To the editor:
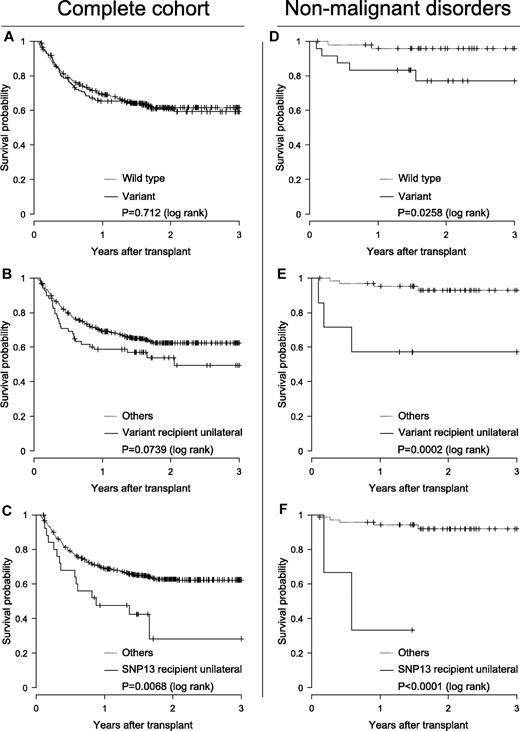
Distinct polymorphisms of NOD2/CARD15 (rs2066844 [SNP08], rs2066845 [SNP12], and rs2066847 [SNP13]) have influence on the incidence of Crohn disease (CD), a chronic inflammatory disorder of the gastrointestinal tract. Similar symptoms of CD and GVHD after allogeneic stem cell transplantation (allo-SCT) inspired Holler and coworkers to initiate a study that actually showed association of these polymorphisms and transplantation outcome.1 Subsequently some groups confirmed unfavorable association,2-5 whereas others did not.6-10 Because the impact of NOD2/CARD15 on SCT is still debatable, we initiated a retrospective multicenter study in pediatric patients (median age 9.8 years [0.2-21 years]) who received allo-SCT. The study was approved by the Goethe-University ethics committee (no. 294/05) and informed consent was obtained according to the Declaration of Helsinki. Genetic variants were analyzed in 567 donor-recipient pairs transplanted between 1996 and 2008. Of these, 446 were HLA-matched and 121 were mismatched. Primary diagnosis comprised hematologic malignancies (n = 472), nonhematologic malignancies (n = 23), and nonmalignant diseases (n = 72). We found polymorphisms in 74 donors (13.1%) and in 70 recipients (12.3%). In 29 (5.1%) cases, both donor and recipient were coincidental variant. The observed genotype frequencies were consistent with the Hardy-Weinberg equilibrium. End points considered in the analysis were overall survival (OS), relapse of disease (REL), treatment-related mortality (TRM), acute GVDH (grades II-IV; II-IV) and chronic GVHD (within day 365). The probability of OS was obtained by the Kaplan-Meier method and cumulative incidences with competing events of TRM, REL, and GVHD according to Kalbfleisch and Prentice using the “survival” and “cmprsk” packages for R 2.9.2 software (www.r-project.org). Differences were tested with the log-rank or the Gray test, respectively. Multivariate analyses were performed using Cox proportional hazard regression analyses of SPSS 15.0, the incidences of categorical parameters were calculated by the Pearson χ2 or the Fisher exact test, and parametric variables were tested by ANOVA or the unpaired t test. The median observation period was 18 months (0.5-119.4 months) and pOS was 61.1% (56.9-65.5%). Clinical risk factors associated with transplantation outcome were diagnoses, stem cell source, HLA match or mismatch, severe acute GVHD, second transplantation, in vivo T-cell reduction, donor leukocyte infusion administration, and type of gastrointestinal decontamination, as revealed by univariate analysis (P < .05). Initial analysis comparing wild-type with variant transplantations did not indicate any association of nod2 variants with adverse outcome. Moreover, coincidental polymorphisms were associated even with favorable outcome, which could be explained by familial aggregation preferably in matched related transplantations. However, detailed analyses of each gene variant with regard to donor- or recipient-sided appearance revealed significant unilateral recipient-side SNP13 association with increased pTRM (P < .01), which remained significant in the multivariate analyses (hazard ratio 2.01 [1.00-4.05], P = .049). The relevance of variants became more apparent for the subcohort of patients with nonmalignant diseases. Albeit comprising heterogeneous diseases, this group was characterized by low exposure to adverse risk factors. OS was significantly affected and decreased in the following order: transplantations with variants, with unilateral recipient-side variants, and with unilateral recipient-side SNP13 (Figure 1).
The impact on survival of nod2 variants. Impact is shown for the complete cohort (n = 576; A-C) and the subcohort of nonmalignant disorders (n = 72; D-F). Kaplan-Meier probability of survival plots as a function of presence ( ) or absence (…) of NOD2/CARD15 polymorphisms (A,D), of presence (
) or absence (…) of NOD2/CARD15 polymorphisms (A,D), of presence ( ) or absence (…) of unilateral recipient-side gene variants (B,E), and of presence (
) or absence (…) of unilateral recipient-side gene variants (B,E), and of presence ( ) or absence (…) of unilateral recipient-side SNP13 (C,F).
) or absence (…) of unilateral recipient-side SNP13 (C,F).
The impact on survival of nod2 variants. Impact is shown for the complete cohort (n = 576; A-C) and the subcohort of nonmalignant disorders (n = 72; D-F). Kaplan-Meier probability of survival plots as a function of presence ( ) or absence (…) of NOD2/CARD15 polymorphisms (A,D), of presence (
) or absence (…) of NOD2/CARD15 polymorphisms (A,D), of presence ( ) or absence (…) of unilateral recipient-side gene variants (B,E), and of presence (
) or absence (…) of unilateral recipient-side gene variants (B,E), and of presence ( ) or absence (…) of unilateral recipient-side SNP13 (C,F).
) or absence (…) of unilateral recipient-side SNP13 (C,F).
In conclusion, we found evidence that NOD2/CARD15 polymorphisms influence outcome after transplantation. However, because statistical correlation was found exclusively for recipient-side polymorphisms, this study does not suggest that NOD2/CARD15 typing might help to optimize donor selection in pediatric allo-SCT.
Authorship
Acknowledgments: This work was supported by the German José Carreras Leukemia Foundation (DJCLS R 06/37v). H. Kreyenberg, A.W., T.K., and P.B. are supported by grants of the LOEWE Zentrum für Zell und Gentherapie Frankfurt am Main.
Contribution: H. Kreyenberg, A.J., C.B., B. Schuster, A.W., T.L.C., E.H., and P.B. designed the study, conducted experimental work, analyzed data, and wrote the manuscript; and B. Strahm, B.K., B.G., A.S., S.B., M.F., C.R., H. Kabisch, P-G.S., D.S., J.F.B., C.M.-K., and T.K. enrolled patients, provided clinical data, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hermann Kreyenberg, JW-Goethe University-Hospital, Centre for Children and Adolescents II/III, Division for Stem Cell Transplantation, Theodor-Stern-Kai 7, 60590 Frankfurt am Main, Germany; e-mail: hermann.kreyenberg@kgu.de.