Abstract
Identification of hematopoietic progenitor cells in the zebrafish (Danio rerio) has been hindered by a lack of functional assays to gauge proliferative potential and differentiation capacity. To investigate the nature of myeloerythroid progenitor cells, we developed clonal methylcellulose assays by using recombinant zebrafish erythropoietin and granulocyte colony-stimulating factor. From adult whole kidney marrow, erythropoietin was required to support erythroid colony formation, and granulocyte colony-stimulating factor was required to support the formation of colonies containing neutrophils, monocytes, and macrophages. Myeloid and erythroid colonies showed distinct morphologies and were easily visualized and scored by their expression of lineage-specific fluorescent transgenes. Analysis of the gene-expression profiles after isolation of colonies marked by gata1:DsRed or mpx:eGFP transgenes confirmed our morphological erythroid and myeloid lineage designations, respectively. The majority of progenitor activity was contained within the precursor light scatter fraction, and more immature precursors were present within the lymphoid fraction. Finally, we performed kinetic analyses of progenitor activity after sublethal irradiation and demonstrated that recovery to preirradiation levels occurred by 14 days after irradiation. Together, these experiments provide the first report of clonal hematopoietic progenitor assays in the zebrafish and establish the number, characteristics, and kinetics of myeloerythroid progenitors during both steady-state and stress hematopoiesis.
Introduction
The majority of cells within the hematopoietic system are postmitotic and relatively short lived, requiring continuous replenishment throughout life. The production of all blood cells is dependent on the actions of hematopoietic stem cells (HSCs), exceedingly rare cells that both self-renew and generate lineage-restricted progenitors. It is through the geometric amplification of these committed progenitors that the vast numbers of mature cells required to sustain life are produced daily. Commitment of HSCs to each of the hematopoietic lineages occurs through a hierarchy of progenitors and precursors, with lineage potential lost with each stepwise differentiation event. The development of mature effector cells from upstream HSCs, multipotent, oligopotent, and unipotent precursors has served as a paradigm for tissue-replenishing stem cell systems.
Whereas long-term reconstitution of lethally irradiated mice remains the standard for HSC function, in vitro culture methods have been instrumental in determining the branchpoints of the hematopoietic tree. The development of clonal in vitro cultures by Metcalf and colleagues in the 1960s enabled the growth of murine bone marrow progenitors1 and the study and quantitation of progenitor number during hematologic disease2 and exposure to irradiation.3 These assays were used to investigate the ontogeny of the developing murine hematopoietic system4 and refined to study human hematopoietic progenitors dysregulated during leukemogenesis.5 Importantly, the use of clonal assays was instrumental for the discovery and validation of colony-stimulating factors (CSFs), secreted proteins that stimulate the specific differentiation of hematopoietic lineages. The ability to isolate, recombinantly produce, and test these factors was a key advance in hematologic research, allowing the sensitive analysis of progenitor differentiation, proliferation, and lineage restriction in the murine and human blood systems. In addition, the clinical use of CSFs has been essential for the treatment of anemia, neutropenia, and thrombocytopenia.
The capability to grow prospective progenitors in vitro and test their differentiation capacity in an unbiased manner has greatly advanced the current understanding of hematopoietic lineage restriction. Prospective isolation of candidate progenitor populations by using antibodies against cell surface markers and FACS, coupled with clonal in vitro analyses, resulted in the identification of multipotent,6,7 oligopotent,6 and monopotent progenitor8,9 intermediates downstream of HSCs in the murine system. In vitro studies with human progenitors have largely validated these findings.10-12
Whereas most investigations of hematopoietic lineage restriction have been performed in mice, precisely how lineage commitment occurs remains somewhat enigmatic. Forward genetic approaches to detect gene functions essential for lineage specification and differentiation may be informative. With the high degree of conservation between the mammalian and teleostean hematopoietic systems, the zebrafish (Danio rerio) may provide a complementary system to make such discoveries through its genetic tractability. Previous mutagenesis screens in zebrafish identified many genes critical for embryonic erythropoiesis.13,14 Ongoing forward genetic screens should similarly reveal undiscovered genetic requirements for the generation of HSCs and their downstream progenitors. Understanding the biology of mutants generated using these approaches, however, requires the development of new assays to ascertain the precise nature of the mutant phenotypes.
To functionally assess the progenitor capability of normal and mutant zebrafish hematopoietic cells, we previously created primary zebrafish kidney stromal (ZKS) cells derived from the main site of hematopoiesis in the adult teleost.15 Culture of hematopoietic progenitor cells on ZKS cells resulted in their continued maintenance15 and differentiation.15-17 This culture system also allowed investigation and chemical rescue of a genetic block in erythroid maturation, confirming the utility of these assays.15 However, because culturing bulk populations of progenitor cells on stroma cannot distinguish between homogeneous multipotent progenitor populations or heterogeneous lineage-restricted populations, it was necessary to investigate the in vitro differentiation potentials of single progenitors by developing sensitive clonal differentiation assays.
In the present study, we describe the first clonal, short-term in vitro assay for zebrafish erythroid and myeloid progenitor cells. We developed methylcellulose culture techniques that support the growth of zebrafish hematopoietic progenitor cells. In addition, we generated recombinant zebrafish cytokines that enable quantification of progenitor number in adults under both steady-state and stress hematopoiesis, enabling a better understanding of hematopoietic stem and progenitor cells in the zebrafish.
Methods
Methylcellulose stock
We prepared 2.0% methylcellulose by adding 20 g of methylcellulose powder (Sigma-Aldrich) to 450 mL of autoclaved H2O, and then we boiled the mixture for 3 minutes. The mixture was allowed to cool to room temperature before adding 2× L-15 powder (Mediatech). The weight of the methylcellulose mixture was adjusted to 1000 g with sterile water. Methylcellulose was allowed to thicken at 4°C overnight before being aliquoted and stored at −20°C.
Methylcellulose clonal assays
Complete methylcellulose medium was prepared by mixing 10 mL of 2.0% methylcellulose stock with 4.9 mL of Dulbecco modified Eagle medium, 2.1 mL of Ham's F-12, 2 mL of embryonic stem cell–qualified fetal bovine serum, 300 μL of HEPES (1M stock), 200 μL of penicillin/streptomycin (5000 U/mL and 5000 μg/mL stock, respectively), 200 μL of l-glutamine (200mM stock), and 40 μL of gentamicin sulfate (50 mg/mL). To perform experiments in triplicate, 3.5 mL of complete methylcellulose was added to round-bottomed 14-mL tubes (BD Biosciences) with 5-mL syringes and 16-gauge needles. Cells were isolated and resuspended in 100 μL of medium15 and added to complete methylcellulose, along with cytokines. To observe separable, individual colonies, cells were resuspended at 1 × 104 to 5 × 104 cells/mL. Carp serum was used at 1%, erythropoietin (Epo) at 0.1 μg/mL, and granulocyte colony-stimulating factor (G-csf) at 0.3 μg/mL. Tubes were tightly capped, and the solution gently vortexed to mix. In triplicate, 1 mL of solution was aliquoted into 35-mm Petri dishes (BD Biosciences). Plates were placed in a humidified 15-cm dish at 32°C and 5% CO2 and removed after 7 days for examination and colony isolation.
Colony-forming unit assays
Hematopoietic colonies were observed and enumerated on a DMI-6000 inverted fluorescent microscope (Leica). Images were processed as described previously.15
Zebrafish stocks and embryos
FACS
Cells were prepared and processed as described previously.20
Cytology
Hematopoietic colonies were plucked from methylcellulose cultures, triturated in PBS, and concentrated by cytocentrifugation at 250g for 5 minutes onto glass slides by using a Shandon Cytospin 4 cytocentrifuge (Thermo Fisher Scientific). Slides were fixed and stained with May-Grünwald Giemsa (Sigma-Aldrich).15
RT-PCR
Generation of recombinant zebrafish cytokines
Recombinant zebrafish Epo was generated as described previously.15 The region corresponding to the mature form of zebrafish G-csf24 was reamplified by PCR from zebrafish heart cDNA by using primers G-csf-forward (5′-CGGCCATGGAGCTCCTGTTCCTCAGAAACTGGA-3′) and G-csf-reverse (5′-CGGCCATGGTCATACACGAATACTGAGGATCCT-3′). The 574-bp product was digested with NcoI, isolated by electrophoresis, and ligated into pETH2a. This expression plasmid was used to transform T7 polymerase-expressing BL21 (DE3) cells. Induction of 19-kDa His-G-csf protein synthesis by isopropyl β-d-thiogalactoside and purification of recombinant protein on a Ni2+-nitrilotriacetic acid agarose column was done according to the manufacturer's procedure (QIAGEN), with modification.25
Irradiation assay
Adult mpx:eGFP; gata1:DsRed double-positive transgenic zebrafish were exposed to 25 Gy of ionizing irradiation from a 137Cs source irradiator as described previously.26 Whole kidney marrow (WKM) was collected at 3, 8, 11, 14, and 21 days after irradiation. The mpx:eGFP; gata1:DsRed double-negative (DN) cells were isolated; added to methylcellulose containing 1% carp serum, 0.1 μg/mL Epo, and 0.3 μg/mL G-csf; and enumerated 7 days after plating.
Microscopy
All images of hemotopoietic colonies (Figures 1B, 3A-B) were taken with a Leica DMI6000B inverted fluorescenct microscope with a 5× objective lens and a 10× eyepiece. Images were captured with a Hamamatsu digital camera (model C7780-20), and processed with Velocity (Version 5.0) software. Assembly of figures was performed with Adobe Photoshop CS. All images of cytocentrifuged, May-Grünwald-Giemsa stained cells (Figure 4A) were taken with an Olympus BX51 upright microscope with a 100× oil objective and a 10× eyepiece. Images were captured with an Olympus DP70 digital camera, and processed with Olympus DP Controller software (Version 2.1.1.183). Assembly of composite image was performed with Adobe Photoshop CS.
Results
Addition of recombinant zebrafish growth factors allows clonal analysis of zebrafish progenitor cells
To develop clonal cultures, we used conditions used in our previous zebrafish stromal cell studies,15 adapting them to semisolid media. To determine whether our methylcellulose cultures would allow colony growth, WKM, the teleostean equivalent of mammalian bone marrow where blood stem and progenitor cells reside, was harvested from mpx:eGFP adult fish. Plating WKM cells in methylcellulose alone resulted in a few small colonies (Figure 1A). The addition of carp serum, shown previously to increase survival and differentiation of zebrafish progenitors cultured on stromal cells,15 had little effect on increasing colony number (Figure 1A); colonies were comprised of only a few cells (Figure 1B).
Zebrafish G-csf and Epo increase colony formation from unfractionated mpx:eGFP WKM. (A) CFUs/100 000 unfractionated WKM cells plated in methylcellulose with combinations of carp serum, G-csf, or Epo added. Bars represent average of at least 3 independent experiments, with error bars representing SD. Black bars represent Mpx:eGFP− colonies and green bars represent Mpx:eGFP+ colonies. (B) Brightfield (top rows) and GFP fluorescent images (bottom rows) of representative colonies enumerated in panel A. All images were taken at 50×. Scale bar in top left panel is 50 μm.
Zebrafish G-csf and Epo increase colony formation from unfractionated mpx:eGFP WKM. (A) CFUs/100 000 unfractionated WKM cells plated in methylcellulose with combinations of carp serum, G-csf, or Epo added. Bars represent average of at least 3 independent experiments, with error bars representing SD. Black bars represent Mpx:eGFP− colonies and green bars represent Mpx:eGFP+ colonies. (B) Brightfield (top rows) and GFP fluorescent images (bottom rows) of representative colonies enumerated in panel A. All images were taken at 50×. Scale bar in top left panel is 50 μm.
We speculated that the semisolid cultures may need more than the growth factors contained in carp serum, so we recombinantly generated the recently described zebrafish G-csf.24 The addition of G-csf caused an increase in colony number (Figure 1A), resulting in small, ruffled colonies reminiscent of the myeloid colonies of mammals. Transgenic mpx:eGFP fish, in which cells express green fluorescent protein (GFP) under the control of the myeloid-specific peroxidase promoter,19 allowed fluorescent visualization of these GFP+ colonies (Figure 1B). Additively, G-csf and carp serum caused a nearly 40-fold increase over carp serum alone and an 8-fold increase over G-csf alone (Figure 1A). Furthermore, the addition of carp serum and G-csf resulted in nearly 100% mpx:eGFP+ colonies (Figure 1A). Colonies had 2 distinct morphologies (Figure 1B; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article): compact colonies with ruffled borders or well-spread colonies that routinely were multicentric. Both colony types were reminiscent of murine granulocytic colonies, and their cellular constituents were uniformly GFP+.
Zebrafish Epo27 was recently identified and shown to stimulate erythroid differentiation in vitro15 . Addition of recombinant Epo to methylcellulose cultures led to an approximately 5-fold expansion of colony number compared with carp serum alone (Figure 1A). Colonies stimulated with Epo had a different appearance than their myeloid counterparts, with a more compact morphology (Figure 1B). More importantly, Epo-stimulated colonies did not express the GFP transgene (Figure 1B; supplemental Figure 1), suggesting that they were not myelomonocytic. Carp serum and Epo together caused a nearly 3-fold expansion of colonies (Figure 1A); colonies were larger and consisted of more cells (Figure 1B). Next, both cytokines and carp serum were combined to determine whether colony number could be increased from WKM. Indeed, addition of all supplements resulted in 720 ± 78 colonies for every 100 000 cells plated (Figure 1A), an approximately 80-fold increase over no cytokines. Importantly, the cells were not all myeloid; approximately 50% of the colonies were GFP+ (Figure 1A-B), with the remainder displaying erythroid-like morphology but not expressing the myeloid-specific transgene (Figure 1B).
Enrichment of stem and progenitor cells from WKM
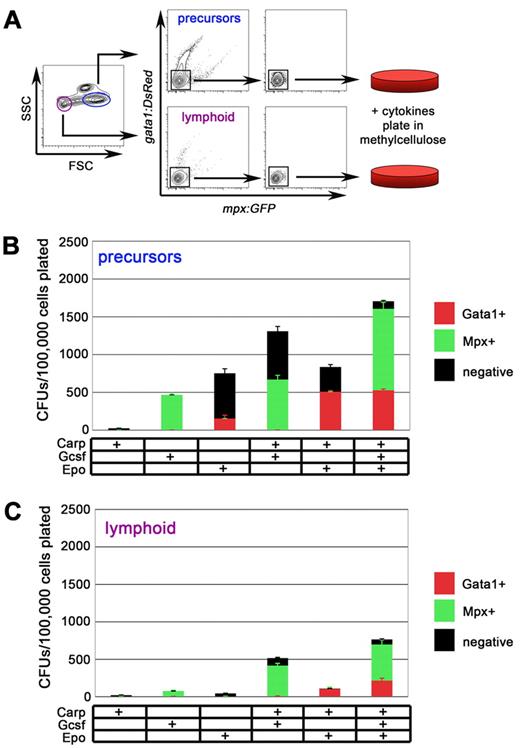
With the culture conditions determined for myeloid and erythroid progenitors, we sought to further purify progenitors from the WKM by light scatter characteristics. Stem and progenitor cells within zebrafish WKM scatter light in characteristic ways.20 HSCs, the keystone cells of the hematopoietic system, are small and agranular, scattering light much like lymphoid cells. Hence, they are contained within a “lymphoid” light scatter population (Figure 2A purple gate).20 More differentiated progenitor cells within zebrafish WKM have been speculated to reside in the “precursor” population, a fraction that contains cells that are more granular and notably larger than HSCs (Figure 2A blue gate). Using mpx:eGFP; gata1:DsRed double transgenic animals, we isolated DN cells from either the lymphoid or the precursor fraction for two reasons. First, erythrocytes can be excluded based on high expression of the erythroid-specific gata1:DsRed transgene. We thus used gata1:DsRed expression to exclude postmitotic erythrocytes that compose up to 50% of the WKM. In addition, we hypothesized that stimulation of DN cells with Epo would induce red fluorescence and stimulation with G-csf would induce green fluorescence, allowing a quantifiable, fluorescence-based readout of myeloerythroid progenitor potential.
Precursor and lymphoid fractions of WKM contain myeloid and erythroid progenitors. (A) Experimental schematic for isolation and culture of mpx:eGFP−; gata1:DsRed− cells from precursor (blue) and lymphoid (purple) fraction of adult WKM. (B) CFUs/100 000 mpx:eGFP−, gata1:DsRed− precursor cells plated in methylcellulose with combinations of carp serum, G-csf, or Epo added. Red bars represent gata1:DsRed+ colonies, green bars represent mpx:eGFP+ colonies, and black bars represent negative colonies generated from the precursor fraction. (C) CFUs/100 000 mpx:eGFP−, gata1:DsRed− lymphoid cells plated in methylcellulose with combinations of carp serum, G-csf, or Epo added. Red bars represent gata1:DsRed+ colonies, green bars represent mpx:eGFP+ colonies, and black bars represent negative colonies generated from the lymphoid fraction. Bars represent average of at least 3 independent experiments, with error bars representing SD.
Precursor and lymphoid fractions of WKM contain myeloid and erythroid progenitors. (A) Experimental schematic for isolation and culture of mpx:eGFP−; gata1:DsRed− cells from precursor (blue) and lymphoid (purple) fraction of adult WKM. (B) CFUs/100 000 mpx:eGFP−, gata1:DsRed− precursor cells plated in methylcellulose with combinations of carp serum, G-csf, or Epo added. Red bars represent gata1:DsRed+ colonies, green bars represent mpx:eGFP+ colonies, and black bars represent negative colonies generated from the precursor fraction. (C) CFUs/100 000 mpx:eGFP−, gata1:DsRed− lymphoid cells plated in methylcellulose with combinations of carp serum, G-csf, or Epo added. Red bars represent gata1:DsRed+ colonies, green bars represent mpx:eGFP+ colonies, and black bars represent negative colonies generated from the lymphoid fraction. Bars represent average of at least 3 independent experiments, with error bars representing SD.
Isolation of DN cells from the precursor fraction resulted in higher numbers of colony-forming units (CFUs) in methylcellulose relative to those from the lymphoid fraction (Figure 2B). Addition of either G-csf or Epo alone resulted in more than 10-fold increases from unfractionated WKM. Colonies stimulated with G-csf or Epo expressed either the myeloid-specific mpx:eGFP or the erythroid-specific gata1:DsRed transgene, respectively. Addition of carp serum to G-csf or Epo increased the total number of colonies generated, but the most CFU numbers were observed when all 3 supplements were added combinatorially to the cultures. Furthermore, the addition of carp serum, G-csf, and Epo permitted visualization of both myeloid (green) and erythroid (red) colonies on the same plate (Figure 2B).
Isolation of DN cells from the lymphoid fraction resulted in similar results, with the highest CFU counts coming from cultures containing carp serum, G-csf, and Epo (Figure 2C). G-csf and carp serum stimulated the formation of mpx:eGFP+ colonies, whereas Epo and carp serum stimulated the formation of gata1:DsRed+ colonies (Figure 2C). However, the colony numbers were markedly lower compared with those generated from the precursor fraction.
To assess whether transgene+ cells contained progenitor activity, we isolated mpx:eGFP+ and gata1:DsRed+ cells from WKM, precursor, or lymphoid fractions, and then we plated cells under optimal conditions. In several experiments we observed only rare, if any, colonies (data not shown). Furthermore, culture of cells from the remaining “myeloid,” “eosinophil,” or “erythroid” light scatter fractions resulted in only a few small colonies, if any. Collectively, these data suggest that the majority of myeloerythroid colony-forming cells are contained within the precursor and lymphoid populations and that they lack appreciable expression of mpx:eGFP and gata1:DsRed transgenes.
Visualization of myeloid and erythroid progenitor differentiation
We speculated that myeloid and erythroid colonies derived from the precursor or lymphoid fractions would be similar when examined for colony morphology, cellular constituents, and gene expression. To address this, we first captured brightfield and fluorescent images of colonies formed under all conditions. As shown in Figure 3A, DN cells plated in methylcellulose from the precursor fraction formed distinct colonies that were distinguishable based on the different cytokines added to the cultures. Similar to unfractionated WKM (Figure 1B), progenitors cultured in G-csf formed small, ruffled colonies. Importantly, plated mpx:eGFP− cells matured into mpx:eGFP+ colonies (Figure 3A). When Epo was added to the cultures, instead of mpx:eGFP+ colonies arising from DN cells, compact gata1:DsRed+ colonies emerged. The addition of carp serum had little effect on the morphology of erythroid cultures (Figure 3A), although it did increase the total number of CFUs formed and the proportion of gata1:DsRed+ colonies present (Figure 2B). Carp serum and G-csf permitted the visualization of GFP+ ruffled and spread colonies in culture (Figure 3A). Finally, the addition of carp serum, G-csf, and Epo in the same cultures resulted in 4 distinct colony types that were easily separable by morphology under brightfield, as well as by expression of lineage-specific transgenes. We visualized tight DsRed+ erythroid colonies, ruffled GFP+ colonies, spread GFP+ colonies, and rare GFP+; DsRed+ mixed colonies (Figure 3A arrowhead). Progenitors isolated from the lymphoid fraction of WKM formed colonies with very similar morphologies (Figure 3B). The only notable difference in these colonies was that they were generally smaller than their precursor counterparts, perhaps because of their lower proliferative capacity.
G-csf encourages myeloid differentiation, whereas Epo encourages erythroid differentiation from zebrafish hematopoietic progenitors. (A) Brightfield images (top row), mpx:eGFP fluorescence (middle row), and gata1:DsRed fluorescence (bottom row) of colonies grown in various growth factor conditions from the precursor fraction of WKM (conditions listed along top row of images). (B) Brightfield images (top row), mpx:eGFP fluorescence (middle row), and gata1:DsRed fluorescence (bottom row) of colonies grown in various growth factor conditions from the lymphoid fraction of WKM (conditions listed along top row of images). All images photographed at magnification 50×. Scale bars in top left panels are 50 μm. Arrowheads in top right panels denote mixed (CFU-granulocyte, erythroid, macrophage colonies) colonies.
G-csf encourages myeloid differentiation, whereas Epo encourages erythroid differentiation from zebrafish hematopoietic progenitors. (A) Brightfield images (top row), mpx:eGFP fluorescence (middle row), and gata1:DsRed fluorescence (bottom row) of colonies grown in various growth factor conditions from the precursor fraction of WKM (conditions listed along top row of images). (B) Brightfield images (top row), mpx:eGFP fluorescence (middle row), and gata1:DsRed fluorescence (bottom row) of colonies grown in various growth factor conditions from the lymphoid fraction of WKM (conditions listed along top row of images). All images photographed at magnification 50×. Scale bars in top left panels are 50 μm. Arrowheads in top right panels denote mixed (CFU-granulocyte, erythroid, macrophage colonies) colonies.
Detailed examination of myeloid and erythroid progenitor differentiation
To verify that DsRed+ colonies were erythroid and that GFP+ colonies were myelomonocytic, we removed, cytocentrifuged, and stained individual colonies for further morphologic examination.
First, compact DsRed+ colonies were isolated from cultures stimulated with carp serum and Epo from the precursor and lymphoid fraction (Figure 4A). Cells isolated from precursor-derived colonies showed erythroid-like morphology, mostly containing dense, stippled chromatin and blastic morphology reminiscent of mammalian erythroblasts (Figure 4A top row, left column). Colonies from the lymphoid fraction contained immature erythroid elements, as well as cells that were lymphoid in appearance (Figure 4A bottom row, left column). In contrast, eGFP+ colonies from cultures stimulated with G-csf and Epo displayed a mixture of myeloid cells. The ruffled colonies from either fraction contained darker-staining myeloid precursor cells and mature neutrophils and monocytes. Examination of the spread colonies from both fractions showed similar cellular constituents, but they differed in the increased number of large, mature macrophages present (Figure 4A right column, top and bottom row).
G-csf encourages myeloid differentiation as assayed by morphology and gene expression of isolated colonies. (A) Cytocentrifuged colonies isolated from precursor (top) and lymphoid (bottom) fraction methylcellulose cultures were stained with May-Grünwald-Giemsa. Tight colonies were isolated from cultures with only carp serum and Epo (left column), whereas ruffled and spread colonies were isolated from cultures containing carp serum and G-csf (right column). All images were photographed at magnification 1000×; scale bar in bottom right is 20 μm. After photographing, cells were cut and pasted from multiple fields to create a composite image. (B) Reverse transcription-PCR analysis of colonies isolated from precursor (top) and lymphoid (bottom) fraction of methylcellulose cultures. Colony morphology is listed on left, and genes assayed are listed along top of gel images.
G-csf encourages myeloid differentiation as assayed by morphology and gene expression of isolated colonies. (A) Cytocentrifuged colonies isolated from precursor (top) and lymphoid (bottom) fraction methylcellulose cultures were stained with May-Grünwald-Giemsa. Tight colonies were isolated from cultures with only carp serum and Epo (left column), whereas ruffled and spread colonies were isolated from cultures containing carp serum and G-csf (right column). All images were photographed at magnification 1000×; scale bar in bottom right is 20 μm. After photographing, cells were cut and pasted from multiple fields to create a composite image. (B) Reverse transcription-PCR analysis of colonies isolated from precursor (top) and lymphoid (bottom) fraction of methylcellulose cultures. Colony morphology is listed on left, and genes assayed are listed along top of gel images.
To examine gene expression within these colonies, we isolated colonies based on morphology from the precursor and lymphoid fractions and subjected them to reverse transcription–PCR analysis (Figure 4B). Cultures stimulated with Epo selectively expressed the erythroid-specific gata1 transcription factor. Neither ruffled nor spread colonies stimulated with G-csf expressed the gata1 gene, instead expressing the myeloid-specific peroxidase gene (mpx) and essential myeloid transcription factor pu.1. Ruffled and spread colonies also expressed the G-csf receptor (gcsfr) and the macrophage colony–stimulating factor receptor (mcsfr), confirming that granulocyte and macrophage precursors and progeny were contained selectively within the myeloid colonies. None of the colonies analyzed showed expression of the lymphoid specific rag1, the T cell–specific lck, or the B cell–specific IgM or pax5 genes (data not shown).
Kinetic analysis of myeloid and erythroid progenitors in response to sublethal irradiation
We speculated that these clonal assays could be used to perform an accurate kinetic analysis of myeloid and erythroid progenitor recovery after whole-body sublethal irradiation of adult zebrafish. Previous studies have shown that after a sublethal dose of 25 Gy, all hematopoietic cells within the kidney are drastically depleted but that they recover over a period of 1 month.26,28,29 These irradiation recovery studies were preformed by analyzing light scatter profiles of WKM cells over time by flow cytometric analysis, and they were validated by cellular morphology. We speculated that we could more accurately assess the recovery of hematopoietic progenitor number with our clonal assays.
To investigate the kinetics of myeloerythroid progenitor recovery, we sublethally irradiated mpx:eGFP; gata1:DsRed double transgenic adult zebrafish at 25 Gy. DN cells were isolated from the lymphoid and precursor WKM light scatter fractions (Figure 2A) at days 3, 8, 11, 14, and 21 after exposure to 25 Gy of ionizing radiation, and we compared these cells with those from nonirradiated fish (day 0). After isolation, cells were plated in methylcellulose with the addition of carp serum, Epo, and G-csf. CFUs were analyzed after 7 days of culture, and all colonies were morphologically similar to those shown in Figure 3. In addition, myelomonocytic colonies were GFP+ and erythroid colonies were DsRed+, in accordance with our previous findings. Analysis of CFU number showed a severe decrease in progenitor activity at 3 days after irradiation (Figure 5A). After 14 days of recovery, myeloerythroid progenitor counts were approximately 50% lower than those of nonirradiated fish; just 7 days later, however, progenitor counts were expanded nearly 3-fold to approximately double the number of preirradiation CFUs (Figure 5A).
Kinetic analysis of myeloerythroid progenitor recovery after sublethal ionizing radiation. (A) CFUs generated from mpx:eGFP; gata1:DsRed DN cells after recovery from 25 Gy irradiation. (B) Ratio of CFUs generated to number of mpx:eGFP; gata1:DsRed DN cells isolated and cultured from the lymphoid (●) and precursor (■) light scatter fractions (as diagrammed in Figure 2A). The x-axis is days after 25 Gy irradiation; day 0 fish were not irradiated. Data points represent the average of 3 individual biologic replicates performed in triplicate, and error bars represent SD.
Kinetic analysis of myeloerythroid progenitor recovery after sublethal ionizing radiation. (A) CFUs generated from mpx:eGFP; gata1:DsRed DN cells after recovery from 25 Gy irradiation. (B) Ratio of CFUs generated to number of mpx:eGFP; gata1:DsRed DN cells isolated and cultured from the lymphoid (●) and precursor (■) light scatter fractions (as diagrammed in Figure 2A). The x-axis is days after 25 Gy irradiation; day 0 fish were not irradiated. Data points represent the average of 3 individual biologic replicates performed in triplicate, and error bars represent SD.
Interestingly, analysis of the ratio of colonies formed to progenitor number isolated indicated that the cells associated with irradiation recovery were located primarily within the lymphoid fraction, from which approximately 80% of plated cells generated either an erythroid or a myeloid colony at 21 days after irradiation (Figure 5B). In addition, these colony types were mainly erythroid, although myeloid and mixed colonies also were increased (supplemental Figure 2A). Whereas the DN cells from the precursor fraction contributed to irradiation recovery, < 5% of the cells plated from this fraction generated erythroid or myeloid colonies at any time point (Figure 5B). Importantly, precursor-derived erythroid, myeloid, and mixed colonies also recovered over the 21 days (supplemental Figure 2B). These data demonstrate that progenitor function can now be precisely quantified using clonal in vitro assays, both during steady state and after hematopoietic stress.
Discussion
The hematopoietic system of higher vertebrates evolved from a common ancestor present in the sea approximately 450 million years ago. The remarkable conservation of hematopoietic lineages, their respective functions, morphologies, and gene-expression programs among disparate vertebrate animals suggest that the fundamental roles played by each lineage were well established in this ancestral precursor.30 Whereas a detailed description of the components and hierarchy of the hematopoietic tree has been well studied in rodents, less attention has been focused on the hematopoietic system of fish. In this report, we have established the basic parameters of hematopoietic progenitor assays in the zebrafish by using a semisolid medium to enable the clonal analyses of myeloid and erythroid progenitors. Clonal assays have been essential in the analyses of mammalian progenitor cells, being used to elucidate the growth factor requirements, proliferative potential, and differentiation potentials of distinct progenitor subsets. Whereas the zebrafish system has been used extensively to investigate the developmental genetics of hematopoiesis, the presence of bona fide progenitor cells has not been formally addressed.
Until recently, it had not been possible to perform clonal semisolid culture assays in the zebrafish system because of a paucity of cytokines to stimulate progenitor cell growth. In our experience, mammalian cytokines have generally not cross-reacted with zebrafish hematopoietic cells, and the identification of zebrafish growth factors has been difficult because of poor sequence homology across evolution. Our previous success in generating and using recombinant zebrafish Epo to expand and differentiate erythroid cells15 on stromal cultures led us to search for additional cytokines that would allow development of clonal assays. With the recent discovery of zebrafish G-csf,24 we reasoned that addition of this factor might be essential to develop multilineage progenitor assays because it is known in mammals to promote the production and survival of granulocytes during normal and stress hematopoiesis.31,32
Although there are very few reports of the in vitro culture of zebrafish hematopoietic cells, other laboratories have cultured fish blood cells. The establishment of goldfish macrophage cell lines,33 rainbow trout macrophage monocyte-like cell lines,34,35 Atlantic salmon macrophage-like cells,36 and carp monocytic cell lines37 has allowed the further investigation of the fish mononuclear phagocytic system. However, there are very few reports describing clonal myeloerythroid progenitor assays. Reports of clonal assays to stimulate B lymphocytes from trout (in soft agar,38 fibrin clots,39 and methylcellulose40 ) and zebrafish B-cell progenitors (in soft agar41 ) have been reported previously, as have clonal assays to examine trout (in soft agar38 and fibrin clots39 ) and carp (in soft agar42 and on stromal cultures43 ) T-cell progenitors. Our previous work using ZKS cells suggested the presence of myeloerythroid progenitors within the zebrafish lymphoid and precursor scatter populations,15 but we could not formally demonstrate the existence of individual progenitors without developing more precise assays.
We thus developed clonal in vitro assays for zebrafish hematopoietic stem and progenitor cells by using an optimized methylcellulose formulation containing recombinant cytokines. As predicted by our previous studies,15,26 we demonstrated the precursor fraction to contain the majority of myeloerythroid CFU activity. Interestingly, we also showed that the lymphoid fraction contains considerable progenitor activity, albeit at 3-fold lower levels than the precursor fraction. We have described previously the presence of some erythroid precursors within the lymphoid scatter fraction.20 These cells, however, are largely made up of small, orthochromatic erythroblasts that are not likely to possess substantial proliferative potential, if similar to their mammalian counterparts. Rather, we believe the CFU activity contained within the lymphoid fraction is because of the presence of rare HSCs. Our previous transplantation studies suggested that all long-term repopulating cells are present within the lymphoid fraction.26 In addition, current estimates using lymphoid-specific transgenic lines generated in our laboratory indicate that approximately 70% of the lymphoid fraction consists of mature B cells, 20% to 25% consists of T and NK cells, and the remainder contains stem and progenitor cells.44 In accord with this finding, progenitor activity within the lymphoid fraction displayed delayed colony-forming kinetics compared with the precursor fraction. For example, although colonies from the progenitor fraction were readily visible by day 5 in culture, lymphoid cultures did not generate visible colonies until at least day 7. In addition, we often noticed that colonies derived from the lymphoid fraction were smaller in size (ie, contained fewer cells) compared with colonies derived from the precursor fraction when analyzed on day 10. Our previous ZKS culture experiments are consistent with these findings.15 The identification of additional zebrafish growth factors that more fully support the differentiation of multipotent hematopoietic progenitors may further distinguish between the CFU potentials contained within each kidney scatter fraction.
Interestingly, the frequency of CFUs generated from zebrafish WKM is comparable to the colony frequency obtained from mouse bone marrow. For example, plating 1 × 105 cells from the bone marrow of C57BL mice generates between 40 and 400 CFUs.45 Although very few studies have been performed in other teleosts, plating identical numbers of WKM cells from rainbow trout generates a range of ∼ 500 to 1600 CFUs, depending on the concentration of trout serum present.40 In our studies, we observed higher numbers than in the mouse but comparable numbers to the trout (720 ± 78). Similar to those in mammals, these colony numbers vary depending on clutch, genetic background, animal age and size, and general health. Age seems to be an important factor in number of progenitors present; experiments in our laboratory indicated that the number of lymphoid and precursor cells in the WKM decreased over time, whereas the number of mature myeloid cells increased (D. Stachura, J. Bertrand, and V. Wittamer, unpublished observation, March, 2010). These findings warrant careful age matching when comparing CFU potential in mutant or diseased animals with wild-type counterparts.
The morphology of zebrafish colonies generated in these clonal assays closely resembles those generated by mouse and human progenitors, showing that colony shape and size is well conserved among zebrafish and mammals. Addition of G-csf to zebrafish progenitors generated ruffled and spread colonies reminiscent of granulocyte macrophage colonies (CFU-GM) in mice46,47 and humans.46 When colonies were stimulated with Epo, we observed compact, tight colonies of maturing erythroid progenitors that (even when grown from nontransgenic wild-type animals) showed a distinctive red hue because of hemoglobinization. Because these erythroid colonies are similar in appearance, size, and cell number to mammalian erythroid CFUs, we term these colonies zebrafish CFU-erythroid (CFU-E). We did not observe colonies resembling mammalian burst-forming unit erythroid colonies, probably because our culture conditions are not optimized for full erythroid proliferation and differentiation. It is also of interest that we observed mixed colonies in our assays, probably the zebrafish equivalents of CFU-granulocyte, erythroid, macrophage colonies (CFU-GEM).
In this study, we used transgenic zebrafish to observe lineage commitment. The isolation and culture of cells negative for DsRed protein driven by the erythroid-specific gata1 promoter showed that only in the presence of Epo did erythropoietic differentiation occur. Similar findings were obtained in mpx:eGFP fish, whereby G-csf specifically encouraged myeloid differentiation. It is important to note that although the mpx:eGFP transgenic animals used in these studies have been reported to specifically mark neutrophils in the zebrafish embryo,19 the mpx transgene also marks other myeloid cell types in adult WKM, because the colonies isolated were uniformly GFP+ and contained neutrophils, monocytes, and macrophages. These data are consistent with the fact that mammalian monocytes express myeloperoxidase (for a review, see van der Veen et al48 ). In addition, human macrophages that localize to atherosclerotic lesions express myeloperoxidase.49 Whereas some GFP expression may result from perdurance of protein expressed in upstream precursor cells, these data collectively suggest that the mpx transgene is expressed outside of the neutrophilic lineage. Further development of these clonal assays and the ability to visualize development in vitro and in real time may help refine our knowledge of lineage-affiliated transgenes. Also, use of additional transgenic lines that employ alternate fluorophores may be useful to visualize lineage determination in real time by using similar clonal assays. Finally, the ability to monitor fluorescence as a readout for lineage commitment and maturation also should enable identification of small molecules, cytokines, or antibodies that play a role in the proliferation or differentiation of hematopoietic progenitors.
The ability to perform clonal hematopoietic assays in the zebrafish will provide the means to quantitatively compare the effects of genetic abnormalities in both the embryo and the adult. However, to support multilineage readouts of clonal progenitors, it is likely that other recombinant growth factors will be required. Using only Epo and G-csf, our current readouts are limited to the erythrocyte, neutrophil, and monocyte-macrophage lineages. We have not observed thrombocytes or eosinophils under any semisolid culture condition, suggesting that additional factors are required to support the survival, proliferation, or differentiation of these lineages. To support the thrombocytic lineage, the zebrafish ortholog of thrombopoietin will probably be required. For eosinophils, it is currently unclear what the growth factor requirements are in fish. Mammalian eosinophils require signaling through interleukin-5 (IL-5) and its receptor for efficient generation. However, extensive searches in fish genomes have failed to identify members of the IL-3 cytokine cluster family of class 1 cytokines, including IL-3, IL-5, and granulocyte-macrophage–colony-stimulating factor (Liongue and Ward31 ; data not shown), indicating that homologs of these genes are not present in teleosts. Because both IL-3 and GM-CSF are key factors in mammalian myeloid cell development, these findings also suggest that G-csf may represent the key ancestral myeloid growth factor in vertebrate evolution. Finally, other growth factors also may be important in support of multilineage hematopoiesis, including stem cell factor and those that signal through the gp130 receptor, which has been identified in the zebrafish.31
In addition to being an invaluable tool to analyze the stepwise lineage restriction of HSCs and their downstream progenitors, these clonal assays should prove useful in the ability to functionally assess the recovery of the hematopoietic system to environmental and chemical insults. We previously developed an FACS-based irradiation recovery assay26 that demonstrated replenishment of the precursor scatter fraction to unirradiated levels by 14 days after irradiation.26,28,29 Our current studies, using functional assessment by colony formation, suggest that the major mediators of irradiation recovery are the HSCs contained within the lymphoid scatter population. At day 21 after irradiation, nearly 80% of the cells within the recovering lymphoid fraction form erythroid colonies, myeloid colonies, or both, with the largest fold-increase being erythroid. In contrast, only 1% of the cells within the recovering precursor population generate colonies. This is markedly different from steady-state conditions in which the precursor fraction is the primary reservoir of myeloerythroid progenitors. However, these findings are consistent with ionizing irradiation eliminating actively dividing cells (ie, hematopoietic progenitors), while having less detrimental effects on relatively quiescent cells, as mammalian HSCs have been demonstrated to be (for a review, see Seita and Weissman50 ). Together, these data suggest that sublethal irradiation recovery in the zebrafish is largely because of the survival, proliferation, and subsequent maturation of HSCs that seem to be relatively radioresistant. Importantly, these data show that even though the precursor fraction begins to recover (as measured by cell counts and FACS-based assays) as early as days 8 to 11 after irradiation, precursor cell counts do not correlate with functional myeloerythroid progenitor activity. Although our data cannot exclude the possibility that cells in the precursor fraction represent hematopoietic progenitors unresponsive to our growth conditions, it is clear that clonal assays will be crucial in more carefully defining and characterizing the biology of zebrafish hematopoietic stem and progenitor cells.
In conclusion, the development of clonal assays in the zebrafish now provides the means to functionally assess mutant animals arising through genetic screens and to further deduce the evolution of growth factor signaling in the vertebrate hematopoietic system. Discovery of additional growth factors should soon result in similar assays to more carefully test the full lineage potentials of putative hematopoietic stem and progenitor cell subsets in the zebrafish.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Julien Bertrand, Yoonsung Lee, and Dawne Page for critical evaluation of the manuscript; Roger Rainville and Lisa Phelps for animal care; and Kerstin Richter for laboratory management.
This study was supported by National Institutes of Health (NIH) grant T32-HL086344 (D.L.S.); GACR (Grant Agency of the Czech Republic) projects 310/08/0878 and 305/10/0953 and Fulbright Scholar Award (P.B.); and the American Society of Hematology, the Arnold and Mabel Beckman Foundation, the Senyei Family Foundation, and NIH grant R01-DK074482 (D.T.).
National Institutes of Health
Authorship
Contribution: D.L.S., O.S., R.P.L., and K.M.B. performed research; D.L.S., P.B., and D.T. designed the research; D.L.S. and D.T. wrote the manuscript; and P.B. and L.I.Z. provided critical reagents for the work and suggestions on experimental design.
Conflict-of-interest disclosure: L.I.Z. is a founder and stockholder of Fate Inc and a scientific advisor for Stemgent. The remaining authors declare no competing financial interests.
Correspondence: David L. Traver, Department of Cellular and Molecular Medicine, Section of Cell and Developmental Biology, University of California at San Diego, La Jolla, CA 92093-0380; e-mail: dtraver@ucsd.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal