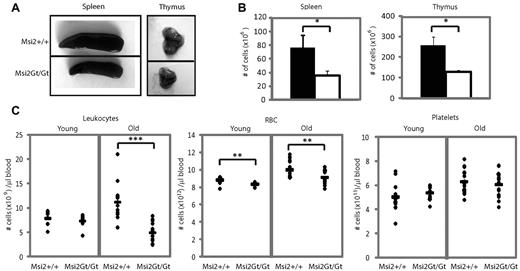
To further characterize the role of Msi2 deletion in blood cell homeostasis, we next analyzed the spleens, thymi, and peripheral blood of knockout animals. As illustrated in Figure 4A, the spleens and thymi of 12-week-old Msi2Gt/Gt mice were smaller and exhibited a decreased cellularity (Figure 4B). In the thymi, all developmental stages were reduced in numbers, including all double-negative cells and double CD4/CD8 positive and single CD4- and CD8-positive cells (supplemental Figure 7A-D). Within the spleens, B cells, macrophages, and granulocytes were also reduced (supplemental Figure 7E-G). Finally, there was a significant decrease in the numbers of circulating leukocytes in old animals, which was mainly accounted for by a decrease in B-cell populations (2.5- to 5-fold) and granulocytes/macrophages (2-fold; supplemental Figure 8A-E). We found a slight but significant decrease of erythrocytes in both young and old animals and no difference in platelet numbers (Figure 4C). In short, the loss of Msi2 leads to a reduction in the size of both thymi and spleens and in the number of circulating lymphoid and myeloid cells, effects that become exacerbated with age.
Analysis of secondary hematopoietic organs and mature blood cells. (A) Images of a representative spleen and thymus from 12-week-old Msi2+/+ and Msi2Gt/Gt mice. (B) Number (±SEM) of nucleated cells in spleen and thymus (n = 11). (C) Hematologic analysis of peripheral blood from young (n = 16) and old (n = 28) Msi2+/+ and Msi2Gt/Gt mice, showing the number of leukocytes, RBCs, and platelets. The black bars represent the mean value for each group. Error bars indicate SEM. *P < .05; **P < .005; ***P < .0005.
Analysis of secondary hematopoietic organs and mature blood cells. (A) Images of a representative spleen and thymus from 12-week-old Msi2+/+ and Msi2Gt/Gt mice. (B) Number (±SEM) of nucleated cells in spleen and thymus (n = 11). (C) Hematologic analysis of peripheral blood from young (n = 16) and old (n = 28) Msi2+/+ and Msi2Gt/Gt mice, showing the number of leukocytes, RBCs, and platelets. The black bars represent the mean value for each group. Error bars indicate SEM. *P < .05; **P < .005; ***P < .0005.
Msi2Gt/Gt primitive progenitors are inhibited in proliferation
The observed decrease in Msi2Gt/Gt mice of primitive hematopoietic progenitors cannot easily be explained by their increased differentiation rate, because there was a reduction in all differentiated derivatives. We therefore tested apoptosis and the rate of cell proliferation in LSK cells from BM of 12- to 14-week-old Msi2Gt/Gt and WT mice. To assay the levels of apoptosis, BM cells were stained with antibodies to detect LSK cells (supplemental Table 1), washed, and stained with Annexin V. The outcome revealed a slight, but insignificant, increase of apoptosis in mutant cells (Figure 5A). For the cell-cycle analyses, BM cells were stained with Ki67 antibody to detect cycling cells and with DAPI to gauge the amount of DNA per cell. Gating on the LSK-cell fraction revealed a minor increase of cells in the G0 and G1 compartments but a significant decrease of cells in the S-G2/M phase of the Msi2Gt/Gt compared with WT cells (Figure 5B-C). Because only a minority of the LSK fraction represent LT-HSCs (approximately 20% in mutant mice, Figure 3D), these data indicate that the reduced numbers of LSK cells in the knockout animals were due, at least in part, to a decreased proliferation rate of ST-HSCs and LMPPs.
Apoptosis and cell-cycle analyses. (A) Apoptosis analysis of freshly isolated BM cells from age-matched 8- to 12-week-old mice. The graph shows the percentage of Annexin V+ cells in LSK cells from Msi2+/+ (n = 5) and Msi2Gt/Gt mice (n = 8). (B) Cell-cycle analysis of cells as above. FACS analysis of cells stained with an antibody that detects proliferating cells (Ki67) and DAPI to determine the relative DNA content on a per-cell basis. (C) Percentage of LSK cells in the G0, G1, and S-G2/M phases (n = 6 for both groups).
Apoptosis and cell-cycle analyses. (A) Apoptosis analysis of freshly isolated BM cells from age-matched 8- to 12-week-old mice. The graph shows the percentage of Annexin V+ cells in LSK cells from Msi2+/+ (n = 5) and Msi2Gt/Gt mice (n = 8). (B) Cell-cycle analysis of cells as above. FACS analysis of cells stained with an antibody that detects proliferating cells (Ki67) and DAPI to determine the relative DNA content on a per-cell basis. (C) Percentage of LSK cells in the G0, G1, and S-G2/M phases (n = 6 for both groups).
Gene-expression analyses of Msi2Gt/Gt LSK cells reveals changes in cell cycle, cell migration, and adhesion genes
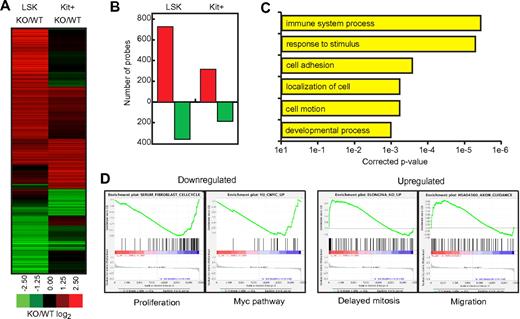
To gain further insights into the defects of hematopoietic progenitors, we determined their gene-expression profile. For this purpose, both LSK cells and Kit+ myeloid progenitors (c-KithighSca-1−Lin−) were sorted from 8-week-old Msi2Gt/Gt and WT mice and their RNAs analyzed using microarrays (Agilent) that detect 41 534 gene probes. Of the 1154 genes that changed > 2 fold in Msi2Gt/Gt LSK or Kit+ cells compared with their WT counterparts, 78% changed in LSK cells and 36% in Kit+ progenitors, with 14% of the probes overlapping between the 2 groups. Therefore, there were 2.5 times more genes with expression that changed specifically in LSK cells compared with those that changed exclusively in myeloid precursors. qRT-PCR was performed for some of the most differentially expressed genes (supplemental Figure 9), confirming the results found by microarrays. For both LSK cells and Kit+ progenitors, the number of probes that became up-regulated was almost twice the number of probes that became down-regulated (Figure 6A-B). Unsupervised clustering of all probes that changed is represented in supplemental Figure 10.
Gene-expression analysis. (A) Heat map of all array probes misregulated at least 2-fold in either LSK or Kit+ Msi2Gt/Gt cells (n = 1399 probes), depicted as the ratio between knockout and WT cells. (B) Number of gene probes that were up-regulated (red bars) or down-regulated (green bars) in Msi2Gt/Gt cells compared with WT cells. (C) Selected Gene Ontology enrichments of probes misregulated in Msi2Gt/Gt LSK cells, showing corrected P values for significance. (D) Examples of GSEA plots obtained from expression change–ranked microarray data. The plots from 2 gene sets enriched in down-regulated genes (Proliferation and Myc pathway) and 2 gene sets enriched in up-regulated genes (Delayed mitosis and Migration) are shown. Within these plots, the green line represents the sliding enrichment score, the black bars demarcate the position of the gene set members within the ranked expression data (up-regulated, red; down-regulated, blue), and the bottom panel represents the log2 expression changes in Msi2Gt/Gt cells compared with WT cells.
Gene-expression analysis. (A) Heat map of all array probes misregulated at least 2-fold in either LSK or Kit+ Msi2Gt/Gt cells (n = 1399 probes), depicted as the ratio between knockout and WT cells. (B) Number of gene probes that were up-regulated (red bars) or down-regulated (green bars) in Msi2Gt/Gt cells compared with WT cells. (C) Selected Gene Ontology enrichments of probes misregulated in Msi2Gt/Gt LSK cells, showing corrected P values for significance. (D) Examples of GSEA plots obtained from expression change–ranked microarray data. The plots from 2 gene sets enriched in down-regulated genes (Proliferation and Myc pathway) and 2 gene sets enriched in up-regulated genes (Delayed mitosis and Migration) are shown. Within these plots, the green line represents the sliding enrichment score, the black bars demarcate the position of the gene set members within the ranked expression data (up-regulated, red; down-regulated, blue), and the bottom panel represents the log2 expression changes in Msi2Gt/Gt cells compared with WT cells.
We next performed a functional annotation analysis of the genes that were differentially expressed. To this end, we further refined our list of misregulated probes in Msi2Gt/Gt LSK cells by removing all entries with P > .05 as determined by Student t test. The 707 genes so obtained were analyzed using GeneSpringGX software (supplemental Table 4). As shown in Figure 6C and supplemental Table 5, the most significant enrichment was in the category “response to external stimulus,” which includes Jun (37× down), Pparg (4.2× down), and Egr1 (4.2× down). There was also a highly significant enrichment for “immune system process,” showing a down-regulation of the Toll receptors/cofactors Tlr7, Tlr12, and Ly86 and the lymphoid marker Rag2, as well as in down-regulated genes belonging to the “cell adhesion, localization, and motion” category. These included Itga2b (CD41) and Itgb3 (CD61), which together form the GpIIbIIIa complex expressed on platelets and HSCs,29 as well as the platelet adhesion molecules Gp1ba (CD42b) and Gp1bb (CD42c). They also contained the myeloid adhesion molecules Itgam (CD11b, Mac-1) and Itgax (CD11c). Among the up-regulated genes were Stab1 (stabilin 1, a macrophage scavenger receptor) and Stab2. The top 4 most regulated genes in the “developmental process” category were Bglap (osteocalcin), Bglap2, Bglap-rs1, and CD36 (thrombospondin receptor; between 7- and 27-fold down-regulation). Whereas CD36 is expressed on platelets, erythrocyte precursors, and monocytes,30 to our knowledge, the expression of osteocalcin and associated proteins in primitive hematopoietic progenitors has not yet been described.
Using the Gene Set Enrichment Analysis (GSEA) tool,31 we obtained information about gene clusters shared between the Msi2Gt/Gt LSK signature and publicly available datasets (Figure 6D and supplemental Table 6). There was a highly significant correlation between genes down-regulated in Msi2Gt/Gt LSKs and several datasets in which cell growth was inhibited or delayed, as well as in Myc-related datasets such as “Myc_targets,” which includes Myc target genes in multiple systems and “Yu_cmyc_up” dataset reporting genes up-regulated in B-cell lymphoma tumors expressing an activated form of Myc. Another group of down-regulated genes was correlated with “oncogenesis and prognosis signature,” notably containing a dataset in which NUP98-HOXA9 was overexpressed in BM cells. This supports the finding that NUP98-HOXA9 directly regulates Msi2 expression, thus contributing to myeloid leukemia induction.14 A significant enrichment was also found for up-regulated genes in the categories “delayed mitosis” and “cell migration.” Other major categories included “PPARG pathway,” “DNA repair,” and “B- and T-cell gene sets.” We found no enrichment of genes involved in apoptosis in either of the 2 approaches used. In summary, bioinformatics analyses showed approximately 700 genes to be up or down-regulated in Msi2Gt/Gt LSK cells and much fewer in myeloid progenitors. Functional annotation of the former showed a strong enrichment for genes involved in the cell cycle and in cell migration and adhesion.
The repopulation capacity of Msi2-defective HSCs is dramatically impaired
To assay the function and quantitatively assess long-term multilineage reconstitution by Msi2Gt/Gt HSCs, we first performed a competitive repopulation experiment with age-matched Msi2Gt/Gt or WT BM cells mixed with CD45.1+ WT cells in the proportions of either 1:1 or 7:3. Equal cell numbers of these mixtures were then injected into irradiated CD45.1/CD45.2 recipients and the peripheral blood of recipient mice was analyzed for the frequency of CD45.2 donor and CD45.1 competitor cells at 4-week intervals over the course of 6 months. The contribution of Msi2Gt/Gt cells to the myeloid, T-cell, and B-cell lineages was dramatically reduced at all time points compared with WT donor-derived cells (Figure 7A and supplemental Figure 11). Analysis of donor type Msi2Gt/Gt cells at 4 weeks after transplantation showed reduced numbers of myeloid and B cells. Essentially no Msi2Gt/Gt cells of any lineage could be detected after 8 weeks of transplantation. To analyze the HSC compartment, the mice were killed after 6 months and LSK cells analyzed. As shown in Figure 7B-C, Msi2Gt/Gt LSK cells were outcompeted by WT-derived LSKs, even when the donor Msi2Gt/Gt cells were in excess. The inability to compete with WT BM was not simply due to a lower content of mutant cells, because the same number of total BM cells from Msi2Gt/Gt or control mice was transplanted, and the difference in the proportion of LSK cells cannot account for the inability to compete with cells from wild-type animals.
BM transplantation experiments. (A-C) Competitive repopulation of Msi2-defective BM cells. Lethally irradiated congenic (CD45.1/2) mice were reconstituted with different ratios of CD45.2+ donor BM cells from either Msi2Gt/Gt or Msi2+/+ (B6) origin with CD45.1 (B6/SJL) competitor BM cells. Mixtures were transplanted and reconstitution monitored by quantifying the percentage of donor-derived CD45.2+ nucleated blood cells in recipients at different times thereafter. Shown are the kinetics of reconstitution of donor-type leukocytes in the peripheral blood (total number of CD45.2+ cells), B cells (B220 antigen), T cells (CD4/CD8 antigens), and myeloid cells (Mac-1/Gr-1 antigens). Shown are 1:1 mixtures of WT donor with competitor cells (squares), 1:1 Msi2 donor with competitor cells (circles), and 7:3 Msi2 donor with competitor cells (triangles). At 24 weeks mice were killed and the contribution of CD45.2 cells to the total BM (B) and LSK (C) population was determined. The data are from 2 independent experiments with 3-4 recipient mice per group. (D-G) Noncompetitive BM transplantation. 107 total BM cells were transplanted into lethally irradiated congenic recipients (CD45.1/2). The percentage of donor-derived cells among total blood leukocytes was determined by flow cytometry. (D) Total leukocyte counts were determined at 8, 16, and 20 weeks after transplantation. Mice were killed 20 weeks after transplantation and BM cells were analyzed. (E) FACS plots are shown for LSKs. (F-G) Histogram showing the total numbers of LSK CD34− (HSCs) and LSK CD34+ (progenitors) in the transplanted animals. Data shown are from 1 experiment with 4 recipient mice per group. Error bars indicate SEM. *P < .05; **P < .005.
BM transplantation experiments. (A-C) Competitive repopulation of Msi2-defective BM cells. Lethally irradiated congenic (CD45.1/2) mice were reconstituted with different ratios of CD45.2+ donor BM cells from either Msi2Gt/Gt or Msi2+/+ (B6) origin with CD45.1 (B6/SJL) competitor BM cells. Mixtures were transplanted and reconstitution monitored by quantifying the percentage of donor-derived CD45.2+ nucleated blood cells in recipients at different times thereafter. Shown are the kinetics of reconstitution of donor-type leukocytes in the peripheral blood (total number of CD45.2+ cells), B cells (B220 antigen), T cells (CD4/CD8 antigens), and myeloid cells (Mac-1/Gr-1 antigens). Shown are 1:1 mixtures of WT donor with competitor cells (squares), 1:1 Msi2 donor with competitor cells (circles), and 7:3 Msi2 donor with competitor cells (triangles). At 24 weeks mice were killed and the contribution of CD45.2 cells to the total BM (B) and LSK (C) population was determined. The data are from 2 independent experiments with 3-4 recipient mice per group. (D-G) Noncompetitive BM transplantation. 107 total BM cells were transplanted into lethally irradiated congenic recipients (CD45.1/2). The percentage of donor-derived cells among total blood leukocytes was determined by flow cytometry. (D) Total leukocyte counts were determined at 8, 16, and 20 weeks after transplantation. Mice were killed 20 weeks after transplantation and BM cells were analyzed. (E) FACS plots are shown for LSKs. (F-G) Histogram showing the total numbers of LSK CD34− (HSCs) and LSK CD34+ (progenitors) in the transplanted animals. Data shown are from 1 experiment with 4 recipient mice per group. Error bars indicate SEM. *P < .05; **P < .005.
To assess whether Msi2Gt/Gt stem cells are intrinsically capable of engraftment and/or differentiation, 107 total BM cells from mutant and control mice were transplanted into lethally irradiated recipients in the absence of competitor cells. Analysis of the peripheral blood for cells of donor origin up to 5 months after transplantation showed efficient engraftment by Msi2Gt/Gt BM cells in the absence of competition at all time points analyzed (Figure 7D). Once engrafted, multilineage differentiation and migration were not abrogated by the lack of Msi2 (supplemental Figure 12). However, there was a significant decrease in the numbers of circulating leukocytes in the mice transplanted with Msi2Gt/Gt BM cells compared with those transplanted with WT cells (Figure 7E).
Twenty-four weeks after transplantation, total LSK numbers were reduced in the mice transplanted with mutant BM cells compared with mice transplanted with WT cells (Figure 7F). Within this population, LSK CD34− (HSC) cells showed a 2-fold decrease in the Msi2Gt/Gt-transplanted mice, whereas the LSK CD34+ (progenitors) decreased more than 4-fold (Figure 7G), indicating that the lower numbers of peripheral blood cells observed may have been due to a defect in the expansion of the progenitor cells.
To rule out any effect of the stromal microenvironment on the LSK phenotype before transplantation that could explain the observed results, we transplanted BM cells from WT CD45.1 animals into lethally irradiated Msi2Gt/Gt animals. Blood analysis after 4 and 8 weeks showed that the expression of the mutated Msi2 in the niche did not influence the ability of the transplanted BM cells to engraft and differentiate into myeloid and lymphoid lineage cells (supplemental Figure 13).
Our data show that Msi2Gt/Gt HSCs can engraft, differentiate, and reconstitute hematopoiesis in lethally irradiated recipients, but only under noncompetitive conditions. Nevertheless, the ability to generate normal numbers of white blood cells was impaired, reflecting the decreased rate of proliferation observed in the LSK population of Msi2Gt/Gt mice during steady-state hematopoiesis. However, we cannot completely rule out a partial defect in the homing capacity of these cells.
Discussion
In the present study, we used a retroviral insertion strategy to identify genes that regulate HSC function. Infection of BM cells with a GFP-tagged Moloney MLV followed by serial transplantations resulted in an enrichment of integration sites adjacent to the Meis1, Abcc1, Fbxo6, and Msi2 genes. We subsequently focused on the function of the Msi2 gene, demonstrating that its ablation leads to a significant reduction of primitive hematopoietic progenitors and leukocytes, and that this phenotype is exacerbated by age. At least part of this effect is mediated by a decreased proliferation ability of ST-HSCs and LMPPs, in which Msi2 is normally expressed at high levels. In addition, we found that Msi2Gt/Gt primitive progenitors have an engraftment defect, because they were dramatically outgrown by wild-type cells in competitive repopulation experiments.
Our study extends the retroviral insertion approach originally developed for the identification of oncogenes that cooperate in leukemia formation32,33 to the discovery of novel genes that are involved in HSC function. The difference is that we have used clonal dominance of hematopoietic cells as a readout instead of leukemia induction, an approach also successfully applied by others.6,34 In all of these studies, the underlying mechanism is thought to consist of the up-regulation of the gene adjacent to the inserted retroviral promoter/enhancer. This hypothesis is supported by the finding that GFP+ hematopoietic cells from secondary recipients harboring an insertion at the Msi2 locus expressed higher levels of Msi2 mRNA. The GFP− cells from these animals were probably caused by retroviral silencing (Figure 1D), which also may be the reason that 2 of the 5 secondary recipient animals transplanted with the infected cell pools did not show an enrichment of the Msi2-driven HSC clone.
Our findings showed that the hematopoietic system of Msi2Gt/Gt mice is severely compromised and that its severity becomes more pronounced with age. The effect is first manifested in young mice, as seen by a significant reduction of LMPP, CLP, and B-cell numbers, as well as granulocytes. Because LMPPs express high levels of Msi2, but not CLPs or more mature cells, and because LMPPs are precursors of both lymphoid and myeloid cells, this suggests that the primary defect resides in the LMPP population. In addition, as the mice age they exhibit a significant decrease in the number of ST-HSCs, CMPs, and GMPs. The reason for the reduction of LMPPs and ST-HSCs can be explained, at least in part, by the finding that their proliferation capacity is impaired (Figure 5B-C). This interpretation is also supported by the observation that the overexpression of Msi2 induces the proliferation of LMPPs but not of LT-HSCs.8
Musashi acts as a regulator of asymmetric cell division of sensory organ progenitors in D melanogaster24 and Msi1 plays an important role in the maintenance of neural stem cells of mice through the translational inhibition of the asymmetric cell division determinant Numb.12 In addition, 2 recent studies showed that Msi2 cooperates with fusion oncoproteins in the induction of acute myeloid leukemia by inhibiting Numb.8,14 Consistent with the idea that Msi2 targets Numb, overexpression of Msi2 in cultured LSK cells induced a significant increase in the proportion of symmetric cell divisions.8 These observations predict that the lack of Msi2 should induce a decrease of HSC self-renewal and an increase in differentiation, causing progressive depletion of the stem cell pool. A similar mechanism has indeed been described for Msi-defective male germline stem cells of D melanogaster, which are lost due to premature differentiation.25 Surprisingly, however, adult Msi2-defective mice have only modestly reduced proportions of LT-HSCs (Figure 3E) and live for more than a year without signs of overt disease. In contrast to these steady-state conditions, Msi2Gt/Gt LSK cells became rapidly depleted after competitive transplantation, possibly because of their proliferative disadvantage and the stress involved in the procedure, leading to the rapid loss of ST-HSCs and LMPPs and to a collapse of the hematopoietic system. Moreover, transplantation under noncompetitive conditions shows that although Msi2Gt/Gt LSKs are able to engraft and differentiate into different lineages, the total numbers of mature cells produced are significantly reduced compared with WT conditions. A possible explanation for this observation is that Msi2 acts primarily on the expansion of multipotent progenitors, although we cannot rule out that it may obscure any defect in the LT-HSC population. Finally, we have to take into consideration that in addition to the observed proliferation defect, the LSK population may have an increased differentiation rate.
Our breeding results showed that approximately half of the Msi2-defective animals did not come to term. Although we do not yet know at what stage in development they died, it indicates that Msi2 has as-yet-unrecognized additional important functions. Whether these involve the formation of HSCs is an interesting question that can now be addressed.
Msi2 may inhibit the translation of proteins other than Numb. Because both an earlier study8 and our own showed an effect of Msi2 on cell proliferation in primitive progenitors, proteins of the cell-cycle machinery are potential targets. These may include transcription factors that negatively regulate the growth of myeloid cells, such as C/EBPa.35,36 Another group of candidate proteins are members of the Ras/ERK/SAPK pathways, based on the observations of Kharas et al8 and our more indirect finding that c-Jun and Egr-1 are strongly down-regulated in knockout LSK cells. How our observations relate to the function of Egr-1 is unclear because Egr-1–deficient HSCs exhibit an increase in their proliferation capacity.37
Finally, as discussed above, our array data suggest that pathways regulating adhesion molecules could be targets of Msi2. Elucidation of the mechanism by which Msi2 regulates the function of primitive hematopoietic progenitors could have important implications not only for understanding how the HSC compartment is regulated, but also how its dysregulation may contribute to the formation of myeloid leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Raúl Méndez, Anna Bigas, Christos Gekas, and Karthik Arumugam for comments to the manuscript; Graf laboratory members for discussions; and Antonio Bernad for the RV-GFP vector.
This work was supported by the Ministerio de Ciencia e Inovacion (SAF2007-63058). F.V. was supported by a grant from Instituto Carlos III-FIS (the Spanish Ministry of Health). E.M.K. was supported by a European Molecular Biology Organization (EMBO) long-term fellowship.
Authorship
Contribution: F.V. initiated the project and designed it together with T.G.; F.V. and L.d.A.-A. performed the retroviral insertion screen and characterization of the knockout mice; E.M.K. analyzed the gene expression arrays; J.F.I. provided technical assistance; W.W. and T.F. created the knockout mice; and L.d.A.-A., F.V., and T.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Graf, PhD, Differentiation and Cancer Program, Center for Genomic Regulation, CRG, Carrer Dr Aiguader, 88, 08003 Barcelona, Spain; e-mail: thomas.graf@crg.es.
References
Author notes
L.d.A.-A. and F.V. contributed equally to this study.