Abstract
TNF-α-converting enzyme (TACE, herein denoted as Adam17) proteolytically sheds several cell-surface inflammatory proteins, but the physiologic importance of the cleavage of these substrates from leukocyte subsets during inflammation is incompletely understood. In this study, we show that Adam17-null neutrophils have a 2-fold advantage in their initial recruitment during thioglycollate-induced peritonitis, and they roll slower and adhere more readily in the cremaster model than wild-type neutrophils. Although CD44 and ICAM-1 are both in vitro substrates of Adam17, their surface levels are not altered on Adam17-null neutrophils. In contrast, L-selectin levels are elevated up to 10-fold in Adam17-null circulating neutrophils, and their accelerated peritoneal influx, slower rolling, and increased adhesion in the cremaster muscle are dependent on L-selectin. Analysis of mixed chimeras shows that enhanced L-selectin levels and accelerated influx were both cell-intrinsic properties of neutrophils lacking Adam17. In contrast, Adam17-null monocytes display no acceleration of infiltration into the peritoneum in spite of elevated L-selectin surface levels, and their peritoneal influx was independent of L-selectin. Therefore, our data demonstrate substrate and myeloid cell-type specificity of Adam17-mediated cleavage of its substrates, and show that neutrophils and monocytes use distinct mechanisms for infiltration of tissues.
Introduction
Recruitment of specific subsets of leukocytes to sites of injury is a complex, multistep process that includes leukocyte tethering and rolling, activation and firm adhesion, and transmigration,1,2 as well as more recently elaborated steps of slow rolling, adhesion strengthening, intraluminal crawling, paracellular and transcellular migration, and migration through the basement membrane into the tissue.3 This process can be characterized as a sequential adhesion cascade that is mediated by quantitative and qualitative changes of several distinct surface receptors on leukocytes and the endothelium. Whereas much has been learned about the mechanisms involved in leukocyte adhesion to endothelial cells, less is known about the molecules responsible for de-adhesion and the transition between the sequential steps. However, transendothelial migration is a very rapid event, and it only takes minutes to reach the subendothelial basement membrane once a leukocyte sticks to the endothelium.4
A useful method to rapidly modulate leukocyte-endothelial interactions is cell-surface proteolysis, a mechanism that can instantly alter the cell-surface protein repertoire.5-7 An array of cell-surface proteins are detected in physiologic fluids as soluble forms representing cleaved extracellular domains.6-9 Included in the known soluble proteins cleaved from the cell surface are proteins that are involved in all steps of leukocyte recruitment, several of which are elevated in the plasma of patients with acute or chronic inflammation.7 This led us to hypothesize that proteolytic shedding of cell-surface proteins on leukocytes and endothelium may regulate leukocyte recruitment and emigration to sites of inflammation.7
In vivo evidence in support of a role for proteolysis in leukocyte recruitment has come from studies using hydroxamic acid–based inhibitors that block the function of matrix metalloproteinases (MMPs) and ADAMs (a disintegrin and metalloproteinases). Intravascular application of a hydroxamate inhibitor to the exteriorized cremaster muscle decreases leukocyte rolling velocity by 40%.10 This inhibitor-induced reduction in rolling was seen in < 5 seconds after injection from a local catheter, demonstrating the immediacy of the effect and its potential physiologic significance. Despite the impact of proteolysis on processes required for leukocyte recruitment, it remains unclear which specific steps in leukocyte recruitment are modulated by proteolysis and whether one or multiple enzymes are involved.
The ADAM family of proteases appears to play a central role in the shedding of cell-surface proteins,7,9,11 and also have the ability to regulate cell-cell interactions.12 Among the 33 members of the ADAM family, Adam17 and Adam10 are the best characterized and are known to cleave many inflammatory cell-surface proteins. In particular, Adam17 regulates the shedding of various inflammatory adhesion molecules, cytokines, and their receptors involved in each step of leukocyte recruitment and activation. These include: L-selectin, ICAM-1, VCAM-1, CD30, CD40, CD44, fractalkine, IL-6 receptor, IL-15 receptor, TNF-α, and TNF-α receptor I and II.7,13 It is difficult to predict the molecular impact of a single enzyme given the multiplicity of substrates cleaved by an individual protease, such as Adam17, and the ability of multiple proteases to shed each of the above substrates.7 Therefore, the potential for overlapping and compensatory functions among these sheddases is significant.
To begin to assess the role of Adam17 in leukocyte recruitment to inflammatory stimuli in vivo, we generated hematopoietic chimeras lacking Adam17 in their circulating cells by repopulation of lethally irradiated C57BL/6 recipients with fetal liver cells. These chimeras were tested in a thioglycollate-induced peritonitis model to examine leukocyte trafficking into the inflamed peritoneal cavity. We show that Adam17−/− circulating neutrophils have a significant advantage in their early infiltration into the peritoneal cavity, whereas Adam17−/− monocytes show no acceleration of infiltration in spite of elevated surface L-selectin levels. Evaluation of neutrophil rolling properties by intravital microscopy further demonstrated that Adam17-null leukocytes roll slower, adhere more readily, and show elevated emigration in inflamed vessels, effects that are L-selectin dependent. No evidence for altered surface levels of the 2 other reported Adam17 substrates, ICAM-1 and CD44, was found. Therefore, Adam17 substrate cleavage is both cell-type specific and context dependent, and has distinct functional effects on specific leukocyte subsets as it controls early neutrophil infiltration at sites of injury in vivo.
Methods
Hematopoietic chimeric mice
Adam17ΔZn/+ heterozygotes (provided by Jacques Peschon and Roy Black, Amgen)14 were backcrossed onto the C57BL/6J background (stock #664; The Jackson Laboratory) for 10 generations. Hematopoietic chimeras were generated by transplanting fetal liver cells from E15.5 Adam17ΔZn/ΔZn, Adam17ΔEx5/ΔEx5, or Adam17+/+ embryos, all on the C57BL/6 background, into lethally irradiated (11 Gy) C57BL/6J recipients. Adam17ΔEx5/ΔEx5 mice generated with a targeting construct designed to delete exon 5 of murine Adam17 will be described in detail elsewhere (C.L.W., Peter J. Gough, Cindy A. Chang, Yonggang Liu, Christina K. Chau, Micheal T. Chin, Thomas N. Wright, and E.W.R., manuscript in preparation). This construct leads to a frame-shift mutation after excision that results in no synthesis of full-length Adam17. Experiments with hematopoietic chimeras generated with fetal livers from Adam17ΔZn/ΔZn or Adam17ΔEx5/ΔEx5 embryos (hereafter denoted as Adam17−/−) gave comparable data. Three to 5 × 106 fetal liver cells from pooled embryos were administered via the retro-orbital sinus. Complete engraftment of all leukocyte lineages (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) was seen at 12 weeks after transplantation (supplemental Figure 1). Study animals were second-generation hematopoietic chimeras repopulated with bone marrow from primary transplants repopulated with fetal liver cells. To generate mixed-hematopoietic chimeras, bone marrow cells for transplantation were a 50:50 mixture of Ly5.1-expressing C57BL/6J bone marrow and either Adam17+/+ or Adam17−/− cells (both Ly5.2+) to repopulate C57BL/6J recipients. All animal procedures were approved by the University of Washington, University of Virginia, La Jolla Institute for Allergy and Immunology, and the University of Münster institutional animal care and use committees.
Flow cytometric analyses
Antibodies for flow cytometric analysis were purchased from either BD Biosciences or from eBioscience. Nonspecific binding was blocked with anti-CD16/32 (BD Fc γ block), and then directly conjugated antibodies were used to detect specific cell-surface markers, including: PE-B220 (RA3-6B2); PE-CD3 (145-2C11); PE- or FITC-Ly6G (1A8); PE-CD62L (MEL-14); FITC- or PerCP-Cy5.5-Ly5.2 (104); PE-Ly5.1 (A20); and PE-, FITC-, PECy5.5-CD11b (M1/70). Stained cells were analyzed on a FACScan (BD Pharmingen), and 10 000-50 000 events were collected for each analysis. Flow data were analyzed using FlowJo 8.1 software (TreeStar).
Peritonitis model
Thioglycollate-induced peritonitis was initiated by intraperitoneal injection of 1 mL of 4% sterile thioglycollate (#2321398; BD Diagnostic). Circulating and peritoneal leukocytes were collected (see supplemental Methods) at different time points after thioglycollate injection, and noninjected mice were used as t = 0 controls. For mice that received the MMP/ADAM inhibitor GM6001 (Elastin Products), the inhibitor was dissolved in 4% carboxymethylcellulose (Sigma-Aldrich) in PBS to make a 1.5 mg/mL solution, and intraperitoneal injection of the GM6001 (200 μL/mouse or 0.3 mg/mouse) was performed 20 minutes before thioglycollate injection. For experiments in which blocking L-selectin (MEL-14) was administered, Fab fragments of the antibodies (see supplemental Methods) or control rat IgG were given via the retro-orbital sinus (30μg in 100μL of PBS/mouse), which was immediately followed by intraperitoneal injection of thioglycollate.
Surgical preparation, intravital microscopy, and leukocyte extravasation
Statistical evaluation
Values are expressed as means ± SD unless stated otherwise. Data were analyzed using Student t test and analyzed using the computer program InStat 2.01 (GraphPad). P < .05 was considered to be significant.
Results
Cell-intrinsic differences lead to accelerated neutrophil infiltration in Adam17−/− chimeras in the early response to thioglycollate-induced peritonitis
Targeted mutation of Adam17 is late embryonically lethal.14 Therefore, to study the function of Adam17 in leukocyte trafficking, mouse hematopoietic chimeras were generated using fetal liver cells from E15.5 Adam17+/+ and Adam17−/− embryos. Stem cells null for Adam17 and wild-type (WT) were comparable in their reconstitution of hematopoietic tissues and circulating cells (supplemental Figure 1 and supplemental Table 1), and detailed analyses of subpopulations of circulating leukocytes revealed no differences in their B cell (B220+), T cell (CD3+), monocyte (CD11b+Ly6G−), and neutrophil (Ly6G+) counts between the 2 chimeric groups (supplemental Table 2). These reconstituted hematopoietic chimeras therefore allowed analysis of how targeted deletion of Adam17 in circulating cells alters the response to inflammatory stimuli.
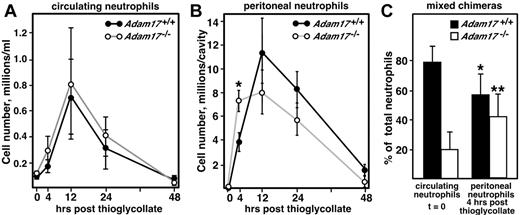
To assess the response of Adam17-null leukocytes to an inflammatory stimulus, the sterile irritant thioglycollate was administered to the peritoneal cavity. Intraperitoneal thioglycollate injection causes transient emigration of neutrophils and monocytes from the bone marrow and circulation into the peritoneal cavity, and neutrophils are the first cells into the peritoneum. Circulating neutrophil numbers in Adam17+/+ and Adam17−/− chimeras increase transiently to a similar extent after thioglycollate challenge (reaching a maximum of 8-fold at 12 hours) before returning to baseline by 48 hours in both groups (Figure 1A). However, the number of neutrophils infiltrating the peritoneal cavity of null chimeras was 1.8- to 2-fold higher than Adam17+/+ chimeras at 4 hours after thioglycollate injection (Figure 1B). This significant increase in neutrophil influx in Adam17-null chimeras suggests that early neutrophil recruitment was accelerated. At 12 hours and later, there was no significant difference in neutrophil recruitment or apoptosis between Adam17+/+ and Adam17−/− chimeras (Figure 1B and supplemental Figure 2). A recent study of neutrophil recruitment during E coli–mediated peritonitis also showed increased neutrophil recruitment 4 hours after administration of bacteria.17 Therefore, 2 distinct models of peritonitis show similar enhancement of early neutrophil recruitment, but the mechanism(s) responsible is unclear.
Neutrophil recruitment is accelerated in Adam17-null hematopoietic chimeras in a model of sterile peritonitis. Neutrophil recruitment was analyzed in the sterile peritonitis model at different time points by evaluation of cell numbers using flow cytometry and an Ly6G antibody to specifically assess neutrophils. Circulating (A) and peritoneal (B) cells were collected (n = 5 per group). Data are representative of 4 experiments, including experiments at additional time points. * P = .02. (C) Mixed chimeras were generated containing 50% each WT (Ly5.1) and Adam17-null (Ly5.2) bone marrow. The relative contribution of Adam17+/+ and Adam17−/− neutrophils to total circulating (unstimulated, t = 0, n = 8) and peritoneal (4 hours after thioglycollate injection, n = 6) were evaluated. *P = .007 and **P = .01 for the change in neutrophil contribution from peritoneal to circulating. Mixed chimeras with different ratios of null and WT were evaluated in 2 other experiments, 1 at 2 hours and 1 at 4 hours after thioglycollate administration, with similar effects observed. All data are expressed as means ± SD.
Neutrophil recruitment is accelerated in Adam17-null hematopoietic chimeras in a model of sterile peritonitis. Neutrophil recruitment was analyzed in the sterile peritonitis model at different time points by evaluation of cell numbers using flow cytometry and an Ly6G antibody to specifically assess neutrophils. Circulating (A) and peritoneal (B) cells were collected (n = 5 per group). Data are representative of 4 experiments, including experiments at additional time points. * P = .02. (C) Mixed chimeras were generated containing 50% each WT (Ly5.1) and Adam17-null (Ly5.2) bone marrow. The relative contribution of Adam17+/+ and Adam17−/− neutrophils to total circulating (unstimulated, t = 0, n = 8) and peritoneal (4 hours after thioglycollate injection, n = 6) were evaluated. *P = .007 and **P = .01 for the change in neutrophil contribution from peritoneal to circulating. Mixed chimeras with different ratios of null and WT were evaluated in 2 other experiments, 1 at 2 hours and 1 at 4 hours after thioglycollate administration, with similar effects observed. All data are expressed as means ± SD.
One mechanism that could induce accelerated neutrophil influx in Adam17-null chimeras is elevated cytokine release. Similar to other models of inflammation, cytokines are primarily released after thioglycollate injection by resident peritoneal macrophages within 2-4 hours of injection.18 Analysis of soluble cytokines in the peritoneal cavity showed a transient increase in cytokine levels in the peritoneal fluid 4 hours after thioglycollate injection (data not shown), but no change in levels of the chemokines MIP-2, MCP-1, or KC were observed (data not shown). TNF-α is a known substrate of Adam17,19,20 and levels of soluble TNF-α in the peritoneal cavity of Adam17-null chimeras were reduced by 90% (Adam17+/+ with 65.9 ± 4.3 pg/cavity vs Adam17−/− with 6.8 ± 0.9 pg/cavity, mean ± SEM, n = 21 and 22, respectively, P < .001), confirming that Adam17 is its major sheddase and that resident peritoneal macrophages are the primary cellular source.
An alternative approach to evaluating enhanced neutrophil emigration is to determine whether Adam17-null neutrophils still show a competitive advantage in the presence of WT cells. To this end, a mixed-hematopoietic chimera model was generated using 50% WT bone marrow cells combined with 50% Adam17−/− bone marrow to repopulate lethally irradiated C57BL/6 recipients. Resultant chimeras were engrafted with both WT cells, which express Ly5.1 on their surface, and Adam17−/− cells, which express Ly5.2 on their surface. This approach normalizes differences in the levels of TNF-α in the peritoneal cavity (mixed chimeras 62.4 ± 4.4 pg/cavity, n = 13, vs Adam17+/+ chimeras 65.9 ± 4.3 pg/cavity, n = 21), and determines whether cell-autonomous differences in the Adam17-null neutrophils enable them to be more rapidly recruited in response to thioglycollate injection. As shown in Figure 1C, the contribution of Adam17-null neutrophils (Ly5.2+Ly6G+) in the peritoneal cavity of the mixed chimeras 4 hours after thioglycollate injection was 2-fold elevated relative to their distribution in the blood (41.6% ± 5.9% in the peritoneal cavity vs 20.5% ± 4.2% in the blood as a percentage of the total, mean ± SEM). In contrast, WT peritoneal neutrophils were reduced approximately 28% relative to their distribution in the blood (57.2% ± 5.9% in the peritoneal cavity vs 78.9% ± 3.8% in the blood as a percentage of the total, mean ± SEM). These data confirm that neutrophils lacking Adam17 on their cell surface showed accelerated emigration to the site of inflammation at an early stage in this model of peritonitis, and suggest that the accelerated emigration is a cell-autonomous effect.
Among 3 neutrophil adhesion substrates, only surface L-selectin levels are elevated on Adam17−/− neutrophils and Adam17 cleavage of L-selectin is context specific
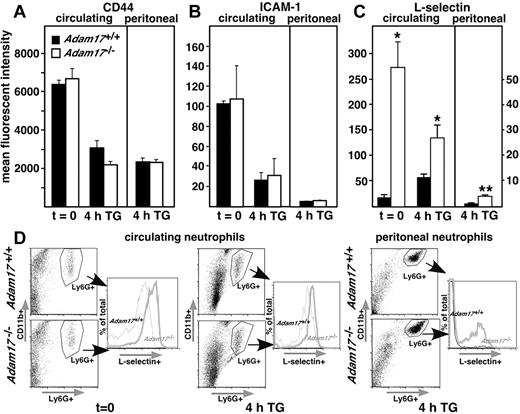
Because Adam17 is a known sheddase of several leukocyte adhesion molecules, including CD-44, ICAM-1 and L-selectin,13,14,21,22 surface levels of these adhesion molecules were evaluated by flow cytometry of Adam17+/+ and Adam17−/− neutrophils. However, as shown in Figure 2A-B, cell-surface levels of CD-44 and ICAM-1 were not significantly different between Adam17+/+ and Adam17−/− chimeras. In contrast, circulating neutrophils of Adam17−/− chimeras showed significantly higher levels of surface L-selectin, with mean fluorescent intensities more than 10-fold higher than those of Adam17+/+ neutrophils without any inflammatory stimulus at t = 0 (Figure 2C-D). At this time, no neutrophils are detected in the peritoneal cavity, as expected. Four hours after thioglycollate injection, surface L-selectin levels on Adam17-null circulating neutrophils were still significantly higher than those of Adam17+/+ neutrophils. In the peritoneum at 4 hours, surface L-selectin levels remained 2- to 3-fold higher on Adam17-null neutrophils, but both groups show a > 90% decrease in L-selectin staining compared with circulating neutrophils (note scale difference for peritoneal cells in Figure 2C). Changes in the cell-surface levels of L-selectin include alterations in mean fluorescent intensity and in the percentage of neutrophils expressing L-selectin in the peritoneum (Figure 2D). Further, the mean fluorescent intensities of L-selectin staining in mixed-hematopoietic chimeras (supplemental Figure 3A) are elevated to a similar extent as Adam17-null hematopoietic chimeras. The mean fluorescent intensity in circulating neutrophils was 342 ± 17 in Adam17-null vs 74.6 ± 7 in WT and in peritoneal neutrophils 53.9 ± 4.6 in Adam17-null vs 8.8 ± 1.0 in WT (mean ± SEM). These data indicate that the regulation of L-selectin levels is also cell autonomous.
Among 3 neutrophil adhesion substrates, only cell-surface levels of L-selectin are significantly elevated on Adam17−/− circulating and peritoneal neutrophils. (A-C) Cell-surface levels of Adam17 adhesion substrates CD-44 (A), ICAM-1 (B), and L-selectin (C) were evaluated on circulating and peritoneal neutrophils by flow cytometry. Data are expressed as means ± SD of mean fluorescent intensity. Significant elevation of cell-surface expression of only L-selectin was observed in Adam17−/− chimeras in both circulating and peritoneal neutrophils (*P = .03 and **P < .01, respectively, n = 5 per group). Multiple replicate experiments showed comparable differences. (D) Representative set of scatter plots is shown (left) for circulating and peritoneal neutrophils (evaluated by CD11b and Ly6G staining) with histograms of percentage of L-selectin+ cells from the total Ly6G+-gated population (right). Data are shown at 4 hours after thioglycollate injection and without thioglycollate injection for circulating neutrophils.
Among 3 neutrophil adhesion substrates, only cell-surface levels of L-selectin are significantly elevated on Adam17−/− circulating and peritoneal neutrophils. (A-C) Cell-surface levels of Adam17 adhesion substrates CD-44 (A), ICAM-1 (B), and L-selectin (C) were evaluated on circulating and peritoneal neutrophils by flow cytometry. Data are expressed as means ± SD of mean fluorescent intensity. Significant elevation of cell-surface expression of only L-selectin was observed in Adam17−/− chimeras in both circulating and peritoneal neutrophils (*P = .03 and **P < .01, respectively, n = 5 per group). Multiple replicate experiments showed comparable differences. (D) Representative set of scatter plots is shown (left) for circulating and peritoneal neutrophils (evaluated by CD11b and Ly6G staining) with histograms of percentage of L-selectin+ cells from the total Ly6G+-gated population (right). Data are shown at 4 hours after thioglycollate injection and without thioglycollate injection for circulating neutrophils.
Our findings demonstrate that surface levels of L-selectin, but not ICAM-1 or CD44, are elevated in the absence of Adam17 in circulating cells. Our data also suggest that L-selectin shedding from neutrophils is only partially dependent on Adam17 in the peritonitis model, with a significant component independent of Adam17 during and/or after emigration into the peritoneum. Our results also raise the possibility that the significant elevation of neutrophil cell-surface L-selectin in Adam17−/− chimeras in the circulation may contribute to their enhanced infiltration into the peritoneum.
Decreased leukocyte rolling velocity and increased adhesion in Adam17−/− chimeras is L-selectin dependent and may contribute to accelerated neutrophil emigration
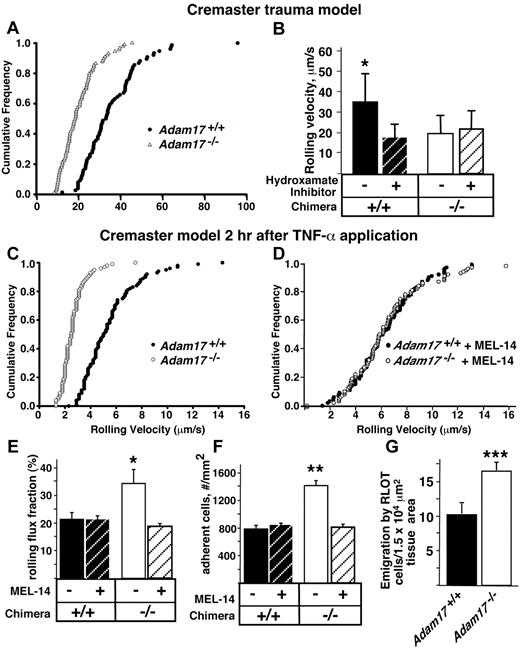
To examine which step(s) in neutrophil recruitment is altered by the absence of Adam17, leukocyte rolling velocity was analyzed in vivo by intravital microscopy of the exteriorized cremaster muscle. The surgical exteriorization leads to a mild inflammatory response that induces a low basal level of leukocyte rolling. Greater than 90% of the leukocytes in this trauma model and reported after local cytokine stimulation are neutrophils.23 In the trauma model, the velocities of rolling leukocytes were significantly reduced in Adam17−/− chimeras (P < .05; Figure 3A). It was previously shown that the addition of a hydroxamate inhibitor, a class of inhibitors with broad inhibitory activity against all metalloproteinases,24,25 significantly reduced leukocyte rolling velocity in C57BL/6 mice.10 Therefore, we added the hydroxamate inhibitor to both WT and Adam17−/− chimeras and it only diminished rolling velocity in the WT chimeras (Figure 4B). The fact that administration of a metalloproteinase inhibitor has no further effect on the rolling velocity of Adam17−/− leukocytes strongly suggests that Adam17 is the primary metalloproteinase regulating this process.
A significant decrease in leukocyte rolling velocity and increased adhesion of Adam17−/− leukocytes in vivo may contribute to their accelerated neutrophil emigration in the peritonitis model. (A) To investigate possible mechanisms involved in the accelerated neutrophil influx, leukocyte rolling velocity was analyzed by intravital microscopy of the exteriorized cremaster muscle of Adam17-null and WT chimeras. The cumulative frequency of velocities of rolling leukocytes in Adam17−/− (▵) and Adam17+/+ (●) chimeras demonstrated a significant reduction in rolling velocity in vivo in Adam17-null chimeras (P < .05). Rolling velocities of at least 75 cells per group (n ≥ 4 mice) were measured. (B) Addition of a hydroxamate metalloproteinase inhibitor diminished the rolling velocity in WT chimeras; however, the inhibitor had no effect on rolling velocity of Adam17−/− chimeras. To model the inflammatory response, TNF-α was administered 2 hours before intravital microscopy and the cumulative frequency of leukocyte rolling velocity was determined (C). (D) Leukocyte rolling velocity was also determined for Adam17+/+ and Adam17−/− chimeras in the presence of Fab fragments of L-selectin antibody MEL-14. The presence or absence of L-selectin MEL-14 Fab fragments was also used to analyze the rolling flux fraction (the number of rolling leukocytes as a fraction of total leukocytes flowing through the venule/unit time; *P = .01 relative to Adam17+/+ chimeras and P = .02 relative to Adam17−/− chimeras with MEL-14; E), and adhesion for Adam17+/+ and Adam17−/− chimeras (**P < .0001 relative to all other parameters; F). (G) Leukocyte extravasation was investigated using reflected light oblique transillumination microscopy. Emigrated cells were determined in an area reaching out 75 mm to each side of the vessel over a distance of 100-mm vessel length (***P = .0002). Data in panels B, E, F, and G are expressed as means ± SD.
A significant decrease in leukocyte rolling velocity and increased adhesion of Adam17−/− leukocytes in vivo may contribute to their accelerated neutrophil emigration in the peritonitis model. (A) To investigate possible mechanisms involved in the accelerated neutrophil influx, leukocyte rolling velocity was analyzed by intravital microscopy of the exteriorized cremaster muscle of Adam17-null and WT chimeras. The cumulative frequency of velocities of rolling leukocytes in Adam17−/− (▵) and Adam17+/+ (●) chimeras demonstrated a significant reduction in rolling velocity in vivo in Adam17-null chimeras (P < .05). Rolling velocities of at least 75 cells per group (n ≥ 4 mice) were measured. (B) Addition of a hydroxamate metalloproteinase inhibitor diminished the rolling velocity in WT chimeras; however, the inhibitor had no effect on rolling velocity of Adam17−/− chimeras. To model the inflammatory response, TNF-α was administered 2 hours before intravital microscopy and the cumulative frequency of leukocyte rolling velocity was determined (C). (D) Leukocyte rolling velocity was also determined for Adam17+/+ and Adam17−/− chimeras in the presence of Fab fragments of L-selectin antibody MEL-14. The presence or absence of L-selectin MEL-14 Fab fragments was also used to analyze the rolling flux fraction (the number of rolling leukocytes as a fraction of total leukocytes flowing through the venule/unit time; *P = .01 relative to Adam17+/+ chimeras and P = .02 relative to Adam17−/− chimeras with MEL-14; E), and adhesion for Adam17+/+ and Adam17−/− chimeras (**P < .0001 relative to all other parameters; F). (G) Leukocyte extravasation was investigated using reflected light oblique transillumination microscopy. Emigrated cells were determined in an area reaching out 75 mm to each side of the vessel over a distance of 100-mm vessel length (***P = .0002). Data in panels B, E, F, and G are expressed as means ± SD.
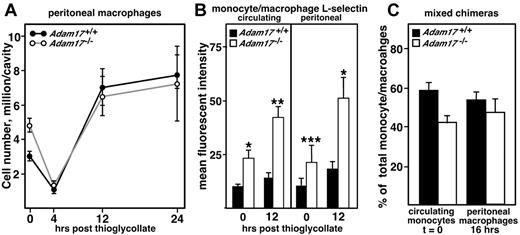
Although surface levels of L-selectin are elevated on Adam17−/− monocytes relative to WT controls, their recruitment to the peritoneal cavity is not accelerated and L-selectin levels do not change on emigration into the peritoneal cavity. As described in the legend to Figure 1, the sterile peritonitis model was used to study monocytic cell recruitment to the peritoneal cavity. (A) Peritoneal cells were collected at the indicated time points after thioglycollate injection (n = 4-5 per group), as well as resident peritoneal cells without stimulation (t = 0). Individual cell numbers were determined and flow cytometry was performed using antibody staining to identify monocytic cells as F4/80+. (B) Cell-surface levels of L-selectin were evaluated on circulating and peritoneal monocytic cells by flow cytometry using antibody staining for CD11b+ and Ly6G− cells. Data are expressed as means ± SD of mean fluorescent intensity (n = 4-5 per group). Statistical significance is indicated (*P < .01 and **P < .02), and these representative data were confirmed in at least 3 replicate experiments. (C) Mixed chimeras were generated containing 50% each WT (Ly5.1) and Adam17-null (Ly5.2) bone marrow. The relative contribution of Adam17+/+ and Adam17−/− monocytes to total circulating monocytes (unstimulated, t = 0, n = 4) and peritoneal macrophages (16 hours after thioglycollate injection, n = 4) were evaluated. Mixed chimeras were evaluated in one other experiment at 24 hours after thioglycollate administration, with similar effects observed. All data are expressed as means ± SD.
Although surface levels of L-selectin are elevated on Adam17−/− monocytes relative to WT controls, their recruitment to the peritoneal cavity is not accelerated and L-selectin levels do not change on emigration into the peritoneal cavity. As described in the legend to Figure 1, the sterile peritonitis model was used to study monocytic cell recruitment to the peritoneal cavity. (A) Peritoneal cells were collected at the indicated time points after thioglycollate injection (n = 4-5 per group), as well as resident peritoneal cells without stimulation (t = 0). Individual cell numbers were determined and flow cytometry was performed using antibody staining to identify monocytic cells as F4/80+. (B) Cell-surface levels of L-selectin were evaluated on circulating and peritoneal monocytic cells by flow cytometry using antibody staining for CD11b+ and Ly6G− cells. Data are expressed as means ± SD of mean fluorescent intensity (n = 4-5 per group). Statistical significance is indicated (*P < .01 and **P < .02), and these representative data were confirmed in at least 3 replicate experiments. (C) Mixed chimeras were generated containing 50% each WT (Ly5.1) and Adam17-null (Ly5.2) bone marrow. The relative contribution of Adam17+/+ and Adam17−/− monocytes to total circulating monocytes (unstimulated, t = 0, n = 4) and peritoneal macrophages (16 hours after thioglycollate injection, n = 4) were evaluated. Mixed chimeras were evaluated in one other experiment at 24 hours after thioglycollate administration, with similar effects observed. All data are expressed as means ± SD.
To further model the inflammatory response, TNF-α was administered 2 hours before intravital microscopy, and leukocyte rolling velocity, rolling flux fraction, and adhesion were measured (Figure 3C,E-F). TNF-α treatment reduced rolling velocities in both groups, with Adam17−/− leukocytes showing an even further 2-fold reduction relative to Adam17+/+ leukocytes; this was associated with a 1.6-fold increase in rolling flux fraction and a 1.8-fold increase in leukocyte adhesion. Further, leukocyte extravasation in the cremaster was elevated 2-fold (Figure 3G), similar to the enhanced emigration of neutrophils in the peritonitis model (Figure 1B). These data demonstrate the critical role of leukocyte Adam17 in limiting the initial rolling and sampling of the endothelial surface with and without cytokine stimulation.
Because surface levels of L-selectin on Adam17−/− circulating neutrophils were significantly elevated (Figure 2C), we investigated whether Fab fragments of the blocking L-selectin antibody MEL-14 could reverse any of the reduction in rolling velocity, increased rolling flux fraction, or increased adhesion observed in Adam17-null leukocytes. Fab fragments of MEL-14 were used to avoid signaling that can be induced by IgG promotion of complex formation. As shown in Figure 3D-F, MEL-14 normalized all of these parameters to the levels of Adam17+/+ leukocytes. These data provide evidence that L-selectin is the primary substrate in which cleavage by Adam17 serves to normally limit leukocyte rolling and adhesion.
Monocyte infiltration is not accelerated in the peritonitis model in spite of elevated surface levels of L-selectin
Adam17 is broadly expressed,19 and significant levels of Adam17 are detected in both neutrophils and macrophages (supplemental Figure 4). Because monocytes and neutrophils share many of the same adhesion molecules, the prediction would be that monocyte infiltration into the peritoneum should also be altered by the absence of Adam17. As shown in Figure 4A, resident peritoneal macrophage cell number was elevated approximately 2-fold in Adam17−/− chimeras, a very reproducible observation that we are currently investigating. However, macrophage cell number in the peritoneal cavities of both Adam17−/− and Adam17+/+ chimeras decreased to the same level within 4 hours of administration of thioglycollate, a time point when the majority of the resident macrophages exit the cavity and migrate to the draining lymph nodes.26 During the monocyte emigration phase that follows, no acceleration of Adam17−/− monocyte infiltration into the peritoneal cavity was observed up to 24 hours after thioglycollate administration. Analysis of mixed-hematopoietic chimeras also failed to convey any competitive advantage for Adam17−/− monocytes in their emigration into the peritoneal cavity at 16 or 24 hours after thioglycollate administration (Figure 4C and data not shown).
The absence of accelerated monocyte influx did not appear to be due to differential effects on monocyte and neutrophil L-selectin cleavage. Cell-surface levels of L-selectin were 2-fold elevated on Adam17-null circulating monocytes compared with WT monocytes (Figure 4B), although Adam17−/− circulating neutrophils showed 10-fold elevation (Figure 2C). Absolute levels of monocytic cell-surface L-selectin were similar in the circulation and in the peritoneal cavity (Figure 4B), including those in mixed-hematopoietic chimeras (supplemental Figure 3B). In contrast, peritoneal neutrophils, both WT and Adam17−/−, had lost > 90% of their cell-surface L-selectin by the time they were in the peritoneal cavity 4 hours after thioglycollate administration (Figure 2C), including mixed chimeras (supplemental Figure 3A). The Adam17-independent loss of surface L-selectin that was seen on neutrophils was not observed with monocyte emigration. Therefore, the acceleration of neutrophil influx into the peritoneal cavity appears to be selective for neutrophils in spite of higher levels of L-selectin expression on both Adam17−/− monocytes and neutrophils.
Blockade of ADAM/MMP–mediated shedding on leukocytes and endothelium also accelerates neutrophil, but not monocyte, infiltration
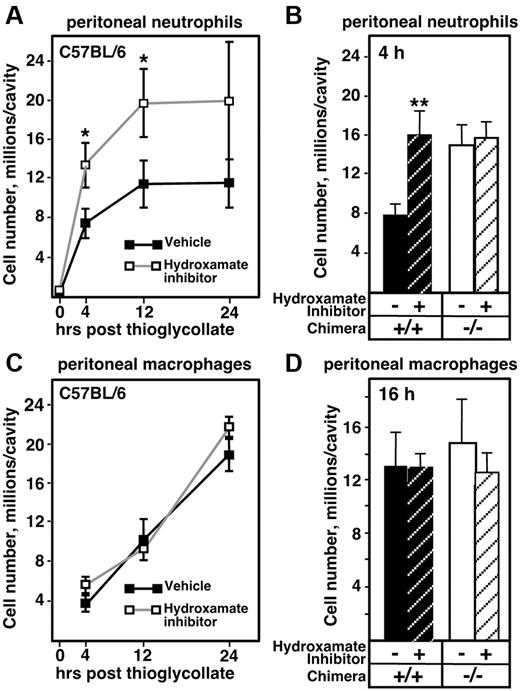
Multiple metalloproteinases have been shown to be capable of cleaving adhesion molecules involved in neutrophil transendothelial migration,7 and our data highlight a role for Adam17 in early neutrophil recruitment, but not in later stages of emigration (Figure 1). Consequently, we investigated whether other metalloproteinases are involved in regulating neutrophil recruitment in the peritonitis model. WT C57BL/6J mice were injected intraperitoneally with hydroxamate inhibitor 20 minutes before and at the time of thioglycollate administration, and the numbers of infiltrating neutrophils were determined. Peritoneal neutrophils in hydroxamate inhibitor–treated mice were 1.8-fold elevated at 4 hours after thioglycollate compared with the vehicle control (Figure 5A); this level of elevation is identical to the extent of accelerated neutrophil influx observed in Adam17−/− mice at this time point (Figure 1B). However, unlike the Adam17−/− chimeras, hydroxamate inhibitor treatment led to a persistent elevation of neutrophil influx that was still 1.7-fold elevated at 12 hours after thioglycollate injection (compare Figure 1B and Figure 5A). Because the administration of a hydroxamate inhibitor leads to the blockade of MMP/ADAM function in all cell types, including leukocytes and endothelial cells, the more persistent inhibition may have been due to the additional blockade in endothelial cells and/or to the involvement of other metalloproteinases.
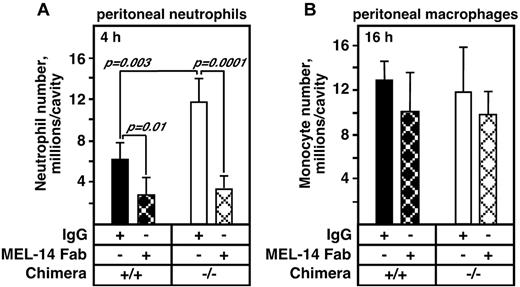
Accelerated neutrophil emigration in Adam17−/− chimeras after thioglycollate injection is completely blocked by the administration of L-selectin antibody, but MEL-14 has no effect on monocyte influx. Fab fragments of the blocking L-selectin antibody MEL-14 or control IgG (30 μg/mouse) were administered just before thioglycollate injection. (A) MEL-14 completely blocked the accelerated neutrophil emigration in Adam17−/− chimeras (n = 5 per group except Adam17+/+ with MEL-14, n = 4). Replicate experiments showed identical results. (B) Monocyte influx into the peritoneal cavity at 16 hours as described in the legend to Figure 4 was not altered by treatment with MEL-14 Fab. All data are expressed as means ± SD.
Accelerated neutrophil emigration in Adam17−/− chimeras after thioglycollate injection is completely blocked by the administration of L-selectin antibody, but MEL-14 has no effect on monocyte influx. Fab fragments of the blocking L-selectin antibody MEL-14 or control IgG (30 μg/mouse) were administered just before thioglycollate injection. (A) MEL-14 completely blocked the accelerated neutrophil emigration in Adam17−/− chimeras (n = 5 per group except Adam17+/+ with MEL-14, n = 4). Replicate experiments showed identical results. (B) Monocyte influx into the peritoneal cavity at 16 hours as described in the legend to Figure 4 was not altered by treatment with MEL-14 Fab. All data are expressed as means ± SD.
The fact that hydroxamate inhibitor leads to acceleration of neutrophil influx at 4 hours that was comparable to that of Adam17−/− chimeras suggests that Adam17 is the primary sheddase controlling early neutrophil recruitment. To test this possibility more directly, Adam17+/+ and Adam17−/− chimeras were injected with thioglycollate with and without the hydroxamate inhibitor, and then neutrophil influx was evaluated after 4 hours. As shown in Figure 5B, treatment with the hydroxamate inhibitor enhanced Adam17+/+ neutrophil influx 2-fold to the same extent as untreated Adam17−/− chimeras. However, the hydroxamate inhibitor did not further increase Adam17−/− neutrophil influx, which is consistent with Adam17 being the primary metalloproteinase enhancing neutrophil emigration.
Because the absence of monocyte Adam17 did not appear to alter early monocyte recruitment, we also investigated whether other metalloproteinases may regulate their emigration by treating C57BL/6 mice (Figure 5C) and Adam17+/+ and Adam17−/− chimeras (Figure 5D) with the hydroxamate inhibitor. However, metalloproteinase inhibition did not alter WT monocyte emigration or infiltration of either Adam17+/+ or Adam17−/− monocytes, further suggesting that proteolytic shedding has distinct effects on neutrophil versus monocyte emigration.
Accelerated ADAM17-null neutrophil emigration is abolished by L-selectin Fab administration
Comparable acceleration of neutrophil emigration at 4 hours in hydroxamate inhibitor–treated mice and Adam17-null chimeras suggests a key role for neutrophil Adam17, and the ability of L-selectin antibody MEL-14 to reverse the slowed rolling, increased rolling flux fraction, and increased adhesion of Adam17-null leukocytes implicates Adam17-mediated cleavage of L-selectin in this process. To determine whether elevated surface L-selectin levels on Adam17-null neutrophils are a significant contributor to their accelerated influx, Adam17+/+ and Adam17−/− hematopoietic chimeras were treated with MEL-14 Fab fragments or control IgG just before thioglycollate injection. Administration of MEL-14 Fab did not alter white blood cell counts or the distribution of any of the circulating leukocyte populations (data not shown). However, MEL-14 Fab administration reduced the influx of Adam17+/+ neutrophils by 50%, and completely blocked the accelerated neutrophil emigration in Adam17−/− chimeras to the level observed in WT chimeras (Figure 6A). Therefore, regulation of surface L-selectin levels by Adam17 appears to be a primary pathway limiting neutrophil rolling and subsequent events leading to early neutrophil emigration after an inflammatory stimulus.
Administration of a hydroxamate inhibitor of MMPs and ADAMs promotes neutrophil emigration to an extent similar to Adam17−/− chimeras, but does not alter monocyte emigration. (A) Neutrophil emigration in C57BL/6 mice treated with a hydroxamate inhibitor or vehicle (n = 4 per group) was monitored as described in the legend to Figure 1. The experiment shown is representative of a total of 3 experiments. *P = .01. (B) Neutrophil emigration into the peritoneal cavity of Adam17+/+ and Adam17−/− chimeras was analyzed in the presence and absence of the hydroxamate inhibitor 4 hours after thioglycollate injection. **P < .0001. (C) Time course of monocyte emigration in C57BL/6 mice in the presence and absence of hydroxamate inhibitor as described in panel A is shown using an antibody to F4/80 to identify macrophages. (D) Monocyte emigration into the peritoneal cavity of Adam17+/+ and Adam17−/− chimeras was analyzed in the presence and absence of the hydroxamate inhibitor 16 hours after thioglycollate injection. **P < .0001. All data are expressed as means ± SD.
Administration of a hydroxamate inhibitor of MMPs and ADAMs promotes neutrophil emigration to an extent similar to Adam17−/− chimeras, but does not alter monocyte emigration. (A) Neutrophil emigration in C57BL/6 mice treated with a hydroxamate inhibitor or vehicle (n = 4 per group) was monitored as described in the legend to Figure 1. The experiment shown is representative of a total of 3 experiments. *P = .01. (B) Neutrophil emigration into the peritoneal cavity of Adam17+/+ and Adam17−/− chimeras was analyzed in the presence and absence of the hydroxamate inhibitor 4 hours after thioglycollate injection. **P < .0001. (C) Time course of monocyte emigration in C57BL/6 mice in the presence and absence of hydroxamate inhibitor as described in panel A is shown using an antibody to F4/80 to identify macrophages. (D) Monocyte emigration into the peritoneal cavity of Adam17+/+ and Adam17−/− chimeras was analyzed in the presence and absence of the hydroxamate inhibitor 16 hours after thioglycollate injection. **P < .0001. All data are expressed as means ± SD.
Although Adam17−/− monocyte levels of L-selectin were elevated, their levels remained similar once they moved into the peritoneal cavity and did not markedly change on Adam17+/+ monocytes either (Figure 4B). Administration of MEL-14 Fab fragments did not alter monocyte infiltration (Figure 6B), which is consistent with the absence of change in surface levels and the failure of metalloproteinase inhibition to alter monocyte influx. Therefore, our studies highlight a key role for Adam17-mediated cleavage of neutrophil influx in limiting their influx to inflammatory sites. Our results also demonstrate that although monocytes have elevated L-selectin surface levels, their emigration to sites of inflammation is independent of L-selectin and distinct from that used by neutrophils.
Discussion
This study demonstrates for the first time specificity in Adam17-dependent regulation of emigration of myeloid-derived leukocytes. Adam17-null monocytes and neutrophils both express higher surface levels of L-selectin, but only neutrophils show an Adam17-dependent, L-selectin–mediated acceleration of influx in response to thioglycollate. Experiments with mixed-hematopoietic chimeras further demonstrate that cell-autonomous characteristics of Adam17-null neutrophils are responsible for their early, accelerated recruitment. The contribution of other metalloproteinases to neutrophil influx into the peritoneum and leukocyte rolling velocity in the cremaster appears to be minimal, because neutrophil phenotype after blockade with a global metalloproteinase inhibitor is indistinguishable from Adam17-null neutrophil characteristics and does not lead to further changes in the null neutrophil infiltration or rolling velocity. In contrast, the absence of Adam17 in monocytes has no impact on monocyte emigration into the peritoneum in the 24 hours after thioglycollate administration, and WT and Adam17-null monocyte influx is independent of L-selectin. Surprisingly, early monocyte influx is also metalloproteinase independent, although monocyte emigration may be dependent on metalloproteinases at later stages of inflammation. It is possible that the differential dependency of neutrophils and monocytes on L-selectin for emigration reflects differences in how they regulate L-selectin cell-surface densities and turnover and/or the use of distinct adhesion pathways.
A recent study of neutrophil recruitment during E coli–mediated peritonitis also observed increased recruitment of Adam17-deficient neutrophils at 4 hours after E coli administration.17 Unlike our results, they observed a blunted neutrophil influx at 24 hours with E coli stimulation; however, the time points analyzed were limited and mechanisms involved in the accelerated influx in the bacterial peritonitis model were not investigated. The mixed genetic background of the mice used for the bacterial peritonitis study may also contribute to the observed differences from our results, because 129SvJ has been shown to have defects in inflammatory responses.27,28 Because signaling through TLRs regulates the response to bacteria, the mechanisms may also be distinct from our studies of the sterile peritonitis model that is independent of TLR4-mediated signaling (data not shown).
Whereas multiple adhesion substrates of Adam17 have been reported in vitro, our results highlight the possibility that, in vivo, Adam17 may only target specific substrates. Neutrophil and monocyte surface levels of L-selectin are clearly elevated in the absence of Adam17, but 2 other adhesion molecules that are reported substrates of Adam17 in vitro, CD44 and ICAM-1,22,29,30 are not altered on either cell in vivo. This study further establishes the in vivo importance of Adam17 and its cleavage of L-selectin in the initial attachment and rolling of circulating neutrophils on inflamed endothelium and in limiting the early accumulation of neutrophils at an inflammatory site. Adam17-null neutrophils roll more slowly than WT neutrophils with or without TNF-α administration, are twice as likely to firmly adhere after TNF-α stimulation, and initially accumulate in 1.8-fold higher numbers, all of which are dependent on L-selectin. Our data also demonstrate differential Adam17-mediated shedding of L-selectin from neutrophils in different contexts: in the circulation and after emigration into the peritoneum. Even though L-selectin levels are elevated 10-fold on Adam17-null circulating neutrophils, > 90% of the neutrophil surface L-selectin is lost after emigration into the peritoneal cavity of both Adam17+/+ and Adam17−/− neutrophils. However, we also show that the L-selectin loss during emigration appears to be specific to neutrophils and is not observed in monocytes.
In studies that first identified the rapid proteolytic shedding of L-selectin, it was suggested that endoproteolytic cleavage of L-selectin could promote leukocyte detachment from the endothelium before entry into tissues.5 Initial tests of this concept used broad-based hydroxamate inhibitors to analyze rolling and adhesion in vitro under flow, and showed a decrease in rolling velocity on purified L-selectin ligand (MECA-79) to 33%-66% of control levels.31 However, the inhibitor had no effect on rolling velocities or transendothelial migration with neutrophils added to cytokine-stimulated human umbilical vein endothelial cells.32 Our current results demonstrate that Adam17 is the primary metalloproteinase limiting normal rolling velocities in vivo (Figure 3B). The elevated surface density of circulating neutrophil L-selectin in Adam17-null chimeras may promote multivalent tethers and thus enhance the conversion of initial tethers into rolling by helping to stabilize the L-selectin tether bond.33 Alternatively, increased surface levels of L-selectin may enhance L-selectin dimer formation, leading to increased numbers of rolling neutrophils and slower rolling.34
Comparison of neutrophil influx in the Adam17-null hematopoietic chimeras with metalloproteinase inhibitor–treated mice revealed comparable acceleration of neutrophil influx in both experimental groups at the 4-hour time point (Figures 1 and 6). These data establish that Adam17 is the primary enzyme targeted by metalloproteinase inhibitors at this initial stage of neutrophil recruitment in the peritonitis model. Analysis of neutrophil L-selectin levels and their context—circulation versus emigration into the peritoneal cavity—further suggest that circulating neutrophil L-selectin levels are Adam17 dependent but peritoneal levels are Adam17 and metalloproteinase independent. Within tissues, neutrophil degranulation is a rich source of serine proteases, and their release may significantly contribute to the loss of L-selectin once they are within the peritoneum. Because some Adam17-independent cleavage of L-selectin can be inhibited by metalloproteinase inhibitors,35 it is possible that there may be a shift in the enzymes controlling L-selectin cleavage as the inflammatory response evolves and/or at different steps in the emigration process. Recently, Adam8 mobilization from the neutrophil granules to the membrane was demonstrated after endothelial adhesion, and Adam8 increased L-selectin shedding.36 Therefore, another ADAM family member may regulate shedding of L-selectin by neutrophils in the absence of Adam17, particularly with firm endothelial adhesion. Coclustering of Adam17 and L-selectin at the trailing edge of neutrophils adherent to E-selectin has also been found for some, but not all, forms of neutrophil activation.37 Consequently, colocalization of Adam17 and L-selectin may be differentially regulated at distinct stages of an inflammatory response.
In summary, proteolytic shedding of neutrophil L-selectin by Adam17 targets the initial interaction of neutrophils with activated endothelium. Because L-selectin cooperates with other selectins and integrins to support neutrophil rolling on inflamed endothelium before firm adhesion and transmigration, perturbation by Adam17-mediated shedding of the initial interaction of neutrophils through L-selectin has the potential to shift the efficiency of the interaction dramatically, as shown by our results. The enhanced efficiency of the L-selectin interaction in the absence of Adam17 leads to slower rolling, with or without cytokine activation, and thus promotes the transition between different steps and increases the effectiveness of each of the subsequent steps in the adhesion cascade. Adam17 acts as the primary gatekeeper to limit the adhesion cascade and thereby restricts recruitment of these potent inflammatory cells that can lead to excessive tissue injury. In contrast to neutrophils, infiltration of Adam17-null monocytes is not altered in spite of elevated L-selectin levels, and therefore neutrophils and monocytes use distinct mechanisms for emigration to sites of inflammation. The cell, substrate, and context specificity of Adam17-mediated cleavage shown in the present study highlight the importance of the underlying regulatory mechanisms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Francis W. Luscinskas for his perspectives and suggestions during the preparation of this manuscript.
This study was supported in part by grants from the National Institutes of Health (HL018645 and HL067267 to E.W.R. and EB002185 to K.L.), from the German Research Foundation (ZA428/3-1 and ZA428/5-1 to A.Z.), and from the University of Washington Nutrition and Obesity Research Center Pilot/Feasibility Program (5P30DK035816 to C.L.W.).
National Institutes of Health
Authorship
Contribution: J.T., I.G., and C.L.W. designed and performed the studies, analyzed the data, and helped to write the manuscript; A.Z. and K.L. designed the intravital microscopy studies and analyzed the data; A.Z. performed the intravital microscopy studies with assistance from C.T.L.; A.S., B.B., and L.-C.H. performed experiments to characterize cytokine release into the peritoneal cavity and analyzed the data; and E.W.R. designed the studies, analyzed the data, and helped to write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elaine W. Raines, University of Washington School of Medicine, Department of Pathology, Harborview Medical Center, 325 Ninth Ave, Box 359675, Seattle, WA 98104; e-mail: ewraines@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal