Abstract
Neutrophils form a vital part of the innate immune response, but at the same time their inappropriate activation contributes to autoimmune diseases. Many molecular components are involved in fine-tuning neutrophil function. We report here the first characterization of the role of ARAP3, a PI3K and Rap-regulated GTPase-activating protein for RhoA and Arf6 in murine neutrophils. We show that neutrophils lacking ARAP3 are preactivated in vitro and in vivo, exhibiting increased β2 integrin affinity and avidity. ARAP3-deficient neutrophils are hyperresponsive in several adhesion-dependent situations in vitro, including the formation of reactive oxygen species, adhesion, spreading, and granule release. ARAP3-deficient cells adhere more firmly under flow conditions in vitro and to the vessel wall in vivo. Finally, loss of ARAP3 interferes with integrin-dependent neutrophil chemotaxis. The results of the present study suggest an important function of ARAP3 downstream of Rap. By modulating β2 integrin activity, ARAP3 guards neutrophils in their quiescent state unless activated.
Introduction
Neutrophils are an essential component of the innate immune responses to invading pathogens and tissue damage. In response to inflammatory stimuli, neutrophils are recruited from the bloodstream to infected tissues, where they produce reactive oxygen species (ROS) and phagocytose and kill pathogens.1 It is vital that the neutrophil response to inflammatory stimuli is well controlled. Leukocyte adhesion deficiency (LAD) patients, in whom the recruitment process is impaired, suffer from repeated, severe bacterial infections. Conversely, excessive neutrophil responsiveness is also detrimental, causing tissue damage and inflammation, as exemplified in autoimmune diseases.
Work aimed at identifying the molecular machinery controlling neutrophils has identified important regulatory players. Rap isoforms regulate adhesion-dependent processes by modulating integrin affinity and avidity.2 Loss of leukocyte Rap1 activation is a cause of LAD-III,3,4 demonstrating the crucial role of this small GTPase in neutrophil control. Agonist-activated PI3Ks generate on activation the lipid second messengers phosphatidylinositol-(3,4,5)-trisphosphate [PtdIns(3,4,5)P3] and PtdIns(3,4)P2. These cause plasma membrane recruitment and activation of PI3K effectors, which regulate multiple enzymes and pathways.5 Known PI3K effectors include regulators of Rho and Arf family small GTPases, GTPase-activating proteins (GAPs), and guanine nucleotide exchange factors. Rho family small GTPases are involved in regulating the neutrophil NADPH oxidase and chemotaxis,6,7 whereas Arf family members regulate phagocytosis, ROS production, and chemotaxis.8,9
ARAP3 was identified from porcine neutrophils in a screen for PtdIns(3,4,5)P3–binding proteins. In vitro, ARAP3 is a PtdIns(3,4,5)P3–dependent Arf6 GAP and a Rap-activated RhoA GAP. In addition, in vivo, PtdIns(3,4,5)P3 drives the recruitment of ARAP3 to the plasma membrane, bringing it into the vicinity of its activator, Rap-GTP, and its substrates, RhoA-GTP and Arf6-GTP.10,11 The remaining ARAP family members (ARAP1/2) share ARAP3's multidomain structure.12,13 We sought to identify ARAP3's physiologic function in neutrophils. Using neutrophils from a conditional Arap3−/− mouse model in biologic assays, we demonstrate that ARAP3 regulates adhesion-dependent processes. Loss of ARAP3 causes the preactivation of neutrophil β2 integrins and hyperresponsiveness in adhesion-dependent situations, feeding into several biologic responses such as adhesion-dependent ROS formation, granule release, and chemotaxis. Whereas Rap activity was not affected by the loss of ARAP3, RhoA activation was increased in an adhesion-dependent setting, suggesting a role for ARAP3 downstream of Rap in neutrophils. Our results suggest a modulatory role for ARAP3 in neutrophils, which prevents their activation in inappropriate situations.
Methods
Unless otherwise stated, materials were obtained from Sigma-Aldrich.
Inducible Arap3−/− mouse model
Generation of the Arap3fl/fl mouse has been described previously.14 For the inducible knockout, Arap3fl/fl mice were crossed with Rosa-ERT2Cre+ mice.15 Cre expression was induced by a single intraperitoneal injection with tamoxifen, as described previously,16 which caused a complete but transient deletion of ARAP3 in bone marrow–derived neutrophils. Mice were housed in open racks or individually ventilated cages in small animal barrier units at the Babraham Institute or Walter-Brendel-Zentrum and used 11 or 12 days after induction. Animal work carried out at the Babraham Institute and Walter-Brendel-Zentrum was approved by United Kingdom Home Office Project licenses PPL80/1875 and PPL80/2335 and Regierung Oberbayern, Germany (AZ55.2-1-54-2531-134/08), respectively.
Neutrophil purification
Analysis of peripheral blood
Tail vein blood was collected in EDTA-coated microvettes (Sarstedt) for analysis using a Vet ABC animal blood cell counter.
ROS production assays
ROS production was measured by chemiluminescence using a luminol-based assay in polystyrene 96-well plates (Berthold Technologies), as described previously.19 Cells (5 × 105) were incubated with luminol (150μM) and HRP (18.75 U/mL) for 10 minutes at 37°C. Where indicated, neutrophils were added manually to wells precoated with fibrinogen (150 μg/mL) or polyRGD (20 μg/mL) with or without murine TNFα (20ng/mL); or to immobilized immune complexes (100μg/mL IgG-BSA). For soluble agonist assays (fMLF [fMet Leu Phe] 10μM final concentration), cells were primed for 1 hour at 37°C in the absence (mock) or presence of mouse TNFα (1000 U/mL) and GM-CSF (100 ng/mL). For all assays, measurements were started immediately and light emission was recorded. Data output was in relative light units (RLUs) per second or total RLUs integrated over the indicated measured periods of time.
Chemotaxis assays
For micropipette chemotaxis assays, neutrophils were resuspended in HBSS, 15mM HEPES, pH 7.4, and 0.05% BSA, and allowed to attach to a glass coverslip. Cells were stimulated at 37°C with a point source of fMLF delivered from a microinjection pipette (Femtotip; Eppendorf) using 50 mbar of pressure, as described previously,18 and monitored by time-lapse imaging for 30 minutes using an inverted Zeiss Axiovert microscope and Axiovision v3.0 software. Chemotaxis assays involving Dunn chambers or EZ-TAXIS chambers were carried out as described previously18,20 using fMLF as the chemoattractant. Chemotaxis in transwells was assayed using 3-μm-pore polycarbonate filter units (Millipore) as described previously.18 Chemotaxis in a 3D collagen matrix was as described previously,21,22 except that neutrophils were prepared by centrifugation over a discontinuous Percoll gradient without prestimulation with TNFα. Dulbecco PBS was used to dilute rat tail collagen (Roche). Cells were tracked using the “manual tracking” plug-in and tracks were analyzed using the “chemotaxis tool” plug-in (Ibidi) in ImageJ v1.37 software.
MAPK, Erk, and PKB activation assays
MAPK, Erk, and PKB activation assays were carried out essentially as described previously,23 with cells plated onto polyRGD-coated or heat-inactivated FCS (hiFCS)–blocked dishes. For details, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Degranulation assay
Release of gelatinase granules after plating onto a fibrinogen-coated surface, or as induced by stimulation with fMLF and cytochalasin B, was detected by zymography, as described previously.24
Analysis of β2 integrins
Surface integrins were visualized by FACS analysis of stained cell populations. Integrin clustering was analyzed by confocal microscopy of unstimulated, stained cells and used as a readout for β2 integrin avidity. To measure integrin affinity, unstimulated cells were allowed to bind to ICAM1-Fc in solution; bound ICAM1-Fc was measured using quantitative immunofluorescence (see supplemental Methods for details).
Reconstitutions
Cohorts of C57B/6 mice were lethally irradiated and subsequently reconstituted by tail vein injection with 4 × 106 bone marrow cells from Arap3 model mice or from sex- and age-matched controls.
Ex vivo flow chamber assay
Rectangular glass capillaries (VitroCom) with a cross-section of 40 μm × 400 μm were coated with rmE-selectin (20 μg/mL), rmCXCL1 (keratinocyte-derived chemokine, 15 μg/mL), and rmICAM-1 (15 μg/mL), and served as ex vivo flow chambers, as described previously25 (see supplemental Methods for details).
Intravital microscopy of the cremaster muscle
Intravital microscopy was performed essentially as described previously26 (see supplemental Methods for details).
Whole mount histology
Cremaster muscle whole mounts were prepared as described previously.25 Leukocytes were Giemsa stained in fixed tissues and analyzed microscopically (see supplemental Methods for details).
Small GTPase activity assays
Results
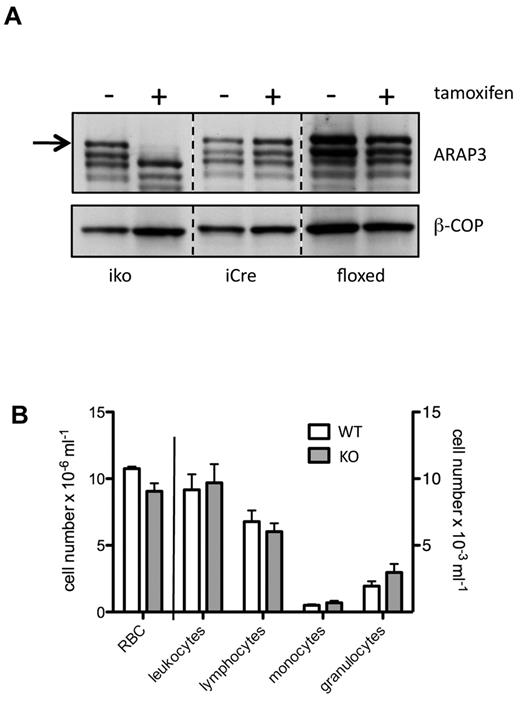
We recently reported that deleting Arap3 in the germline causes embryonic lethality.14 To analyze the function of ARAP3 in neutrophils, we crossed Arap3fl/fl mice with mice expressing a tamoxifen-inducible Cre (ERT2Cre). A single Cre induction caused the temporary loss of ARAP3 protein from bone marrow–derived neutrophils of Arap3fl/flERT2Cre+ animals (Figure 1A). ARAP3 protein expression was not affected by injecting tamoxifen into Arap3fl/fl or into Arap3+/+ERT2Cre+ control mice, or by mock inducing (vehicle control) any of these mice (Figure 1A). ARAP1 and ARAP2, which are expressed only weakly in murine bone marrow–derived neutrophils, were not affected by inducibly deleting Arap3 (data not shown). Similarly, peripheral blood cell counts from tamoxifen- or mock-induced Arap3fl/flERT2Cre+ animals were not affected (Figure 1B).
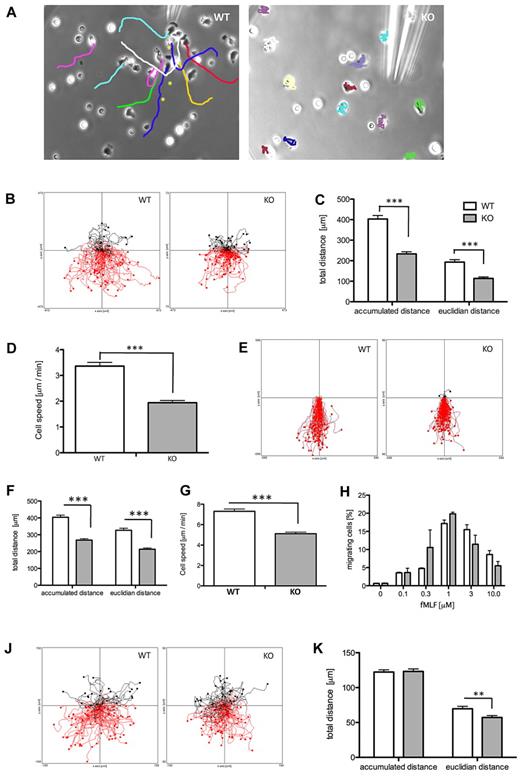
Arap3 is inducibly knocked out in neutrophils. (A) Bone marrow–derived neutrophils were isolated from femurs and tibias of tamoxifen- and mock-induced Arap3fl/flERT2Cre+ (iko), Arap3+/+ERT2Cre+ (iCre), and Arap3fl/fl (floxed) mice. Cells were boiled in sample buffer and proteins were separated by SDS-PAGE, transferred to PVDF membranes, and subjected to blotting using an anti-ARAP3 antiserum (ARAP3).10 Blots were reprobed using β-COP as a loading control (β-COP); 1 × 106 cells were loaded per lane. (B) Peripheral blood cells from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice were analyzed using a Vet ABC animal blood cell counter. Data shown are pooled from 2 separate experiments (means ± SEM; n = 18).
Arap3 is inducibly knocked out in neutrophils. (A) Bone marrow–derived neutrophils were isolated from femurs and tibias of tamoxifen- and mock-induced Arap3fl/flERT2Cre+ (iko), Arap3+/+ERT2Cre+ (iCre), and Arap3fl/fl (floxed) mice. Cells were boiled in sample buffer and proteins were separated by SDS-PAGE, transferred to PVDF membranes, and subjected to blotting using an anti-ARAP3 antiserum (ARAP3).10 Blots were reprobed using β-COP as a loading control (β-COP); 1 × 106 cells were loaded per lane. (B) Peripheral blood cells from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice were analyzed using a Vet ABC animal blood cell counter. Data shown are pooled from 2 separate experiments (means ± SEM; n = 18).
ARAP3 regulates adhesion-dependent formation of ROS
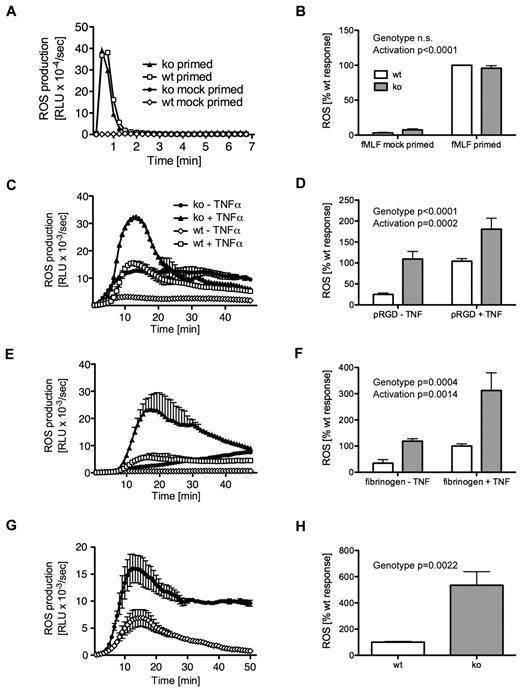
Neutrophils produce ROS on appropriate stimulation. PI3Ks and Rho, Rap, and Arf family small GTPases are involved in regulating the phagocyte oxidase.6,9,30 We found no difference in ROS production by neutrophils from tamoxifen- or mock-induced Arap3fl/flERT2Cre+ mice or controls after stimulation with the soluble agonist fMLF (Figure 2A-B and supplemental Figure 1A for controls), nor with the nonphysiologic, soluble stimulus phorbol 12-myristate 13-acetate (data not shown). This indicated that the machinery required for ROS production was intact in neutrophils lacking ARAP3.
Increased adhesion-induced ROS production in ARAP3-deficient neutrophils. Bone marrow–derived neutrophils from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice were prepared (A-B), primed with TNFα and GM-CSF or mock primed, and (all) preincubated with luminol as described in “ROS assays.” Cells (5 × 105) were plated into 96-well plates containing fMLF as a soluble stimulus (A-B) or plates that had been coated with polyRGD (C-D), fibrinogen (E-F), or a BSA–anti-BSA immune complex (G-H), and light emission was recorded using a Berthold Microluminat Plus luminometer. Data were recorded in duplicate and at least 3 independent experiments were performed. Data (mean ± range) from a representative experiment performed with cells from tamoxifen- and mock-induced Arap3fl/fERT2Cre+ mice are shown in panels A, C, E, and G. Data shown in panels B, D, F, and H represent accumulated light emission (mean ± SEM) from the pooled performed experiments (n = 3) expressed as a percentage of the response in mock-induced neutrophils of each particular experiment. Raw data were analyzed by 2-way ANOVA with Bonferroni post tests (B,D,F) and by t tests (Mann-Whitney; H).
Increased adhesion-induced ROS production in ARAP3-deficient neutrophils. Bone marrow–derived neutrophils from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice were prepared (A-B), primed with TNFα and GM-CSF or mock primed, and (all) preincubated with luminol as described in “ROS assays.” Cells (5 × 105) were plated into 96-well plates containing fMLF as a soluble stimulus (A-B) or plates that had been coated with polyRGD (C-D), fibrinogen (E-F), or a BSA–anti-BSA immune complex (G-H), and light emission was recorded using a Berthold Microluminat Plus luminometer. Data were recorded in duplicate and at least 3 independent experiments were performed. Data (mean ± range) from a representative experiment performed with cells from tamoxifen- and mock-induced Arap3fl/fERT2Cre+ mice are shown in panels A, C, E, and G. Data shown in panels B, D, F, and H represent accumulated light emission (mean ± SEM) from the pooled performed experiments (n = 3) expressed as a percentage of the response in mock-induced neutrophils of each particular experiment. Raw data were analyzed by 2-way ANOVA with Bonferroni post tests (B,D,F) and by t tests (Mann-Whitney; H).
Neutrophils attached to several surfaces generate long-lasting, β2 integrin–dependent ROS when costimulated with an additional proinflammatory stimulus (eg, a cytokine or bacterial product).31,32 In contrast, the synthetic multivalent integrin ligand polyRGD does not require any costimulation.23 We found that adhesion-dependent ROS production after plating cells onto polyRGD-coated surfaces was increased in tamoxifen-induced Arap3fl/flERT2Cre+ neutrophils compared with controls (Figure 2C-D and supplemental Figure 1B) in the presence or absence of TNFα. We observed the same trend with neutrophils plated onto fibrinogen-coated surfaces, again with or without costimulation with TNFα (Figure 2E-F and supplemental Figure 1C). This suggested that β2 integrins in the ARAP3-deficient neutrophils might be present in a preactivated state, able to produce adhesion-dependent ROS even in the absence of costimulation with a proinflammatory cytokine.
Immune complexes activate neutrophils by signaling through cell-surface Fc receptors. This also induces ROS production,24 a function that is critical for autoimmune inflammatory disorders. Fc receptors and integrins share signaling intermediates.33 We assessed ROS production after plating tamoxifen-induced Arap3fl/flERT2Cre+ neutrophils (and the relevant controls) in immobilized immune complex–coated wells (BSA–anti-BSA). In agreement with the results described in the previous paragraph, we observed a heightened response when cells lacking ARAP3 were plated onto immune complex–coated surfaces (Figure 2G-H and supplemental Figure 1D). In summary, ROS production due to integrin (or Fc-receptor) engagement, but not to stimulation with a soluble agonist, was up-regulated in neutrophils lacking ARAP3.
Loss of ARAP3 causes increased responses in adhesion-dependent processes in neutrophils
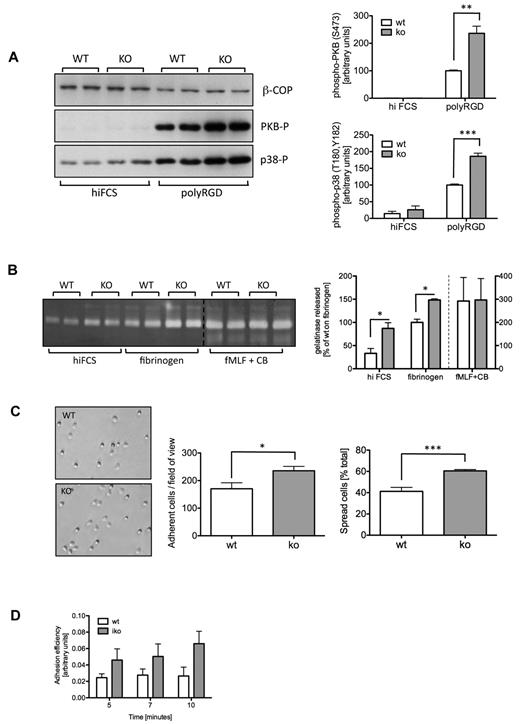
Prompted by the results obtained with the ROS assays, we investigated whether ARAP3 might also be important for other events downstream of integrin engagement in neutrophils. We analyzed the phosphorylation status of protein kinase B (PKB, also called Akt) and of p38 MAPK, 2 downstream components of outside-in signaling. We plated neutrophils onto dishes that had been blocked with hiFCS or coated with polyRGD. Both kinases were hyperphosphorylated in tamoxifen-induced Arap3fl/flERT2Cre+ neutrophils, but not in controls, after plating on polyRGD (Figure 3A and supplemental Figure 2A).
Integrin-dependent events are up-regulated in ARAP3-deficient neutrophils. (A) Integrin-dependent signaling. Bone marrow–derived neutrophils were prepared from mock (WT) and tamoxifen-induced (KO) Arap3fl/flERT2Cre+ mice. Cells (5×10−6) were left to adhere to hiFCS- or polyRGD-coated tissue culture plastic to induce intracellular signaling before being scraped into lysis buffer. Lysates were subjected to SDS-PAGE and immunoblotted for phospho-PKB (Ser473), phospho-p38 (Thr180 and Tyr182), and β-COP as a loading control. A representative blot is shown on the left. Blots were quantified using ImageJ v1.37 software, and pooled data from 3 independent experiments are plotted (top right, phospho-PKB; bottom right, phospho-p38), expressed as a percentage of the response obtained with mock-induced neutrophils plated onto polyRGD (data ± SEM). (B) Gelatinase granule release. Neutrophils were prepared as in panel A, and plated in wells that had been coated with hiFCS or fibrinogen in the presence of 20 ng/mL of TNFα. For a positive control, neutrophils were stimulated with 1μM fMLF in the presence of 10μM cytochalasin B (CB). Supernatants from all wells were used for zymography. Stained gels were quantified using ImageJ v1.37 software. A representative stained zymography gel is shown on the left. The positive control samples were not loaded immediately adjacent to adhesion-induced samples. For ease of viewing, the 2 parts of the gel were pasted here, as indicated by a dotted line. Pooled data from 3 independent experiments are plotted (data ± SEM) on the right. (C) Adhesion and spreading. Neutrophils were prepared as in panel A, and 5 × 106 cells were plated into 24-well plates that had been coated with 20 μg/mL of polyRGD, left to adhere for 10 minutes, and fixed. In washed plates, adherent (phase dark) and spread cells (phase light) were counted in 4 randomly chosen fields of view from each individual experiment, and the percentage of spread cells of total numbers was determined. Example photos are shown on the left; on the right are plotted data combined from 3 independent experiments (data ± SEM). Statistical analysis was by paired t tests performed on the raw data. *P < .05; **P < .01; ***P < .001. (D) Ex vivo flow chamber studies were performed with tamoxifen-induced Arap3fl/flERT2Cre+ (iko) and wild-type animals (wt) using microflow chambers (0.4 × 0.04 mm) coated with rmE-selectin (20 μg/mL), rmCXCL1 (15 μg/mL), and rmICAM-1 (15 μg/mL). Flow chambers were recorded for 10 minutes, and adhesion efficiency (adherent cells/mm2 × WBC) was calculated (data ± SEM; n = at least 5 chambers per group).
Integrin-dependent events are up-regulated in ARAP3-deficient neutrophils. (A) Integrin-dependent signaling. Bone marrow–derived neutrophils were prepared from mock (WT) and tamoxifen-induced (KO) Arap3fl/flERT2Cre+ mice. Cells (5×10−6) were left to adhere to hiFCS- or polyRGD-coated tissue culture plastic to induce intracellular signaling before being scraped into lysis buffer. Lysates were subjected to SDS-PAGE and immunoblotted for phospho-PKB (Ser473), phospho-p38 (Thr180 and Tyr182), and β-COP as a loading control. A representative blot is shown on the left. Blots were quantified using ImageJ v1.37 software, and pooled data from 3 independent experiments are plotted (top right, phospho-PKB; bottom right, phospho-p38), expressed as a percentage of the response obtained with mock-induced neutrophils plated onto polyRGD (data ± SEM). (B) Gelatinase granule release. Neutrophils were prepared as in panel A, and plated in wells that had been coated with hiFCS or fibrinogen in the presence of 20 ng/mL of TNFα. For a positive control, neutrophils were stimulated with 1μM fMLF in the presence of 10μM cytochalasin B (CB). Supernatants from all wells were used for zymography. Stained gels were quantified using ImageJ v1.37 software. A representative stained zymography gel is shown on the left. The positive control samples were not loaded immediately adjacent to adhesion-induced samples. For ease of viewing, the 2 parts of the gel were pasted here, as indicated by a dotted line. Pooled data from 3 independent experiments are plotted (data ± SEM) on the right. (C) Adhesion and spreading. Neutrophils were prepared as in panel A, and 5 × 106 cells were plated into 24-well plates that had been coated with 20 μg/mL of polyRGD, left to adhere for 10 minutes, and fixed. In washed plates, adherent (phase dark) and spread cells (phase light) were counted in 4 randomly chosen fields of view from each individual experiment, and the percentage of spread cells of total numbers was determined. Example photos are shown on the left; on the right are plotted data combined from 3 independent experiments (data ± SEM). Statistical analysis was by paired t tests performed on the raw data. *P < .05; **P < .01; ***P < .001. (D) Ex vivo flow chamber studies were performed with tamoxifen-induced Arap3fl/flERT2Cre+ (iko) and wild-type animals (wt) using microflow chambers (0.4 × 0.04 mm) coated with rmE-selectin (20 μg/mL), rmCXCL1 (15 μg/mL), and rmICAM-1 (15 μg/mL). Flow chambers were recorded for 10 minutes, and adhesion efficiency (adherent cells/mm2 × WBC) was calculated (data ± SEM; n = at least 5 chambers per group).
In neutrophils, integrin outside-in signaling leads to degranulation, firm adhesion, and spreading. To determine whether ARAP3 is involved in these functions, we plated mock- and tamoxifen-induced Arap3fl/flERT2Cre+ neutrophils in hiFCS-blocked and fibrinogen-coated plastic wells and quantified gelatinase granule release. Gelatinase granule release was increased in tamoxifen-induced Arap3fl/flERT2Cre+ neutrophils (Figure 3B and supplemental Figure 2B), but not in controls, upon plating the cells on fibrinogen. Control stimulation with a powerful soluble stimulus (fMLF in combination with cytochalasin B) did not lead to an increased release of gelatinase granules in ARAP3-deficient cells (Figure 3B and supplemental Figure 2B), indicating that the cellular machinery required for granule release was unaffected.
We analyzed cell adhesion and spreading 10 minutes after plating neutrophils onto dishes coated with integrin ligands and found increased adhesion and spreading of tamoxifen-induced Arap3fl/flERT2Cre+ neutrophils compared with controls (Figure 3C shows adhesion and spreading after plating onto polyRGD and supplemental Figure 2C shows the controls). The same trend was observed when cells were plated onto fibrinogen (data not shown).
We analyzed adhesion of peripheral blood neutrophils to immobilized E-selectin, ICAM1, and CXCL1 ex vivo in a flow chamber system,25 a setup that represents a more accurate reflection of the situation of neutrophils in the bloodstream because of the constant shear forces. ARAP3-deficient cells again adhered better to the coated chamber surface (Figure 3D). Our results show that intracellular events downstream of integrin activation were up-regulated in Arap3−/− neutrophils (outside-in signaling).
Loss of ARAP3 regulates β2 integrin affinity and avidity but not surface expression
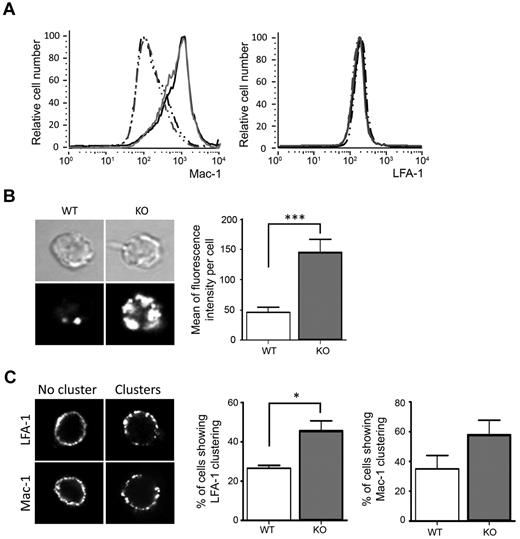
We focused on the 2 major neutrophil β2 integrins, LFA-1 and Mac-1, to identify the reason for the observed increased responses of adhesion-dependent neutrophil functions. The total amount of Mac-1 in neutrophils that did or did not contain ARAP3 was unaltered (supplemental Figure 3A). Similarly, surface LFA-1 and Mac-1 on neutrophils that had or had not been prestimulated with TNFα was nearly identical on tamoxifen- and mock-induced Arap3fl/flERT2Cre+ neutrophils (Figure 4A), suggesting that the differences in biologic activities observed were not due to an increased number of surface integrins.
Higher affinity and avidity of β2 integrins on ARAP3-deficient neutrophils. (A) Surface Mac-1 and LFA-1 were analyzed by FACS from flushed bone marrow cells from mock- and tamoxifen-induced Arap3fl/flERT2Cre+ mice that had or had not been prestimulated with 20 ng/mL of TNFα at 37°C before being labeled with PE-conjugated anti-GR1, APC-conjugated anti–Mac-1, and FITC-conjugated anti–LFA-1. For FACS analysis, GR1-positive cells were gated and Mac-1 and LFA-1 staining was measured; results were analyzed using FlowJo v6.4.7 software. Cells from 27 tamoxifen- and mock-injected mice were analyzed in 5 separate experiments. Representative traces are shown. Gray lines represent cells from tamoxifen-induced and black lines from mock-induced Arap3fl/flERT2Cre+ mice; broken lines represent unstimulated samples and full lines TNFα-stimulated samples. (B) Binding of unstimulated, bone marrow–derived neutrophils from mock-induced (WT) or tamoxifen-induced (KO) Arap3fl/flERT2Cre+ mice to ICAM1-Fc in solution as a readout of affinity was measured by quantitative immunofluorescence using an FV1000 confocal microscope (Olympus) with a 60× objective, as detailed in “Analysis of β2 integrins.” Mean fluorescence intensity of 88 WT and 148 KO cells pooled from 3 separate experiments are plotted (right) and representative examples are shown (left). (C) Mac-1 and LFA-1 distribution in unstimulated neutrophils in suspension was visualized microscopically. Forty cells were analyzed for each condition in each of 3 experiments; representative examples are shown (left). Cells were scored for integrin clustering as a readout for high avidity status. Integrated values obtained from 3 separate experiments are shown graphically (see also supplemental Figure 3 for an analysis of integrin clustering using ImageJ v1.37 software). (B-C) Statistical analysis by paired t test. *P < .05; ***P < .001.
Higher affinity and avidity of β2 integrins on ARAP3-deficient neutrophils. (A) Surface Mac-1 and LFA-1 were analyzed by FACS from flushed bone marrow cells from mock- and tamoxifen-induced Arap3fl/flERT2Cre+ mice that had or had not been prestimulated with 20 ng/mL of TNFα at 37°C before being labeled with PE-conjugated anti-GR1, APC-conjugated anti–Mac-1, and FITC-conjugated anti–LFA-1. For FACS analysis, GR1-positive cells were gated and Mac-1 and LFA-1 staining was measured; results were analyzed using FlowJo v6.4.7 software. Cells from 27 tamoxifen- and mock-injected mice were analyzed in 5 separate experiments. Representative traces are shown. Gray lines represent cells from tamoxifen-induced and black lines from mock-induced Arap3fl/flERT2Cre+ mice; broken lines represent unstimulated samples and full lines TNFα-stimulated samples. (B) Binding of unstimulated, bone marrow–derived neutrophils from mock-induced (WT) or tamoxifen-induced (KO) Arap3fl/flERT2Cre+ mice to ICAM1-Fc in solution as a readout of affinity was measured by quantitative immunofluorescence using an FV1000 confocal microscope (Olympus) with a 60× objective, as detailed in “Analysis of β2 integrins.” Mean fluorescence intensity of 88 WT and 148 KO cells pooled from 3 separate experiments are plotted (right) and representative examples are shown (left). (C) Mac-1 and LFA-1 distribution in unstimulated neutrophils in suspension was visualized microscopically. Forty cells were analyzed for each condition in each of 3 experiments; representative examples are shown (left). Cells were scored for integrin clustering as a readout for high avidity status. Integrated values obtained from 3 separate experiments are shown graphically (see also supplemental Figure 3 for an analysis of integrin clustering using ImageJ v1.37 software). (B-C) Statistical analysis by paired t test. *P < .05; ***P < .001.
Integrin ligand binding affinity and avidity are regulated by inside-out signaling. Integrins reversibly change their tertiary structure between low-, intermediate-, and high-affinity status, and they reversibly cluster, modulating their binding avidity.34 To compare β2 integrin affinity of neutrophils from tamoxifen- or mock-induced Arap3fl/flERT2Cre+ mice in the absence of affinity status–specific antibodies for murine β2 integrins, we allowed cells to bind to ICAM1 in solution and analyzed binding using quantitative immunofluorescence on cells that had settled on glass slides after fixation. ARAP3-deficient cells bound to ICAM1 significantly more strongly than controls (Figure 4B), whereas cells isolated from tamoxifen and mock-induced Arap3+/+ERT2Cre+ mice behaved in the same manner (supplemental Figure 3B). We next analyzed the avidity of LFA-1 and Mac-1 by staining neutrophils kept in solution using antibodies for these surface integrins. After fixation, cells were allowed to settle on slides for microscopic analysis. LFA-1 was significantly more clustered in ARAP3-deficient neutrophils than in controls (Figure 4C and supplemental Figure 3C); this trend was also observed for Mac-1, whereas cells from tamoxifen- and mock-induced Arap3+/+ERT2Cre+ mice displayed similar distributions of both LFA-1 and Mac-1 (supplemental Figure 3D). In summary, β2 integrin affinity and avidity were increased in ARAP3-deficient neutrophils, indicating increased inside-out signaling.
ARAP3 regulates chemotaxis
We analyzed chemotaxis in neutrophils that did or did not express ARAP3. We initially performed “needle chemotaxis” assays in which a chemoattractant-filled micropipette coupled to a microinjector set to a constant, slow stream was inserted into a glass-bottomed dish containing adherent neutrophils, causing the formation of a steep gradient of chemoattractant. Control neutrophils adopted a polarized shape and migrated consistently toward the tip of the micropipette, but neutrophils from tamoxifen-induced Arap3fl/flERT2Cre+ mice were unable to chemotax toward fMLF in this assay (Figure 5A and supplemental Videos 1-2; controls are shown in supplemental Figure 4A). These neutrophils extended a pseudopod in one direction for a short time, then retracted this pseudopod and generated another one in a different direction and so forth, making little if any net progress in any direction in the process. This indicated that neutrophils lacking ARAP3 had a severe chemotaxis defect, although they clearly reacted to being stimulated with fMLF.
ARAP3 controls integrin-dependent chemotaxis. Bone marrow–derived neutrophils were prepared from mock-induced (WT) and tamoxifen-induced (KO) Arap3fl/flERT2Cre+ mice as detailed in “Neutrophil purification.” (A) Cells were allowed to adhere to glass-bottomed dishes before dishes were filled with buffer and a micropipette filled with 1μM fMLF in buffer was inserted. A slow, constant flow of fMLF was initiated using a microinjection system, and cell movements were followed by phase-contrast time-lapse imaging over 30 minutes, taking an exposure every 30 seconds using a Zeiss Axiovert 200 microscope with a 32× objective and Axiovision v3.0 software. The needle was moved away from the cells when they threatened to migrate into the micropipette and potentially block it (WT cells only). Individual cells were tracked using the manual tracking plug-in for ImageJ. The final images of representative videos containing the tracks are shown. For the WT cells, asterisks indicate previous positions of the micropipette tip. (B-D) Chemotaxis in Dunn chambers. Cells were allowed to migrate toward 300nM fMLF in Dunn chambers and their movements were recorded by time-lapse imaging using 5× magnification. (B) Pooled tracks of individual cells obtained from experiments carried out on 3 separate days with separate cell preparations were plotted using the Ibidi chemotaxis tool plug-in for ImageJ. The source of fMLF is at the bottom. The tracks were analyzed using the chemotaxis tool's statistics feature. Accumulated and Euclidean distances (C) as well as cell speed (D) of WT and KO cells are plotted (mean ± SEM). Data were analyzed using t tests (Mann-Whitney), and differences were found to be statistically significant (P < .001). (E-G) Neutrophils from mock-induced (WT) and tamoxifen-induced (KO) Arap3fl/flERT2Cre+ mice were used in chemotaxis assays in the EZ-TAXIS chamber. Cells were lined up at the bridge of an EZ-TAXIS chamber and allowed to migrate toward 3μM fMLF while being followed by time-lapse imaging using a BD pathway reflection microscopy system. (E) Cell movements were tracked and analyzed as for Dunn chamber chemotaxis; cell tracks from pooled cells tracked in 2 separate experiments are shown. (F-G) Tracks were analyzed using the statistical features of the Ibidi chemotaxis tool, and plotted results show pooled data from 3 experiments performed on separate days with separate cell preparations. Accumulated and Euclidean distances traveled by WT and KO cells (left) and their cell speeds were significantly different, as shown by t test (P < .001). (H) Transwell chemotaxis. Flushed bone marrow cells from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice were placed in the top well of polycarbonate transwells with 3-μm pores. The bottom chambers contained the indicated concentrations of fMLF. Cells that migrated through the filters in 40 minutes were counted. Graph shows pooled data (mean ± SEM) obtained from 3 individual experiments performed on separate days with separate cell preparations. Stimulation with fMLF caused significant activation of chemotaxis (P < .0001), whereas there was no significant difference between WT and KO cells (P = .5098; 2-way ANOVA with Bonferroni post test). (J-K) Chemotaxis in a 3D collagen matrix. Bone marrow neutrophils from tamoxifen-induced (KO) and mock-induced (WT) Arap3fl/flERT2Cre+ mice were mixed with type I collagen in custom-made chambers. After allowing the collagen to polymerize, creating a 3 mg/mL matrix, gels were overlaid with 0.5μM fMLF and the chambers sealed. Gradients were allowed to develop and chemotaxing cells were followed by time-lapse imaging for 15 minutes, taking an exposure every 30 seconds. Cells were tracked through the frames using the manual tracking and chemotaxis tools plug-ins in ImageJ v1.37. The tracks in panel J represent pooled tracks from randomly chosen cells from 3 independent runs performed in one day. The graph in panel K represents the pooled accumulated and Euclidean distances traveled by the randomly tracked cells from 3 independent runs of WT and KO cells in experiments performed on 3 separate days with separate cell preparations (mean ± SEM). Data were analyzed using t test (Mann-Whitney); **P < .01.
ARAP3 controls integrin-dependent chemotaxis. Bone marrow–derived neutrophils were prepared from mock-induced (WT) and tamoxifen-induced (KO) Arap3fl/flERT2Cre+ mice as detailed in “Neutrophil purification.” (A) Cells were allowed to adhere to glass-bottomed dishes before dishes were filled with buffer and a micropipette filled with 1μM fMLF in buffer was inserted. A slow, constant flow of fMLF was initiated using a microinjection system, and cell movements were followed by phase-contrast time-lapse imaging over 30 minutes, taking an exposure every 30 seconds using a Zeiss Axiovert 200 microscope with a 32× objective and Axiovision v3.0 software. The needle was moved away from the cells when they threatened to migrate into the micropipette and potentially block it (WT cells only). Individual cells were tracked using the manual tracking plug-in for ImageJ. The final images of representative videos containing the tracks are shown. For the WT cells, asterisks indicate previous positions of the micropipette tip. (B-D) Chemotaxis in Dunn chambers. Cells were allowed to migrate toward 300nM fMLF in Dunn chambers and their movements were recorded by time-lapse imaging using 5× magnification. (B) Pooled tracks of individual cells obtained from experiments carried out on 3 separate days with separate cell preparations were plotted using the Ibidi chemotaxis tool plug-in for ImageJ. The source of fMLF is at the bottom. The tracks were analyzed using the chemotaxis tool's statistics feature. Accumulated and Euclidean distances (C) as well as cell speed (D) of WT and KO cells are plotted (mean ± SEM). Data were analyzed using t tests (Mann-Whitney), and differences were found to be statistically significant (P < .001). (E-G) Neutrophils from mock-induced (WT) and tamoxifen-induced (KO) Arap3fl/flERT2Cre+ mice were used in chemotaxis assays in the EZ-TAXIS chamber. Cells were lined up at the bridge of an EZ-TAXIS chamber and allowed to migrate toward 3μM fMLF while being followed by time-lapse imaging using a BD pathway reflection microscopy system. (E) Cell movements were tracked and analyzed as for Dunn chamber chemotaxis; cell tracks from pooled cells tracked in 2 separate experiments are shown. (F-G) Tracks were analyzed using the statistical features of the Ibidi chemotaxis tool, and plotted results show pooled data from 3 experiments performed on separate days with separate cell preparations. Accumulated and Euclidean distances traveled by WT and KO cells (left) and their cell speeds were significantly different, as shown by t test (P < .001). (H) Transwell chemotaxis. Flushed bone marrow cells from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice were placed in the top well of polycarbonate transwells with 3-μm pores. The bottom chambers contained the indicated concentrations of fMLF. Cells that migrated through the filters in 40 minutes were counted. Graph shows pooled data (mean ± SEM) obtained from 3 individual experiments performed on separate days with separate cell preparations. Stimulation with fMLF caused significant activation of chemotaxis (P < .0001), whereas there was no significant difference between WT and KO cells (P = .5098; 2-way ANOVA with Bonferroni post test). (J-K) Chemotaxis in a 3D collagen matrix. Bone marrow neutrophils from tamoxifen-induced (KO) and mock-induced (WT) Arap3fl/flERT2Cre+ mice were mixed with type I collagen in custom-made chambers. After allowing the collagen to polymerize, creating a 3 mg/mL matrix, gels were overlaid with 0.5μM fMLF and the chambers sealed. Gradients were allowed to develop and chemotaxing cells were followed by time-lapse imaging for 15 minutes, taking an exposure every 30 seconds. Cells were tracked through the frames using the manual tracking and chemotaxis tools plug-ins in ImageJ v1.37. The tracks in panel J represent pooled tracks from randomly chosen cells from 3 independent runs performed in one day. The graph in panel K represents the pooled accumulated and Euclidean distances traveled by the randomly tracked cells from 3 independent runs of WT and KO cells in experiments performed on 3 separate days with separate cell preparations (mean ± SEM). Data were analyzed using t test (Mann-Whitney); **P < .01.
For a more quantifiable response, we performed chemotaxis assays in Dunn chambers. For these assays, cells moved on a “bridge” through a 20-μm-deep gap between 2 glass surfaces in a shallow gradient of chemoattractant.35 Unexpectedly, Arap3−/− neutrophils were able to move directionally in Dunn chambers (Figure 5B). However, tracking the chemotaxing cells and analyzing the tracks confirmed that neutrophils from tamoxifen-induced Arap3fl/flERT2Cre+mice had a significantly reduced ability to chemotax toward fMLF in terms of Euclidean distance (the shortest distance between the start and end points) and total accumulated distance covered, and also in their speed (Figure 5C-D). As expected, there were no significant differences between neutrophils from tamoxifen- or mock-induced Arap3+/+ERT2Cre+ control mice in the Dunn chamber assays (supplemental Figure 4B).
Neutrophils from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice in EZ-TAXIS chambers36 in which cells were lined up at a narrow gap (5-μm depth) through which they squeezed when exposed to a shallow gradient of chemoattractant (fMLF; Figure 5E) exhibited the same trend. Interestingly, the differences between neutrophils from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice chemotaxing in EZ-TAXIS chambers were more subtle than in those measured in the Dunn chamber assays (Figure 5F-G).
Several in vitro studies with neutrophils lacking integrins21,37,38 or in which integrin function had been inhibited using antibodies or chelators39 have shown that integrins are required for migration on 2D but not in 3D substrates (or when “chimneying” between close glass surfaces). To our knowledge, this issue has not been addressed in depth in cells with increased integrin activity. We wondered whether the different results we obtained in the different chemotaxis assays might reflect the fact that we were measuring different mixtures of integrin-dependent and integrin-independent chemotaxis. We reasoned that needle chemotaxis assayed highly integrin-dependent chemotaxis on a 2D glass coverslip, whereas Dunn and EZ-TAXIS chambers, both of which analyze chemotaxis of cells squeezing through more or less narrow gaps, might assay mixtures of 2D and 3D chemotaxis. To determine whether ARAP3 might be most important for integrin-dependent chemotaxis, we performed chemotaxis assays in integrin-independent setups in which cells migrated in 3D substrates. We measured chemotaxis toward fMLF in Boyden chambers (transwells), in which the cells squeeze through 3-μm polycarbonate pores, which has previously been documented as being integrin independent,40 and found no significant differences between ARAP3-deficient and control neutrophils (Figure 5H). We also analyzed neutrophil chemotaxis in a 3D collagen matrix (Figure 5J), which has previously been shown to support chemotaxis of entirely integrin-deficient leukocytes.21 Interestingly, analysis of tracks from neutrophils from tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice migrating in the collagen gels indicated that there was no difference in the total accumulated distances migrated (or in the speed, data not shown) in the 2 groups of cells, but Euclidean distances were different (Figure 5K). Hence, ARAP3-deficient cells ended up significantly nearer to their starting points than controls, suggesting a directionality defect in ARAP3-deficient cells. Neutrophils from tamoxifen- or mock-induced Arap3+/+ERT2Cre+ mice behaved identically (supplemental Figure 4C).
In summary, our experiments show that ARAP3-deficient neutrophils displayed a context-dependent chemotaxis defect in vitro. Our data suggest an integrin-dependent component to ARAP3-regulated chemotaxis, which affects the total distance traveled by the cells in a substrate-dependent fashion. They also suggest an additional directional chemotaxis defect in neutrophils lacking ARAP3, which appears to be integrin independent.
ARAP3 regulates adhesion in vivo
During inflammation, the recruitment of neutrophils that circulate in the bloodstream in a quiescent, nonadhesive state follows a well-established cascade of adhesion and activation steps,41 starting with the capture of free-flowing neutrophils to the inflamed endothelium, followed by rolling along the endothelium. Both capture and rolling are mediated by selectins. During rolling, endothelial-expressed chemokines, together with selectin-mediated signaling events, trigger the activation of neutrophil-expressed β2 integrins (inside-out signaling), which leads to further slowing down and eventually to firm neutrophil arrest on the endothelium. This is followed by steady strengthening of the adhesive contacts and neutrophil spreading along the endothelium, postarrest steps that require interactions between neutrophil integrins and their endothelial ligands (outside-in-signaling). Finally, neutrophils start crawling along the luminal surface of the inflamed endothelium in search of an appropriate exit point out into the tissue.
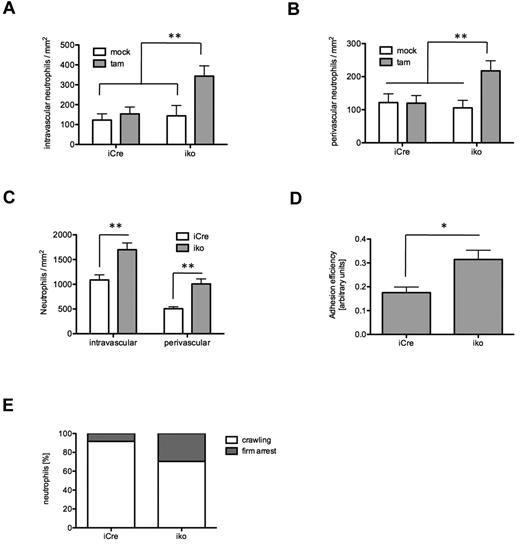
To address the relevance of our observations in vivo, we analyzed neutrophil recruitment in mouse cremaster muscle venules in the absence of ARAP3. Because our in vitro assays suggested a preactivated state of β2 integrins in ARAP3-deficient neutrophils, we evaluated baseline adhesion and extravasation of neutrophils in unstimulated cremaster muscle whole mounts. To prevent trauma-induced inflammatory changes, tamoxifen- and mock-induced Arap3fl/flERT2Cre+ and Arap3+/+ERT2Cre+ mice were killed before preparation of the cremaster muscle. In agreement with the previous in vitro adhesion and flow chamber assays, intravascular adhesion was increased in tamoxifen-induced Arap3fl/flERT2Cre+ animals compared with controls, which exhibited low numbers of adherent intravascular neutrophils under these conditions (Figure 6A). In addition, the baseline number of perivascular neutrophils was also found to be higher in ARAP-3–deficient animals compared with controls (Figure 6B and supplemental Figure 5). These findings corroborate our in vitro data indicating that deletion of ARAP3 causes preactivation of neutrophils.
ARAP3 regulates neutrophil adhesion in vivo. Cremaster muscle experiments were performed on tamoxifen- and mock-induced Arap3fl/flERT2Cre+ (iko) and Arap3+/+ERT2Cre+ (iCre) bone marrow chimeras. Baseline levels of intravascular neutrophil adhesion (A) and neutrophil extravasation (B) were histologically evaluated in untreated, Giemsa-stained cremaster muscle whole mounts (13-17 vessels per group). (C) TNFα (500 ng intrascrotal)–stimulated neutrophil recruitment was assessed in cremaster muscle whole mounts of tamoxifen-induced Arap3+/+ERT2Cre+ (22 vessels in 3 animals) and Arap3fl/flERT2Cre+ mice (37 vessels in 4 mice) by quantification of intravascular and extravascular leukocytes 3 hours after injection. (D) Intravital microscopy of the TNFα-stimulated cremaster muscle was performed to assess adhesion efficiency—(adherent cells/mm2)/systemic WBC count—in postcapillary venules of tamoxifen-induced Arap3+/+ERT2Cre+ (11 vessels in 3 animals) and Arap3fl/flERT2Cre+ mice (18 vessels in 5 animals). (E) For evaluation of intraluminal crawling, time-lapse intravital microscopy of cremaster muscle venules (at least 3 mice per group) were recorded via CCD camera (20 frames per minute) and analyzed offline using the manual tracking plug-in of ImageJ. All data are given as means ± SEM. Data were analyzed using Kruskal-Wallis 1-way ANOVA on ranks with the Dunn post hoc test for multiple comparison or the Mann-Whitney rank-sum test for pairwise comparison. *P < .05; ** P < .01. (Detailed information on microcirculatory parameters of intravital experiments is provided in supplemental Table 1.)
ARAP3 regulates neutrophil adhesion in vivo. Cremaster muscle experiments were performed on tamoxifen- and mock-induced Arap3fl/flERT2Cre+ (iko) and Arap3+/+ERT2Cre+ (iCre) bone marrow chimeras. Baseline levels of intravascular neutrophil adhesion (A) and neutrophil extravasation (B) were histologically evaluated in untreated, Giemsa-stained cremaster muscle whole mounts (13-17 vessels per group). (C) TNFα (500 ng intrascrotal)–stimulated neutrophil recruitment was assessed in cremaster muscle whole mounts of tamoxifen-induced Arap3+/+ERT2Cre+ (22 vessels in 3 animals) and Arap3fl/flERT2Cre+ mice (37 vessels in 4 mice) by quantification of intravascular and extravascular leukocytes 3 hours after injection. (D) Intravital microscopy of the TNFα-stimulated cremaster muscle was performed to assess adhesion efficiency—(adherent cells/mm2)/systemic WBC count—in postcapillary venules of tamoxifen-induced Arap3+/+ERT2Cre+ (11 vessels in 3 animals) and Arap3fl/flERT2Cre+ mice (18 vessels in 5 animals). (E) For evaluation of intraluminal crawling, time-lapse intravital microscopy of cremaster muscle venules (at least 3 mice per group) were recorded via CCD camera (20 frames per minute) and analyzed offline using the manual tracking plug-in of ImageJ. All data are given as means ± SEM. Data were analyzed using Kruskal-Wallis 1-way ANOVA on ranks with the Dunn post hoc test for multiple comparison or the Mann-Whitney rank-sum test for pairwise comparison. *P < .05; ** P < .01. (Detailed information on microcirculatory parameters of intravital experiments is provided in supplemental Table 1.)
To further analyze the functional relevance of ARAP3 in vivo under inflammatory conditions and to exclude a possible effect of ARAP3 in the endothelial compartment, we evaluated neutrophil adhesion and extravasation in TNFα-stimulated cremaster muscle whole mounts of tamoxifen-induced Arap3fl/flERT2Cre+ and Arap3+/+ERT2Cre+ bone marrow chimeras. Stimulation with TNFα resulted in an increased recruitment of neutrophils in both groups compared with baseline conditions. However, the number of ARAP3-deficient, adherent intravascular neutrophils was significantly higher than that of controls. There were also more perivascular ARAP3-deficient cells (Figure 6C). We next directly evaluated intravascular adhesion in postcapillary venules of the cremaster muscle by intravital microscopy. Microvascular and hemodynamic parameters were similar between the groups (supplemental Table 1). These experiments showed an increased adhesion efficiency using the equation (adherent cells/mm2)/systemic WBCs in ARAP3-deficient neutrophils compared with controls (Figure 6D). More adherent ARAP3-deficient neutrophils stayed firmly attached and showed a decreased tendency to crawl intraluminally compared with controls (Figure 6E and supplemental Videos 3-4). In agreement with observations from chemotaxis assays in 3D matrices in vitro, extravascular ARAP3-deficient cells were clearly able to migrate interstitially.
ARAP3 does not modulate Rap activity, but affects RhoA in neutrophils
Rap1 has previously been shown to modulate integrin affinity and avidity in several settings.2 We measured Rap1 activity in neutrophils that were kept in suspension or that had been allowed to adhere to polyRGD. We saw a robust activation of Rap1 on plating cells on polyRGD, but there was no significant difference in Rap1 activity between cells lacking ARAP3 and controls (Figure 7A). This indicated that Rap1 activity was not affected by the presence or absence of ARAP3 in neutrophils.
Assaying activities of small GTPases. Bone marrow–derived neutrophils were prepared from tamoxifen-induced (KO) and mock-induced (WT) Arap3fl/flERT2Cre+ mice. Neutrophils were or were not plated onto polyRGD. Ten minutes later, cells were lysed using ice-cold lysis buffer. (A-B) Clarified lysates were used for pull-down assays to determine GTP-bound fractions of small GTPases: Rap1 pull-downs using GST-Ral GDS beads are shown in panel A and Arf6 pull-downs using GST-MT2 beads are shown in panel B. After incubation of baits with lysate, beads were washed, boiled in sample buffer, and proteins were subjected to SDS-PAGE and immunoblotted using anti-Rap1 (A) and anti-Arf6 (B) antibodies. Graphs show pooled data (means ± SEM) obtained from 3 independent experiments. Photographs of representative experiments are shown. Two different exposure lengths of the same blot are shown in the Arf6 pull-down panel because there was a large difference between unstimulated and stimulated samples (dotted line). For the same reason, the 2 conditions were quantified independently. (C) Clarified lysates were used for RhoA G-LISA assays. To allow comparison of 4 independent experiments, suspension readings were equalized (left graph). Statistical analyses were carried out with the raw data using 2-way ANOVA with Bonferroni post tests or paired t tests for pairwise comparisons. *P < .05.
Assaying activities of small GTPases. Bone marrow–derived neutrophils were prepared from tamoxifen-induced (KO) and mock-induced (WT) Arap3fl/flERT2Cre+ mice. Neutrophils were or were not plated onto polyRGD. Ten minutes later, cells were lysed using ice-cold lysis buffer. (A-B) Clarified lysates were used for pull-down assays to determine GTP-bound fractions of small GTPases: Rap1 pull-downs using GST-Ral GDS beads are shown in panel A and Arf6 pull-downs using GST-MT2 beads are shown in panel B. After incubation of baits with lysate, beads were washed, boiled in sample buffer, and proteins were subjected to SDS-PAGE and immunoblotted using anti-Rap1 (A) and anti-Arf6 (B) antibodies. Graphs show pooled data (means ± SEM) obtained from 3 independent experiments. Photographs of representative experiments are shown. Two different exposure lengths of the same blot are shown in the Arf6 pull-down panel because there was a large difference between unstimulated and stimulated samples (dotted line). For the same reason, the 2 conditions were quantified independently. (C) Clarified lysates were used for RhoA G-LISA assays. To allow comparison of 4 independent experiments, suspension readings were equalized (left graph). Statistical analyses were carried out with the raw data using 2-way ANOVA with Bonferroni post tests or paired t tests for pairwise comparisons. *P < .05.
We previously showed that ARAP3 regulates the small GTPases Arf6 and RhoA. We therefore performed activity assays with neutrophils kept in suspension or plated onto polyRGD. Measuring global Arf6-GTP in lysates from these neutrophils, we saw a dramatic activation of Arf6 upon adhesion to polyRGD, but we did not detect any significant differences between the 2 genotypes (Figure 7B). When analyzing RhoA, we saw an increased activity in cells plated onto polyRGD. Although the extent of activation was variable between experiments, the RhoA activation in ARAP3-deficient cells was increased every time (Figure 7C), whereas RhoA activation in neutrophils from tamoxifen- and mock-induced Arap3+/+ERT2Cre+ mice was very similar (supplemental Figure 6). In summary, our data suggest that ARAP3 modulates RhoA in an adhesion-dependent context. These findings are in agreement with our previous work identifying ARAP3 as a Rap-regulated RhoA GAP.11
Discussion
ARAP3, one of 24 PtdIns(3,4,5)P3–binding proteins identified from porcine neutrophils,10 is abundant in human and mouse neutrophils. The present study represents the first characterization of ARAP3 function in these highly specialized immune cells. Because germline deletion of Arap3 is embryonically lethal,14 we used a tamoxifen-inducible, ubiquitous Cre to delete Arap3 conditionally in the adult mouse to efficiently (although transiently) delete Arap3 in bone marrow–derived neutrophils. Our mouse model was unsuitable for any longer-term in vivo experiments such as models of autoimmune diseases. In addition, we observed variable basal peritoneal leukocyte numbers even in intraperitoneally mock-injected control mice, rendering our model unsuitable for assessment of leukocyte recruitment after thioglycollate-induced sterile peritonitis. Given that Cre expression can in some circumstances lead to nonspecific artifacts, tamoxifen induction was kept to a minimum by routinely comparing tamoxifen- and mock-induced Arap3fl/flERT2Cre+ mice with tamoxifen- and mock-induced Arap3+/+ERT2Cre+ mice, controlling for tamoxifen and for Cre expression. None of the phenotypes described for ARAP3-deficient neutrophils were due to tamoxifen or to inducing Cre. However, in agreement with previous studies finding nonspecific effects caused by expression of Cre,42,43 we observed a significant loss of erythrocytes in the bone marrow of tamoxifen-induced Arap3fl/flERT2Cre+ and Arap3+/+ERT2Cre+ mice 12 days after tamoxifen induction (supplemental Figure 7). This side effect was temporary, because no differences in bone marrow erythrocytes were found 6 weeks after induction (data not shown). Because we observed no differences in circulating blood cells, we are confident that this temporary loss of bone marrow erythrocytes had no effect on any of the measurements we carried out.
We noted increased responses with ARAP3-deficient neutrophils in all adhesion-dependent responses that were tested, including in vitro assays carried out with purified bone marrow–derived neutrophils, ex vivo assays in flow chambers, and in vivo observations in the cremaster muscle system. A response was triggered even in ARAP3-deficient, unstimulated cells both in vitro (eg, increased ROS production in response to plating onto fibrinogen in the absence of TNFα and increased gelatinase granule release in cells plated in heat-inactivated FCS-blocked wells) and in vivo (eg, increased baseline adhesion in unstimulated cremaster muscle venules). We measured no differences in surface Mac-1 and LFA-1, the most abundant, constitutively expressed neutrophil integrins. Instead, both the affinity and the avidity of these β2 integrins were significantly increased in cells lacking ARAP3 (inside-out signaling). Our data support 2 conclusions: (1) both inside-out and outside-in signaling could be altered independently of one another, or (2) the increased responses observed in events downstream of integrin ligation could, at least in part, be a direct consequence of the increased inside-out signaling we measured.
In addition to regulating responses that are classically thought of as downstream of integrin ligation, we observed a striking, context-dependent chemotaxis defect in ARAP3-deficient neutrophils. The literature describes integrin-dependent and integrin-independent modes of cell migration. Integrin-dependent migration involves force generation because of integrin-dependent, transient adhesions to a typically 2D substratum, whereas integrin-independent migration involves amoeboid squeezing of cells through narrow pores of a 3D matrix, propelled by the force of a polarized actomyosin cytoskeleton. Relevant experiments have been carried out either with healthy human neutrophils in which integrin function was inhibited, using chelators or blocking antibodies,39 or with cells taken from LAD patients.37 More recently, analysis of chemotaxis with integrin-deficient murine neutrophils supported very convincingly the conclusions drawn in these earlier studies.21,38 Fewer studies have addressed the integrin dependency of chemotaxis in cells with hyperactive integrins, as is the case in ARAP3-deficient neutrophils. We conclude that in ARAP3-deficient neutrophils, integrin-dependent chemotaxis (needle chemotaxis, in which cells migrate on a glass coverslip) is defective because the adhesive contacts formed with the glass coverslip are too tight. Chemotaxis in Dunn or EZ-TAXIS chambers represent intermediates between integrin-dependent and integrin-independent chemotaxis, with an increasing amount of chimneying between the glass coverslips forming the bridge performed by cells the tighter the gap between the 2 coverslips. This explains why we observed a smaller defect with ARAP3-deficient neutrophils in EZ-TAXIS chambers with their narrow gaps (which were previously used to assay neutrophil chemotaxis in the presence of chelating agents40 ) than in Dunn chambers, in which the gap is significantly wider. Finally, chemotaxis in integrin-independent circumstances (eg, in a 3D collagen gel or a polycarbonate transwell in vitro or in interstitial migration in vivo) was not affected in ARAP3-deficient cells in terms of the total accumulated distance traveled by the neutrophils. Therefore, varying integrin dependency in different chemotaxis assays is the most likely explanation for the reproducible yet seemingly contradictory results we obtained with these different chemotaxis assays. We propose that ARAP3 is required for integrin-dependent chemotaxis, but is dispensable for integrin-independent chemotaxis. In addition, our data hint that there may be an additional defect in directionality of chemotaxis. ARAP3-deficient cells failed to persistently polarize in the orientation of the chemoattractant in needle chemotaxis assays. In addition, despite equal total accumulated distances, ARAP3-deficient neutrophils migrated shorter Euclidean distances in 3D matrices. Future work will be directed at establishing the nature of any directionality defect in chemotaxis of ARAP3-deficient neutrophils.
When analyzing cremaster muscle venules, we observed increased numbers of intravascular, but also perivascular, ARAP3-deficient leukocytes, suggesting increased extravasation. However, given the increased adhesion to the endothelium, it is conceivable that transmigration of a fraction of these cells could have masked a mild transmigration defect. Unfortunately, we were unable to address this possibility experimentally because we could not use the sterile peritonitis model.
Having identified PI3K as major regulator of ARAP3,10,14 the phenotype of ARAP3-deficient neutrophils was somewhat surprising. We previously showed that ARAP3 is regulated by Rap-GTP in addition to PI3K.11 Interestingly, Rap proteins have been demonstrated to be major modulators of integrin inside-out signaling in lymphocytes, macrophages, neutrophils, and platelets, affecting integrin affinity and avidity (although not surface expression), ligand binding, and cell spreading.30,44-46 Rap activity was not affected in neutrophils devoid of ARAP3, indicating (in agreement with our previous work11 ) that ARAP3 is not upstream of Rap.
We assessed Arf6-GTP in neutrophils kept in solution or plated onto polyRGD, which led to significant Arf6 activation. We did not notice any differences between ARAP3-deficient neutrophils and controls. Given that ARAP3 is just one of several Arf6 GAPs, and that we analyzed global Arf6 activity, this negative result was not very surprising. It is conceivable that an analysis of localized Arf6 activity would reveal differences that are hidden behind the robust activation of Arf6 on plating neutrophils onto polyRGD.
When assaying RhoA activity, we noticed that the increase measured in ARAP3-deficient cells was subtly increased compared with controls in each experiment (on average, 1.6-fold). RhoA has been shown to regulate leukocyte integrin activation in several situations.47-50 In thymocytes, RhoA was shown to be required for optimal integrin activation by Rap1,51 indicating that RhoA can be part of Rap-dependent inside-out activation of integrins. Given that Rap regulates ARAP3 as a Rho GAP and that ARAP3-deficient neutrophils are characterized by heightened β2 integrin activity and hyperresponsiveness in adhesion-dependent processes, we speculate that Rap activates ARAP3 as a Rho GAP to fine-tune integrin activity in neutrophils. Because we know that PI3K activity is required for ARAP3 activation, it is likely to function as a coincidence detector for PtdIns(3,4,5)P3 and Rap-GTP. It will be interesting to dissect the regulatory inputs of PI3K and Rap into ARAP3 in the future.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Simon Walker for help with image analysis, Nick Ktistakis for the anti-βCOP antibody, Michelle Janas for help with procedures, Anthony Green (Cambridge Institute for Medical Research, Cambridge, United Kingdom) for use of a blood cell counter, and John Ferguson, Su Kulkarni, and Michael Sixt (Institute of Science and Technology, Klosterneuberg, Austria), and Klaus Ley (La Jolla Institute for Allergy and Immunology, La Jolla, CA) for helpful discussions.
This work was supported by the Biotechnology and Biological Sciences Research Council and the Medical Research Council (G0700740). S.V. holds a David Phillips Fellowship (BB/C520712).
Authorship
Contribution: L.G., K.E.A., C.N., M.S., and S.V. designed experiments; L.G., K.E.A., C.N., T.M., L.N., M.S., and S.V. performed experiments; T.L. provided reagents; L.G., C.N., A.S.-P., P.T.H., M.S., L.S., and S.V. analyzed and/or interpreted data; and S.V. and M.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sonja Vermeren (née Krugmann), The Inositide Laboratory, The Babraham Institute, Babraham Research Campus, Cambridge CB22 3AT, United Kingdom; e-mail: sonja.vermeren@bbsrc.ac.uk.
References
Author notes
L.G. and K.E.A. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal