Abstract
The impact of early human cytomegalovirus (HCMV) replication on leukemic recurrence was evaluated in 266 consecutive adult (median age, 47 years; range, 18-73 years) acute myeloid leukemia patients, who underwent allogeneic stem cell transplantation (alloSCT) from 10 of 10 high-resolution human leukocyte Ag-identical unrelated (n = 148) or sibling (n = 118) donors. A total of 63% of patients (n = 167) were at risk for HCMV reactivation by patient and donor pretransplantation HCMV serostatus. In 77 patients, first HCMV replication as detected by pp65-antigenemia assay developed at a median of 46 days (range, 25-108 days) after alloSCT. Taking all relevant competing risk factors into account, the cumulative incidence of hematologic relapse at 10 years after alloSCT was 42% (95% confidence interval [CI], 35%-51%) in patients without opposed to 9% (95% CI, 4%-19%) in patients with early pp65-antigenemia (P < .0001). A substantial and independent reduction of the relapse risk associated with early HCMV replication was confirmed by multivariate analysis using time-dependent covariate functions for grades II to IV acute and chronic graft-versus-host disease, and pp65-antigenemia (hazard ratio = 0.2; 95% CI, 0.1-0.4, P < .0001). This is the first report that demonstrates an independent and substantial reduction of the leukemic relapse risk after early replicative HCMV infection in a homogeneous population of adult acute myeloid leukemia patients.
Introduction
Human cytomegalovirus (HCMV), a member of the β-herpesvirus family, is a ubiquitous opportunistic pathogen. Symptomatic HCMV infection predominantly occurs in immunocompromised hosts, such as patients after allogeneic hematopoietic stem cell transplantation (alloSCT), whereas symptomatic infection of healthy persons is rare.1 Inapparent HCMV viremia as a potential prestage of manifest HCMV systemic or organ disease can be detected as early as 10 to 14 days after alloSCT and may last for several weeks but usually resolves by early preemptive treatment with nucleoside antiviral agents, such as ganciclovir.2 HCMV blood replication is routinely monitored by HCMV-related external matrix protein pp65-antigenemia in peripheral blood cells or quantification of serum HCMV nucleic acids concentrations using sensitive polymerase chain reaction-based techniques.3,4 Early detection of inapparent replicative HCMV infection together with its preemptive antiviral treatment has clearly led to a marked reduction of life-threatening HCMV disease after alloSCT, whereby it has become an exceptional single cause of nonrelapse mortality.2 Nevertheless, even in the current era of effective prophylactic and preemptive therapy, HCMV reactivation is still regarded as a contributing cause of death after alloSCT because of virally mediated immunosuppression, an increased risk of acute graft-versus-host diseases (GVHD), or suppression of graft hematopoiesis.2,5,6
A new aspect of the pretransplantation HCMV serostatus has recently emerged by observations of Behrendt et al, who found a significantly higher leukemic relapse risk after alloSCT in pediatric acute leukemia or myelodysplastic syndrome patients with a negative pretransplantation HCMV donor and recipient serostatus compared with patients with a positive pretransplantation HCMV serology of donors and/or recipients.7
This prompted us to evaluate the potential impact of early HCMV replication in a homogeneous and well-defined population of 266 consecutive adult patients with acute myeloid leukemia (AML), who underwent alloSCT from fully human leukocyte Ag-A, B, C, DRB1, DQB1 matched donors after myeloablative preparative regimens using T-cell replete grafts over a 12-year period. This study demonstrates a strong and independent decrease of the long-term leukemic relapse risk in patients with early HCMV replication across all disease-specific risk features and thus challenges a reassessment of the biologic relevance of HCMV in alloSCT.
Methods
Patient eligibility
The study population encompasses 266 AML patients who underwent alloSCT from human leukocyte Ag-identical sibling donors (n = 118) or unrelated donors (n = 148). This patient cohort was selected from a total of 387 AML patients (thus composing 69% of all AML patients) who received alloSCT between September 1997 and December 2009 at our department according to the following criteria: (1) complete allele identity at the human leukocyte Ag loci A, B, C, DRB1, and DQB1 as confirmed by high-resolution genotyping in unrelated donor/recipient pairs and by low resolution genotyping with documented familial segregation of the 10 human leukocyte Ag antigens in sibling pairs as previously published8 ; (2) availability of the pretransplantation HCMV antibody serostatus of patients and their donors; (3) performance of a myeloablative preparative regimen according to the definition of the European Blood and Marrow Transplantation Cooperative Group9 ; (4) transplantation of unmanipulated bone marrow or blood stem cell grafts; (5) pharmacologic immune prophylaxis of acute GVHD without antibody-based in vivo T-cell depletion; (6) no application of prophylactic ganciclovir or immunoglobulin preparations in the entire posttransplantation course; (7) blood product substitutions from HCMV-seronegative blood donors for patient and donor pairs with a negative pretransplantation HCMV serology; (8) regular, at least once weekly, monitoring of HCMV pp65-antigenemia until week 16 after alloSCT; (9) bone marrow examinations within 2 weeks before initiation of the preparative regimen to define the pretransplantation disease stage; (10) availability of sufficient information on the primary disease etiology and (11) on the primary karyotype or on qualitative analysis of disease-specific molecular markers in case of normal karyotype AML for patients in complete remission (CR) according to the diagnostic standards in the respective time periods; (12) availability of cytogenetic and/or molecular analysis of disease-specific markers of marrow cells in patients with active disease pretransplantation; and (13) no use of prophylactic or adoptive donor lymphocyte infusions in patients without posttransplantation hematologic leukemic relapse. These rigid and conservative criteria were chosen to exclude procedural inhomogeneities as far as possible, which may have influenced the risk of HCMV replication and interfered with hematologic and/or immune reconstitution or leukemic relapse. A clear-cut definition of hematologic and genotypic prognostic disease features was regarded as indispensible for the evaluation of the influence of HCMV antigenemia on leukemic relapse across distinct prognostic AML subgroups.10
All patients gave their written informed consent to all aspects of the stem cell transplantation procedure and family donors to the donation process according to protocols approved by the Institutional Review Board on Medical Ethics at the University Hospital of Essen, which are in accordance with the standards of Good Clinical Practice and the Declaration of Helsinki. Patient, donor, disease, and transplantation characteristics according to the development of HCMV antigenemia are summarized in Table 1.
Patient, donor, disease, and transplant characteristics according to the development of post-transplantation pp65-antigenemia
| . | All patients . | Patients with pp65-antigenemia . | Patients without pp65-antigenemia . | P . |
|---|---|---|---|---|
| Patients, no. (%) | 266 (100) | 77 (29) | 189 (71) | — |
| Male patients, no. (%) | 137 (52) | 38 (49) | 99 (52) | NS |
| Median patient age, y (range) | 47 (18-73) | 47 (19-68) | 46 (18-73) | NS |
| Patient age, y, no. (%) | ||||
| Younger than 20 | 8 (3) | 1 (1) | 7 (4) | |
| 20-40 | 90 (34) | 28 (36) | 62 (33) | NS |
| Older than 40 | 168 (63) | 48 (62) | 120 (63) | |
| Median donor age, y (range) | 40 (10-70) | 40 (12-70) | 40 (10-70) | NS |
| Donor type, no. (%) | ||||
| Identical sibling | 118 (44) | 29 (38) | 89 (47) | NS |
| Matched unrelated | 148 (56) | 48 (62) | 100 (53) | |
| Donor/patient sex, no. (%) | ||||
| Female/male | 28 (11) | 8 (10) | 20 (11) | NS |
| Other combinations | 238 (89) | 69 (90) | 169 (89) | |
| Donor/patient HCMV serology, no. (%) | ||||
| Negative/negative | 99 (37) | 4 (5) | 95 (50) | < .0002 |
| Positive/positive | 125 (47) | 57 (74) | 68 (36) | |
| Positive/negative | 8 (3) | 2 (3) | 6 (3) | |
| Negative/positive | 34 (13) | 14 (18) | 20 (11) | |
| Negative/negative | 99 (37) | 4 (5) | 95 (50) | < .0001 |
| Other combinations | 167 (63) | 73 (95) | 94 (50) | |
| Disease etiology, no. (%) | ||||
| De novo | 222 (83) | 64 (83) | 158 (84) | NS |
| Secondary | 26 (19) | 11 (14) | 15 (8) | |
| Therapy-related | 18 (7) | 2 (3) | 16 (8) | |
| Disease stage, no. (%) | ||||
| First remission | 123 (46) | 34 (44) | 89 (47) | |
| Second remission | 61 (23) | 18 (23) | 43 (23) | NS |
| Other stages | 82 (31) | 25 (31) | 57 (30) | |
| Disease genetic group, no. (%)* | ||||
| Favorable | 25 (9) | 4 (5) | 21 (11) | NS |
| Intermediate | 147 (55) | 47 (61) | 100 (53) | |
| Adverse | 94 (35) | 26 (34) | 68 (36) | |
| Stem cell source, no. (%) | ||||
| Bone marrow | 45 (17) | 10 (13) | 35 (19) | NS |
| Peripheral blood | 221 (83) | 67 (87) | 154 (81) | |
| Preparative regimen, no. (%) | ||||
| TBI + chemotherapy | 164 (62) | 40 (52) | 124 (66) | NS |
| Chemotherapy alone | 102 (38) | 37 (48) | 65 (34) | |
| Immune prophylaxis, no. (%) | ||||
| sMTX + CsA | 259 (97) | 74 (96) | 185 (98) | NS |
| MMF + CsA | 7 (3) | 3 (4) | 4 (2) |
| . | All patients . | Patients with pp65-antigenemia . | Patients without pp65-antigenemia . | P . |
|---|---|---|---|---|
| Patients, no. (%) | 266 (100) | 77 (29) | 189 (71) | — |
| Male patients, no. (%) | 137 (52) | 38 (49) | 99 (52) | NS |
| Median patient age, y (range) | 47 (18-73) | 47 (19-68) | 46 (18-73) | NS |
| Patient age, y, no. (%) | ||||
| Younger than 20 | 8 (3) | 1 (1) | 7 (4) | |
| 20-40 | 90 (34) | 28 (36) | 62 (33) | NS |
| Older than 40 | 168 (63) | 48 (62) | 120 (63) | |
| Median donor age, y (range) | 40 (10-70) | 40 (12-70) | 40 (10-70) | NS |
| Donor type, no. (%) | ||||
| Identical sibling | 118 (44) | 29 (38) | 89 (47) | NS |
| Matched unrelated | 148 (56) | 48 (62) | 100 (53) | |
| Donor/patient sex, no. (%) | ||||
| Female/male | 28 (11) | 8 (10) | 20 (11) | NS |
| Other combinations | 238 (89) | 69 (90) | 169 (89) | |
| Donor/patient HCMV serology, no. (%) | ||||
| Negative/negative | 99 (37) | 4 (5) | 95 (50) | < .0002 |
| Positive/positive | 125 (47) | 57 (74) | 68 (36) | |
| Positive/negative | 8 (3) | 2 (3) | 6 (3) | |
| Negative/positive | 34 (13) | 14 (18) | 20 (11) | |
| Negative/negative | 99 (37) | 4 (5) | 95 (50) | < .0001 |
| Other combinations | 167 (63) | 73 (95) | 94 (50) | |
| Disease etiology, no. (%) | ||||
| De novo | 222 (83) | 64 (83) | 158 (84) | NS |
| Secondary | 26 (19) | 11 (14) | 15 (8) | |
| Therapy-related | 18 (7) | 2 (3) | 16 (8) | |
| Disease stage, no. (%) | ||||
| First remission | 123 (46) | 34 (44) | 89 (47) | |
| Second remission | 61 (23) | 18 (23) | 43 (23) | NS |
| Other stages | 82 (31) | 25 (31) | 57 (30) | |
| Disease genetic group, no. (%)* | ||||
| Favorable | 25 (9) | 4 (5) | 21 (11) | NS |
| Intermediate | 147 (55) | 47 (61) | 100 (53) | |
| Adverse | 94 (35) | 26 (34) | 68 (36) | |
| Stem cell source, no. (%) | ||||
| Bone marrow | 45 (17) | 10 (13) | 35 (19) | NS |
| Peripheral blood | 221 (83) | 67 (87) | 154 (81) | |
| Preparative regimen, no. (%) | ||||
| TBI + chemotherapy | 164 (62) | 40 (52) | 124 (66) | NS |
| Chemotherapy alone | 102 (38) | 37 (48) | 65 (34) | |
| Immune prophylaxis, no. (%) | ||||
| sMTX + CsA | 259 (97) | 74 (96) | 185 (98) | NS |
| MMF + CsA | 7 (3) | 3 (4) | 4 (2) |
TBI indicates total body irradiation; sMTX, short-course methotrexate; CsA, cyclosporine A; MMF, mycophenolate mofetil; —, not applicable; and NS, not significant (P ≥ .01).
Favorable indicates t(8;12) or RUNX1-RUNX1T1; inv(16)(p13q22); t(16;16)(p13q22) or CBFB-MYH11; t(15;17); normal karyotype and mutated NPM1 without FLT3-ITD or mutated CEBPA.10 Intermediate indicates cytogenetic or molecular genetic abnormalities not classified as favorable or adverse.10 Adverse indicates inv(3)(q21q26.2) or t(3;3)(q21;q26.2) or RPN1-EVI1; t(6;9)(p23;q34) or DEK-NUP214; t(v;11)(v;q23) or MLL rearranged; −5 or del(5q); −7; abnl(17p); complex karyotype.10
HCMV pp65 antigenemia and preemptive antiviral therapy
HCMV-related matrix protein pp65-antigenemia was routinely monitored twice weekly during the in-patient posttransplantation course until discharge, and thereafter in weekly intervals until week 16 after alloSCT as previously published.11 HCMV pp65-antigenemia monitoring was generally started when a white blood cell count of 500 per microliter had been reached. Results were given as the number of HCMV pp65 antigen-expressing cells per 5 × 105 white blood cells. HCMV replication was assumed if at least 25 HCMV pp65 antigen-positive cells were detectable at 2 consecutive time points. Preemptive intravenous induction therapy using twice daily 5 mg/kg of patient body weight ganciclovir for at least 7 days was initiated in all patients with documented antigenemia, followed by an intravenous or oral maintenance therapy until 2 consecutive negative antigenemia samples had been obtained. In case of severe neutropenia or in ganciclovir-refractory patients, foscarnet or cidofovir was applied at the discretion of the treating physician.
Evaluation of the pretransplantation disease-specific prognostic profile, hematologic relapse, and of acute and chronic GVHD
Standard marrow morphology and hematologic criteria were used for the evaluation of the pretransplantation and posttransplantation disease stage, posttransplantation treatment response, and leukemic relapse after transplantation. For patients in CR at the time of admission to alloSCT, the cytogenetic and/or molecular genetic prognostic disease profile was deduced from these findings at the primary diagnosis, whereas for patients with active pretransplantation disease, it was based on evaluations of marrow samples taken before the initiation of the preparative regimen. The prognostic categorization of the genetic disease profile relied on the cytogenetic and molecular genetic detection methods representing the diagnostic standards in the respective time periods of this study and was retrospectively performed according to the recommendations of the European Leukemia Network expert panel.10 The diagnosis of leukemic relapse was also replenished by studies of disease-specific cytogenetic and/or molecular genetic anomalies of marrow cells, whenever applicable. Hematologic leukemic relapse was diagnosed in patients who were in CR before or attained CR after alloSCT. During the first year after alloSCT, marrow examinations were routinely performed at days 28 and 100, and at 6, 9, and 12 months thereafter. In patients surviving without leukemic relapse beyond 1 year, marrow examinations were performed every 6 months during the next 2 years or whenever clinically indicated. Acute GVHD was assumed if its clinical signs and symptoms developed within the first 100 days after alloSCT. The diagnosis of chronic GVHD was based on its occurrence later than 100 days after alloSCT together with the fulfillment of the clinical diagnostic criteria. The diagnosis and clinical grading of acute and chronic GVHD followed the commonly accepted criteria.12,13
Patient care and transplant characteristics
From the start of the preparative regimen until discharge, all patients were treated in reverse isolation single rooms equipped with high-efficacy particular air filtration. Additional measures for infection prevention and for supportive therapy complied with our previously published institutional standards.14 The myeloablative preparative regimen in patients up to the age of 50 years consisted of fractionated total body irradiation from a 60cobalt source at daily fractions of 2.5 Gy on 4 consecutive days (total body dose, 10 Gy with reduction of the central lung dose to 8 Gy) followed by either 2 consecutive daily fractions of 60 mg/kg intravenous cyclophosphamide (total dose 120 mg/kg of body weight) or by 5 daily fractions of 30 mg/m2 intravenous fludarabine (total dose 150 mg/m2 of body surface area). Four consecutive daily oral doses of 1 mg/kg of body weight in 6-hour intervals of busulfan (total dose, 16 mg/kg of body weight) or 3 consecutive daily intravenous doses of 14 g/m2 treosulfan (total dose 42 g/m2 of body surface area) in combination with 5 daily fractions of 30 mg/m2 intravenous fludarabine (total dose 150 mg/m2 of body surface area) were generally applied in patients older than 50 years.
For all red blood cell and platelet transfusions, irradiated and in-line leukocyte-filtered products were exclusively used in the entire posttransplantation course. The immune pharmacologic prophylaxis of acute GVHD consisted of blood-level adjusted continuous intravenous cyclosporine starting at an initial daily dose of 3 mg/kg cyclosporine as described previously.15 Cyclosporine was generally combined with short-course methotrexate or in exceptional patients with mycophenolate-mofetil. Before discharge, intravenous cyclosporine was substituted orally to keep target through blood levels in the range of 150 to 250 ng/mL as detected by a monoclonal assay for the parent compound. In patients without clinical signs and symptoms of GVHD, tapering of cyclosporine was initiated by day 100 after transplantation. First-line treatment of grades II to IV acute GVHD consisted of 2 mg/kg of body weight intravenous prednisolone over 5 to 7 days, which was gradually tapered in 3-day intervals in responding patients thereafter. In patients with steroid-refractory acute GVHD, polyclonal or monoclonal anti-T-lymphocyte globulin preparations were added over 5 to 7 days according to the discretion of the treating physician. Outpatient follow-up visits were scheduled at least once a week during the first 2 months after discharge and were then gradually prolonged depending on the clinical situation. All long-term surviving patients of this study are seen at least once yearly in the outpatient department.
Study endpoints and statistical analysis
Differences in frequencies of discrete variables were tested by the 2-sided Fisher exact test. The Wilcoxon rank-sum test was used to test differences of continuous variables. For the comparison of time-to-event endpoints, for which no competing event had to be considered (ie, overall survival [OS] and event-free survival [EFS]), the probabilities of events over time were calculated by the product-limit method, and heterogeneity of time-to-event distribution functions was compared by the log-rank test.16 To account for interactions of competing events on leukemic relapse (ie, death without relapse), nonrelapse mortality (NRM; ie, leukemic relapse), and on pp65-antigenemia (ie, leukemic relapse or NRM in patients without pp65-antigenemia), the probabilities of events over time were estimated by cause-specific cumulative incidence rates.17 For the comparison of cumulative incidence rates between patient subsets, the time to events was compared by proportional hazards general linear model (PHGLM) analysis of the cause-specific hazard functions using the 2-sided Wald test.18 In addition, multivariate PHGLM analysis on the endpoints of leukemic relapse, NRM, OS, and EFS was performed.19 In all multivariate PHGLM analyses on these endpoints, the dichotomous pretransplantation HCMV serologic risk constellation (0 indicates donor and recipient seronegative; and 1, donor and/or recipient seropositive), the disease stage (0 indicates complete remission 1 [CR1]; CR2; and other stages), the categorized disease duration (0 indicates up to 12 months; and 1, > 12 months), the genetic disease risk group (0 indicates favorable; 1, intermediate I + II; and 2, adverse), the stem cell source (0 indicates bone marrow cells; and 1, blood stem cells), the donor type (0 indicates identical sibling; and 1, matched unrelated donor), the categorized patient age (0 indicates younger than 20 years; 1, 20-40 years; and 2, older than 40 years), and the European Blood and Marrow Transplantation risk score were included as categorical covariates.20 Acute GVHD (0 indicates grades 0 to I; and 1, grades II to IV), chronic GVHD (0 indicates absent; and 1, present), and pp65-antigenemia (0 indicates no; and 1, yes) were included as time-dependent covariates with the time interval from alloSCT (day 0) until the first occurrence of the respective event or censoring in model building. All PHGLM analyses were performed using stepwise forward and backward selection procedures, and only those covariates with a significance level < 1% were allowed to enter the model building procedure. Further, only those covariates that attained a significant level < 1% after adjustment for the other significant covariates selected by the forward and by the backward model building procedure were regarded significant in the final models. To account for potential interactions of pp65-antigenemia and grades II to IV acute GVHD on leukemic relapse, univariate and multivariate day 100 landmark analysis was additionally performed, which was restricted to 238 patients (89% of the total patient population), who survived without relapse by day 100 after alloSCT. The hazard ratio and its 95% confidence interval (CI) were derived for each significant covariate included in the final PHGLM models. Statistical analysis and presentation were performed using Statistical Analysis Software procedures and macros (SAS user's guide statistics, release 9.22). Date of the final analysis was December, 10, 2010.
Results
Pretransplantation HCMV serology and pp65-antigenemia
In 73 of 167 patients (44%) in whom a positive pretransplantation HCMV serostatus of donors and/or recipients had been documented, first pp65-antigenemia occurred between 25 and 108 days (median, 46 days) after alloSCT, resulting in a cause-specific cumulative incidence of pp65-antigenemia of 44% (95% CI, 37%-52%) for these patients. Notably, even 4 of 99 patients with a negative pretransplantation HCMV donor and recipient serostatus developed pp65-antigenemia between 41 and 66 days after alloSCT, corresponding to a cause-specific cumulative incidence of 4% (95% CI, 1%-11%). Thus, given that no HCMV transmission had taken place after transplantation, the pretransplantation donor and/or recipient HCMV serology was false negative in at least 4% of patients in this study.
Acute GVHD and pp65-antigenemia
Grades II to IV acute GVHD developed in 187 patients (70%). The cause-specific cumulative incidence of pp65-antigenemia was 35% (95% CI, 29%-42%) for these patients opposed to 15% (95% CI, 9%-26%) for 79 patients with grades 0 to I acute GVHD (P < .0001). If considering only those 167 patients with a positive pretransplantation HCMV serostatus of donors and/or recipients, the cause-specific cumulative incidence of pp65-antigenemia increased to 52% (95% CI, 44%-62%) for the 117 patients with grades II to IV acute GVHD compared with 24% (95% CI, 15%-39%) for 50 patients with grades 0 to I acute GVHD (P < .0001). Thus, the development of grades II to IV acute GVHD increased the risk of pp65-antigenemia more than 2-fold in this study. Of the 65 patients with grades II to IV acute GVHD, in whom pp65-antigenemia occurred, the clinical diagnosis of acute GVHD preceded the detection of pp65-antigenemia in 59 patients (91%).
Leukemic relapse, pretransplantation HCMV serology, and pp65-antigenemia
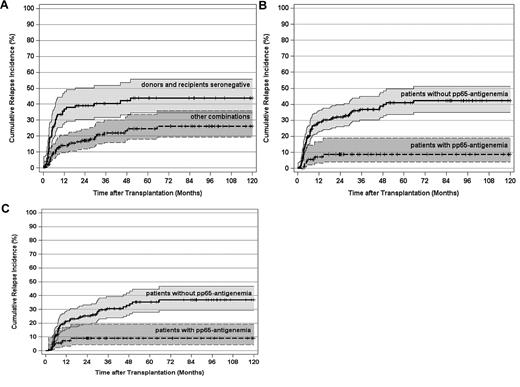
In 75 of 266 patients (28%), a hematologic leukemic relapse was documented at a median of 157 days (range, 44-1998 days) after transplantation corresponding to a cause-specific cumulative incidence of posttransplantation leukemic relapse at 10 years (10-year cumulative incidence of hematologic relapse [CIR]) of 33% (95% CI, 27%-40%) in this study. For the 99 patients with a negative pretransplantation donor and recipient HCMV serostatus, the 10-year CIR was 44% (95% CI, 35%-56%) opposed to 26% (95% CI, 19%-36%) for the 167 patients with a positive pretransplantation HCMV serostatus of donors and/or recipients (P < .0001; Figure 1A).
CIR and 95% CI stratified by pretransplantation HCMV serostatus and post-transplantation HCMV pp65-antigenemia. (A) CIR by pretransplantation HCMV serostatus of donors and recipients (P < .0001). (B) CIR by post-transplantation pp65-antigenemia (P < .0001). (C) CIR by post-transplantation pp65-antigenemia of patients surviving free of relapse at day 100 after transplantation (P < .0001). Tick marks indicate patients surviving free of relapse or competing events.
CIR and 95% CI stratified by pretransplantation HCMV serostatus and post-transplantation HCMV pp65-antigenemia. (A) CIR by pretransplantation HCMV serostatus of donors and recipients (P < .0001). (B) CIR by post-transplantation pp65-antigenemia (P < .0001). (C) CIR by post-transplantation pp65-antigenemia of patients surviving free of relapse at day 100 after transplantation (P < .0001). Tick marks indicate patients surviving free of relapse or competing events.
When stratifying the 10-year CIR by pp65-antigenemia, it was only 9% (95% CI, 4%-19%) for the 77 patients, who actually developed pp65-antigenemia. In contrast, the 10-year CIR was 42% (95% CI, 35%-51%) for the 189 patients without detectable antigenemia (P < .0001; Figure 1B). Thus, early posttransplantation HCMV replication as detected by pp65-antigenemia was associated with a > 4-fold lower long-term leukemic relapse risk compared with a nonreplicative HCMV infectious course in this study. Notably, compared with all patients with a positive pretransplantation HCMV serostatus of donors and/or recipients, the detection of pp65-antigenemia was associated with a 3-fold lower leukemic relapse risk (Figure 1A-B). Conversely, the 10-year CIR of patients with a seronegative pretransplantation donor and recipient HCMV serostatus was not significantly different opposed to patients with a positive pretransplantation HCMV serostatus of donors and/or recipients, in whom a replicative HCMV infection was not detected (data not shown). A landmark analysis on the 238 patients (89%), who survived free of relapse by day 100 after transplantation demonstrated that the 10-year CIR was still 4-fold lower for those patients, in whom a replicative HCMV infection had been documented within the first 100 days after alloSCT (P < .0001; Figure 1C). Multivariate analysis confirmed a strong and independent relapse risk reduction by pp65-antigenemia in all study patients as well as in those 238 patients, who survived free of relapse beyond day 100 after alloSCT (Table 2). Taken together, this indicates that early HCMV replication is required to exert the as yet undefined HCMV replication-associated antileukemic mechanisms.
Proportional hazards general linear model analysis on the leukemic relapse risk
| . | All patients . | P . | Patients surviving free of relapse at day 100 . | P . | Patients with grades II to IV acute GVHD . | P . | Patients with grades II to IV acute GVHD surviving free of relapse at day 100 . | P . |
|---|---|---|---|---|---|---|---|---|
| No. of patients | 266 | — | 238 | — | 187 | — | 168 | — |
| Covariates* | ||||||||
| Disease stage† | 1.6 (1.2-2.1) | < .0005 | 1.5 (1.1-2.0) | < .009 | 1.6 (1.1-2.2) | < .008 | — | NS |
| Acute GVHD† | — | NS | — | NS | — | — | — | — |
| Chronic GVHD† | 0.2 (0.1-0.4) | < .0001 | 0.3 (0.1-0.5) | < .0001 | 0.2 (0.1-0.3) | < .0001 | 0.2 (0.1-0.4) | < .0001 |
| HCMV pp65-antigenemia† | 0.2 (0.1-0.4) | < .0001 | 0.2 (0.1-0.6) | < .0001 | 0.1 (0.04-0.4) | < .0002 | 0.2 (0.1-0.5) | < .002 |
| . | All patients . | P . | Patients surviving free of relapse at day 100 . | P . | Patients with grades II to IV acute GVHD . | P . | Patients with grades II to IV acute GVHD surviving free of relapse at day 100 . | P . |
|---|---|---|---|---|---|---|---|---|
| No. of patients | 266 | — | 238 | — | 187 | — | 168 | — |
| Covariates* | ||||||||
| Disease stage† | 1.6 (1.2-2.1) | < .0005 | 1.5 (1.1-2.0) | < .009 | 1.6 (1.1-2.2) | < .008 | — | NS |
| Acute GVHD† | — | NS | — | NS | — | — | — | — |
| Chronic GVHD† | 0.2 (0.1-0.4) | < .0001 | 0.3 (0.1-0.5) | < .0001 | 0.2 (0.1-0.3) | < .0001 | 0.2 (0.1-0.4) | < .0001 |
| HCMV pp65-antigenemia† | 0.2 (0.1-0.4) | < .0001 | 0.2 (0.1-0.6) | < .0001 | 0.1 (0.04-0.4) | < .0002 | 0.2 (0.1-0.5) | < .002 |
— indicates not applicable; and NS, not significant (P ≥ .01).
Proportional hazards general linear models with forward and backward selection of covariates; time-dependent covariates: time intervals to grades II to IV acute GVHD, chronic GVHD, and HCMV pp65-antigenemia; and further covariates included in all PHGLM analyses: stratified patient age; patient/donor sex match; pretransplantation risk score; donor type; graft source; pretransplantation HCMV donor and recipient serology; and disease genetic group.
Hazard ratio (HR) and 95% CI after adjustment for all significant (P < .01) covariates in the final models.
Interactions between acute GVHD, pp65-antigenemia, and leukemic relapse
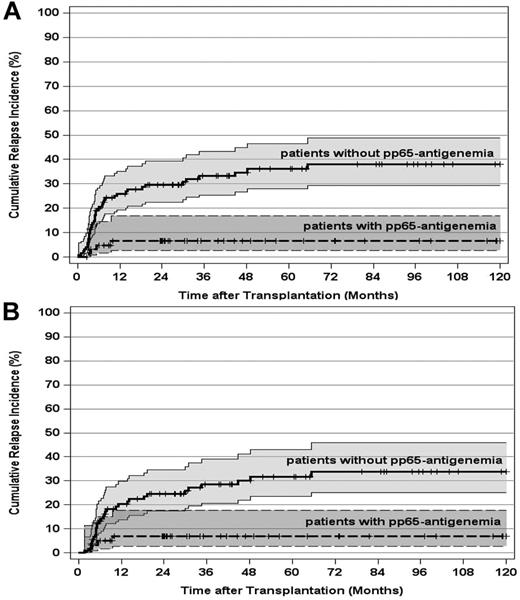
Because grades II to IV acute GVHD or its treatment strongly promoted HCMV replication, the impact of pp65-antigenemia on the leukemic relapse risk was separately compared in the 187 patients, who developed grades II to IV acute GVHD in this study. Again, the occurrence of pp65-antigenemia led to a significant reduction of the 10-year CIR from 38% (95% CI, 29%-49%) for grades II to IV acute GVHD patients without pp65-antigenemia to 7% (95% CI, 3%-17%) for patients with pp65-antigenemia (P < .0005; Figure 2A). A day 100 landmark analysis of this patient subset confirmed the significant long-term reduction of the leukemic relapse risk from 34% (95% CI, 25%-46%) for patients without pp65-antigenemia to 7% (95% CI, 3%-18%) for patients with pp65-antigenemia (P < .0005; Figure 2B). Multivariate analysis on the leukemic relapse risk, using time-dependent covariate functions for pp65-antigenemia and chronic GVHD, further validated that the occurrence of HCMV replication is an independent and strong predictor of the relapse risk in patients with grades II to IV acute GVHD. Besides pp65-antigenemia, the disease stage and the development of chronic GVHD were included in the final model (Table 2). The same analysis performed on the 168 patients who developed grades II to IV acute GVHD and survived free of relapse by day 100 completely corroborated the results obtained for all grades II to IV acute GVHD patients (Table 2).
CIR and 95% CI stratified by post-transplantation HCMV pp65-antigenemia in patients with grades II to IV acute GVHD. (A) CIR by post-transplantation pp65-antigenemia in all patients who developed grades II to IV acute GVHD (P < .0005). (B) CIR by post-transplantation pp65-antigenemia of patients who developed grades II to IV and survived free of relapse at day 100 after transplantation (P < .0005). Tick marks indicate patients surviving free of relapse or competing events.
CIR and 95% CI stratified by post-transplantation HCMV pp65-antigenemia in patients with grades II to IV acute GVHD. (A) CIR by post-transplantation pp65-antigenemia in all patients who developed grades II to IV acute GVHD (P < .0005). (B) CIR by post-transplantation pp65-antigenemia of patients who developed grades II to IV and survived free of relapse at day 100 after transplantation (P < .0005). Tick marks indicate patients surviving free of relapse or competing events.
Interactions between disease-specific prognostic factors, pp65-antigenemia, and leukemic relapse
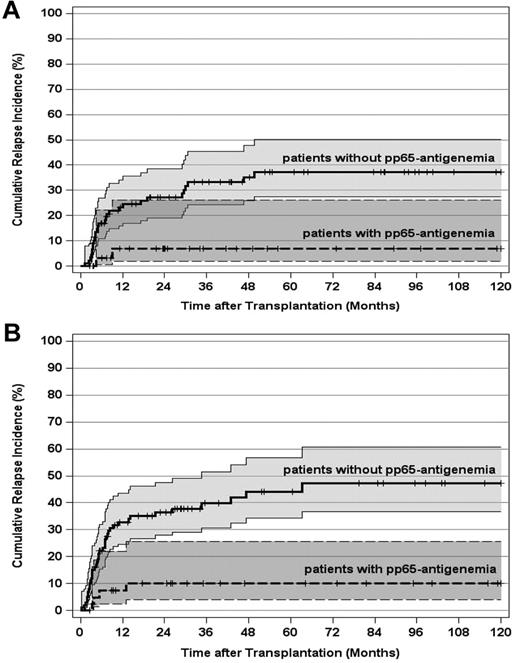
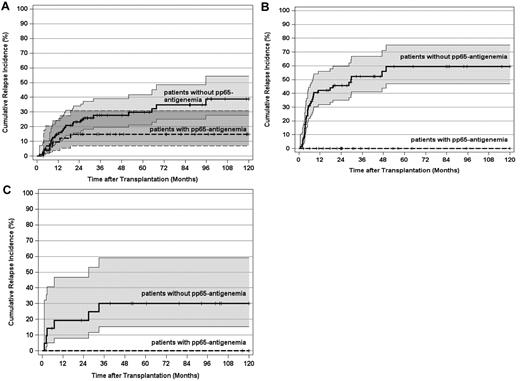
As expected, the pretransplantation disease stage had a paramount impact on the leukemic relapse risk in this study. Therefore, we separately evaluated the importance of pp65-antigenemia for 123 patients transplanted in CR1 and for those 143 patients who underwent alloSCT beyond CR1. For CR1 patients, the 10-year CIR declined from 37% (95% CI, 28%-50%) in patients without pp65-antigenemia to 7% (95% CI, 2%-26%) in patients with pp65-antigenemia (Figure 3A; P < .002). The corresponding values for patients in advanced AML stages were 47% (95% CI, 37%-61%) and 10% (95% CI, 4%-26%), respectively (Figure 3B; P < .0001). In a second step, the influence of pp65-antigenemia on the relapse risk was analyzed in patient subsets according to the genetically defined prognostic disease profile. For 147 patients (55%) with an intermediate karyotype, the 10-year CIR declined from 39% (95% CI, 28%-54%) in patients without pp65-antigenemia to 15% (95% CI, 7%-31%) in patients with pp65-antigenemia (Figure 4A; P < .03). This effect was even more pronounced in 94 patients with an adverse karyotype, in whom the 10-year CIR reached 59% (95% CI, 48%-75%) without pp65-antigenemia. In contrast, all 26 patients with an adverse karyotype who developed antigenemia remained relapse-free irrespective of disease stage (Figure 4B; P < .0001). The same applied to favorable karyotype patients with pp65-antigenemia, but the total number of patients (n = 25) and events (n = 6) in this subset was by far too small to demonstrate a significant difference of the relapse risk by posttransplantation HCMV replication (Figure 4C).
CIR and 95% CI stratified by post-transplantation HCMV pp65-antigenemia and disease stage. (A) CIR by post-transplantation pp65-antigenemia of patients transplanted in first remission (P < .002). (B) CIR by post-transplantation pp65-antigenemia of patients transplanted after first remission (P < .0001). Tick marks indicate patients surviving free of relapse or competing events.
CIR and 95% CI stratified by post-transplantation HCMV pp65-antigenemia and disease stage. (A) CIR by post-transplantation pp65-antigenemia of patients transplanted in first remission (P < .002). (B) CIR by post-transplantation pp65-antigenemia of patients transplanted after first remission (P < .0001). Tick marks indicate patients surviving free of relapse or competing events.
CIR and 95% CI stratified by post-transplantation HCMV pp65-antigenemia and disease genetic risk category. (A) CIR by post-transplantation pp65-antigenemia of patients with an intermediate genetic risk (P < .03). (B) CIR by post-transplantation pp65-antigenemia of patients with an adverse genetic risk (P < .0001). (C) CIR by post-transplantation HCMV antigenemia of patients with a favorable genetic risk (not significant). Tick marks indicate patients surviving free of relapse or competing events.
CIR and 95% CI stratified by post-transplantation HCMV pp65-antigenemia and disease genetic risk category. (A) CIR by post-transplantation pp65-antigenemia of patients with an intermediate genetic risk (P < .03). (B) CIR by post-transplantation pp65-antigenemia of patients with an adverse genetic risk (P < .0001). (C) CIR by post-transplantation HCMV antigenemia of patients with a favorable genetic risk (not significant). Tick marks indicate patients surviving free of relapse or competing events.
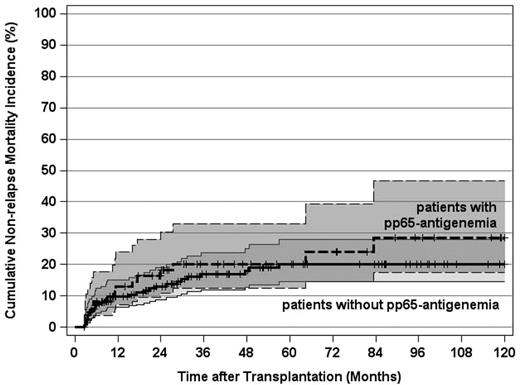
Nonrelapse mortality and pp65-antigenemia
In contrast to the prevailing view that HCMV infections increase the risk of NRM after alloSCT,5 this study revealed no evidence for this perception because the CI rates of NRM of patients with or without pp65-antigenemia were superimposable (Figure 5). By multivariate analysis on NRM as the endpoint, 2 decisive predictors were identified: the pretransplantation disease stage and the development of grades II to IV acute GVHD, whereas neither the pretransplantation serologic HCMV risk status nor posttransplantation HCMV replication had any influence on this endpoint. (Table 3). Thus, as exemplified by this study, the significance of the pretransplantation HCMV serostatus and, in particular, of replicative HCMV infections for NRM needs to be reassessed in the era of early diagnosis and preemptive antiviral therapy after alloSCT.
Cumulative incidence of nonrelapse mortality and 95% CI stratified by post-transplantation HCMV pp65-antigenemia (not significant). Tick marks indicate surviving patients or competing events.
Cumulative incidence of nonrelapse mortality and 95% CI stratified by post-transplantation HCMV pp65-antigenemia (not significant). Tick marks indicate surviving patients or competing events.
Proportional hazards general linear model analysis on NRM, OS, and EFS
| . | NRM . | P . | OS . | P . | EFS . | P . |
|---|---|---|---|---|---|---|
| No. of patients | 266 total patients | — | 266 total patients/253 patients surviving at day 100 | — | 266 total patients/238 patients surviving free of events at day 100 | — |
| Covariates* | ||||||
| Disease stage† | 1.7 (1.2-2.4) | < .002 | 1.6 (1.3-2.0) | < .0001 | 1.7 (1.3-2.0) | < .0001 |
| 1.6 (1.2-2.0) | < .0001 | 1.6 (1.2-2.0) | < .0002 | |||
| Acute GVHD† | 3.8 (1.6-9.3) | < .003 | 2.1 (1.3-3.2) | < .002 | 1.9 (1.2-2.9) | < .003 |
| 1.8 (1.1-2.8) | < .01 | 2.0 (1.2-3.3) | < .007 | |||
| Chronic GVHD† | — | NS | 0.2 (0.1-0.4) | < .0001 | 0.2 (0.1-0.4) | < .0001 |
| 0.3 (0.2-0.4) | < .0001 | 0.3 (0.2-0.4) | < .0001 | |||
| HCMV pp65-antigenemia† | — | NS | 0.4 (0.3-0.7) | < .0008 | 0.4 (0.2-0.6) | < .0001 |
| 0.5 (0.3-0.9) | < .01 | 0.4 (0.2-0.7) | < .0008 |
| . | NRM . | P . | OS . | P . | EFS . | P . |
|---|---|---|---|---|---|---|
| No. of patients | 266 total patients | — | 266 total patients/253 patients surviving at day 100 | — | 266 total patients/238 patients surviving free of events at day 100 | — |
| Covariates* | ||||||
| Disease stage† | 1.7 (1.2-2.4) | < .002 | 1.6 (1.3-2.0) | < .0001 | 1.7 (1.3-2.0) | < .0001 |
| 1.6 (1.2-2.0) | < .0001 | 1.6 (1.2-2.0) | < .0002 | |||
| Acute GVHD† | 3.8 (1.6-9.3) | < .003 | 2.1 (1.3-3.2) | < .002 | 1.9 (1.2-2.9) | < .003 |
| 1.8 (1.1-2.8) | < .01 | 2.0 (1.2-3.3) | < .007 | |||
| Chronic GVHD† | — | NS | 0.2 (0.1-0.4) | < .0001 | 0.2 (0.1-0.4) | < .0001 |
| 0.3 (0.2-0.4) | < .0001 | 0.3 (0.2-0.4) | < .0001 | |||
| HCMV pp65-antigenemia† | — | NS | 0.4 (0.3-0.7) | < .0008 | 0.4 (0.2-0.6) | < .0001 |
| 0.5 (0.3-0.9) | < .01 | 0.4 (0.2-0.7) | < .0008 |
— indicates not applicable; and NS, not significant (P ≥ .01).
Proportional hazards general linear models with forward and backward selection of covariates; time-dependent covariates: time intervals to grades II to IV acute GVHD, chronic GVHD, and HCMV pp65-antigenemia; and further covariates included in all PHGLM analyses: stratified patient age; patient/donor sex match; pretransplantation risk score; donor type; graft source; pretransplantation HCMV donor and recipient serology; and disease genetic group.
Hazard ratio (HR) and 95% CI after adjustment for all significant (P < .01) covariates in the final models.
Survival and pp-65 antigenemia
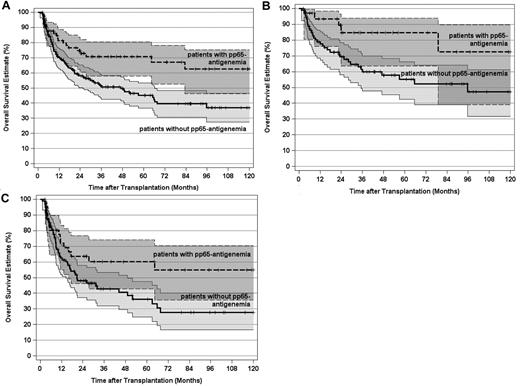
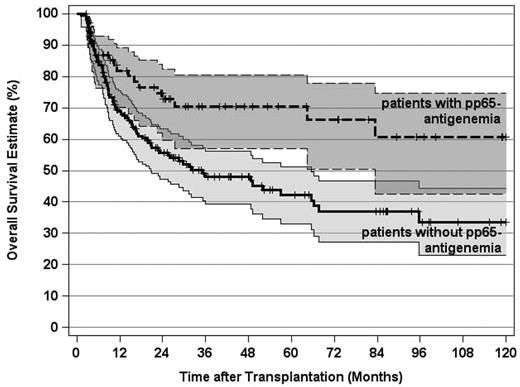
Because posttransplantation pp65-antigenemia markedly reduced the leukemic relapse risk without increasing NRM, it appeared obvious that replicative HCMV infections should also improve OS in this study. Indeed, the 10-year OS estimate for the 77 patients who developed pp65-antigenemia was significantly superior opposed to this estimate for the 189 patients without posttransplantation HCMV replication (Figure 6A). The beneficial effect of pp65-antigenemia on OS was detectabe in CR1 patients as well as in more advanced disease stage patients (Figure 6B-C). Likewise, in 241 patients with an intermediate or adverse genetic disease profile, the 10-year OS estimate was significantly superior if posttransplantation pp65-antigenemia had been detected (Figure 7). Multivariate analysis confirmed an independent and strong beneficial effect of pp65-antigenemia on the 10-year OS estimate, which was even more pronounced for the 10-year EFS estimate and also persisted in the 100-day multivariate landmark analysis on these endpoints (Table 3). Hence, the reduction of the leukemic relapse risk observed after posttransplantation pp65-antigenemia translated into improved long-term OS and EFS here. It is of note that no independent influence of the pretransplantation HCMV serostatus was evident by multivariate analysis on these endpoints after adjustment for significant covariates in the final models. Table 4 summarizes the causes of death in the patient subsets without or with pp65-antigenemia.
OS estimates and 95% CI stratified by post-transplantation HCMV pp65-antigenemia and disease stage. (A) OS by post-transplantation pp65-antigenemia of all patients (P < .005). (B) OS by post-transplantation pp65-antigenemia of patients transplanted in first remission (P < .03). (C) OS by post-transplantation pp65-antigenemia of patients transplanted after first remission (P < .05). Tick marks indicate surviving patients.
OS estimates and 95% CI stratified by post-transplantation HCMV pp65-antigenemia and disease stage. (A) OS by post-transplantation pp65-antigenemia of all patients (P < .005). (B) OS by post-transplantation pp65-antigenemia of patients transplanted in first remission (P < .03). (C) OS by post-transplantation pp65-antigenemia of patients transplanted after first remission (P < .05). Tick marks indicate surviving patients.
OS estimates and 95% CI stratified by post-transplantation HCMV pp65-antigenemia in patients with an intermediate or adverse genetic disease category (P < .004). Tick marks indicate surviving patients.
OS estimates and 95% CI stratified by post-transplantation HCMV pp65-antigenemia in patients with an intermediate or adverse genetic disease category (P < .004). Tick marks indicate surviving patients.
Death rates and leading causes of death according to the development of post-transplantation pp65-antigenemia
| . | All patients . | Patients with pp65-antigenemia . | Patients without pp65-antigenemia . | P . |
|---|---|---|---|---|
| Overall death rate* | 113/266 (42) | 21/77 (27) | 92/189 (49) | < .002 |
| Relapse-related* | 67/266 (25) | 6/77 (8) | 61/189 (32) | < .0001 |
| Non–relapse-related* | 46/266 (17) | 15/77 (19) | 31/189 (16) | NS |
| Until day 100† | 7/46 (15) | 2/15 (13) | 5/31 (16) | NS |
| After day 100† | 39/46 (85) | 13/15 (87) | 26/31 (84) | NS |
| Acute GVHD with or without fatal infections† | 14/46 (30) | 4/15 (27) | 10/31 (32) | NS |
| Chronic GVHD with or without fatal infections† | 11/46 (24) | 4/15 (27) | 7/31 (23) | NS |
| Fatal infections without GVHD† | 7/46 (15) | 2/15 (13) | 5/31 (16) | NS |
| Hepatic VOD† | 3/46 (7) | 1/15 (7) | 2/31 (6) | NS |
| Bronchiolitis, COLD† | 4/46 (9) | 2/15 (13) | 2/31 (6) | NS |
| Cardiomyopathy, myocardial infarction† | 4/46 (9) | 1/15 (7) | 3/31 (10) | NS |
| PML† | 2/46 (4) | 1/15 (7) | 1/31 (3) | NS |
| HIV infection† | 1/46 (2) | — | 1/31 (3) | — |
| . | All patients . | Patients with pp65-antigenemia . | Patients without pp65-antigenemia . | P . |
|---|---|---|---|---|
| Overall death rate* | 113/266 (42) | 21/77 (27) | 92/189 (49) | < .002 |
| Relapse-related* | 67/266 (25) | 6/77 (8) | 61/189 (32) | < .0001 |
| Non–relapse-related* | 46/266 (17) | 15/77 (19) | 31/189 (16) | NS |
| Until day 100† | 7/46 (15) | 2/15 (13) | 5/31 (16) | NS |
| After day 100† | 39/46 (85) | 13/15 (87) | 26/31 (84) | NS |
| Acute GVHD with or without fatal infections† | 14/46 (30) | 4/15 (27) | 10/31 (32) | NS |
| Chronic GVHD with or without fatal infections† | 11/46 (24) | 4/15 (27) | 7/31 (23) | NS |
| Fatal infections without GVHD† | 7/46 (15) | 2/15 (13) | 5/31 (16) | NS |
| Hepatic VOD† | 3/46 (7) | 1/15 (7) | 2/31 (6) | NS |
| Bronchiolitis, COLD† | 4/46 (9) | 2/15 (13) | 2/31 (6) | NS |
| Cardiomyopathy, myocardial infarction† | 4/46 (9) | 1/15 (7) | 3/31 (10) | NS |
| PML† | 2/46 (4) | 1/15 (7) | 1/31 (3) | NS |
| HIV infection† | 1/46 (2) | — | 1/31 (3) | — |
VOD indicates veno-occlusive disease; COLD, chronic obstructive lung disease; PML, progressive multifocal leukencephalopathy; HIV, human immunodeficiency virus (sexually transmitted after transplantation); NS, not significant; and —, not applicable.
Proportion of patients (%).
Proportion of deceased patients (%).
Discussion
The seroprevalence of antibodies against HCMV in hematopoietic stem cell donors and transplant recipients is an established prognostic factor for the outcome of alloSCT as a paramount consequence of an increased nonrelapse-related mortality associated with latent pretransplantation HCMV infections. Although a plethora of scientific publications dealing with different settings of alloSCT has substantiated an adverse effect of a positive pretransplantation HCMV serostatus of donors and/or recipients (for review, see Boeckh and Nichols5 ), only sparse information on the prognostic impact of replicative posttransplantation HCMV infections on alloSCT outcome is currently available. Early observations that replicative HCMV infections may also have a beneficial effect on transplantation outcome because of a substantial decrease of the leukemic relapse risk after alloSCT go back to the mid 1980s, a time period in which reliable and fast detection methods for replicative HCMV infections as well as effective antiviral treatment against HCMV were not yet available.21-23 However, these early observations derived from very limited patient cohorts have not been reconfirmed so far. For a meaningful evaluation of the potential influence of posttransplantation replicative HCMV infections on the leukemic relapse risk, numerous prerequisites regarding patient and donor characteristics, disease features, transplantation procedures, and strategies to prevent HCMV disease after transplantation have to be considered to reduce potential confounding factors, which may interfere with this endpoint. The present study was therefore stringently restricted to a comparatively large cohort of AML patients with well-defined prognostic disease features, which underwent alloSCT without in vivo or ex vivo T-cell depletion after myeloablative preparative regimens using a uniform long-term strategy for the detection and preemptive therapy of early HCMV replication. For the first time, it demonstrates that the development of HCMV replication after alloSCT, as detected by pp65-antigenemia, is a strong and independent predictor of a reduced leukemic relapse risk, which also translates into superior long-term survival in early and advanced disease stages of AML. Furthermore, the higher antileukemic effect after posttransplantation HCMV replication was notable across all prognostic genetic disease groups and was particularly pronounced in patients with adverse genetic disease features. As previously shown for a smaller and more heterogeneous pediatric patient population from a single institution,7 the pretransplantation HCMV serostatus also predicted a reduced relapse risk for seropositive donor and/or recipient pairs in the present study, but this effect was largely restricted to those patients who actually developed posttransplantation HCMV replication. The fact that HCMV replication followed the development of grades II to IV acute GVHD in the majority of affected patients in this study does not argue against an independent antileukemic effect of HCMV replication because the relapse risk was still > 5-fold lower for those grades II to IV acute GVHD patients, who actually developed pp65-antigenemia opposed to those who did not. The same comparison for grades II to IV acute GVHD patients who survived free of relapse by day 100 after alloSCT corroborated the higher long-term antileukemic effect of posttransplantation HCMV replication. In addition, multivariate analysis using time-dependent covariate functions for the development of grades II to IV acute and chronic GVHD as well as for pp65-antigenemia confirmed that, besides the disease stage and the development of chronic GVHD, only HCMV replication was an independent predictor of a reduced relapse risk. Hence, it appears reasonable to assume that HCMV replication by itself has promoted the strong long-term antileukemic effect observed in this study.
Potential mechanisms by which HCMV replication may exert a long-term antileukemic effect after alloSCT are elusive at present. One study in a heterogeneous patient cohort, which underwent CD34 cell-selected human leukocyte Ag-identical sibling donor alloSCT, described a 3-fold higher leukemic relapse risk for patients with persisting HCMV antigenemia, and this was further associated with a poor in vitro lymphocyte proliferation response to HCMV antigen.24 This observation raises the possibility that the quality of the T-cell immune response to HCMV after alloSCT is also a surrogate for the strength of donor T cell–mediated antileukemic effects.24,25 Contrary to this study, a clear-cut beneficial effect of early HCMV antigenemia on the overall leukemic relapse risk was demonstrable in this study population, which can be explained by several substantial distinctions between these studies. These, among others, rely on underlying diseases, donor sources, and, in particular, on the use of unmanipulated versus CD34 cell-selected grafts. We have previously shown that CD34 cell-selected grafts are associated with a substantially delayed reconstitution of peripheral blood naive and memory CD4+ helper T cells and, possibly more importantly, with a low rate of donor T-cell chimerism at 3 months after sibling donor alloSCT compared with unmanipulated marrow or blood stem cell grafts.26 It has further been shown that a deficient HCMV-specific CD4+ T-cell immune response within the first 30 to 50 days after transplantation is associated with a particularly high risk of HCMV reactivation and, in addition, that the transfer of adaptive immunity to HCMV during alloSCT appears to be dependent on the specificity and phenotype of HCMV-specific T cells in the donor.27,28 Thus, given that donor T cells also constitute the major cellular compartment of the putative antileukemic effect associated with HCMV antigenemia in the present study, this would have been largely offset in a transplantation setting using CD34 cell-selected grafts.
A beneficial effect of HCMV-seropositive donors on the leukemic relapse risk has also been suggested by a relatively small study in patients after unmanipulated alloSCT after reduced-intensity conditioning.29 Based on previous observations that a reduced relapse incidence was particularly prominent after human leukocyte Ag-identical sibling donor marrow transplantation in a subgroup of human leukocyte Ag-A2–positive donor/recipient pairs, it was supposed that cross-reactivity between HCMV-peptide specific human leukocyte Ag-A2 restricted cytotoxic T cells and human leukocyte Ag-A2 restricted minor histocompatibility or leukemia-associated antigens expressed on leukemic cells might mediate a higher antileukemic effect associated with HCMV-seropositive donors.30 This hypothesis, however, has not been confirmed by several other investigators, including one definitive retrospective study with appropriate sample size published by Erard et al.31
We have primarily restricted this study to patients with AML because it may well be that a putative “virus-versus-leukemia” effect of replicative HCMV infections after alloSCT is particularly operative in AML patients. It has been shown previously that primary AML marrow blasts can contain fairly large HCMV copy numbers in a significant proportion of affected patients.32 Thus, HCMV infection of residual AML marrow blasts may similarly fade to a latent state as latent HCMV infections in other tissues after alloSCT. This in turn may lead to presentation of HCMV-induced peptides by AML blasts, which can serve as targets for specific cytotoxic donor cells. This hypothesis is supported by the work of Fletcher et al who reported that HCMVs induce changes in cell surface lymphocyte function-associated antigen-3 expression.33 Indeed, they found that natural killer cells lyse cytomegalovirus-infected cells with the rate of lysis of cytomegalovirus-infected cells correlating with HCMV-induced changes in cell lymphocyte function-associated antigen-3 expression.33
Alternatively or in addition, minor histocompatibility and/or leukemia-associated antigen expression of infected AML blasts might be up-regulated during HCMV replication, rendering them more vulnerable to allogeneic cytotoxic donor cells. These potential mechanisms may also apply to HCMV-seronegative patients who are primarily infected through the stem cell graft from a seropositive donor.
Furthermore, it may be that other potential antileukemic mechanisms exist, such as possible direct cytotoxic effects of replicative HCMV infections or of preemptive antiviral therapy on residual AML blasts. However, if such a direct cytotoxic effect exists, it may be limited considering that replicative HCMV infections during induction chemotherapy of AML are not uncommon but, according to current knowledge, neither HCMV replication nor preemptive antiviral therapy has an apparent influence on remission induction or duration in this particular situation.34
Interestingly, we have not observed the described antileukemic effects of HCMV in patients with AML after herpes simplex virus, varicella zoster virus, or Epstein-Barr virus replications after transplantation, so that we have to assume that this effect might be restricted to HCMV only (data not shown).
Because the present study results have been obtained by a retrospective analysis, they await confirmation by prospective evaluations of the influence of HCMV replication on the leukemic relapse risk in different disease and stem cell transplantation settings. This would open the perspective for a “controlled” induction of HCMV replication in particular for patients with a high relapse risk to augment the antileukemic efficacy of alloSCT by the putative “virus-versus-leukemia” effect.
In conclusion, this is the first report that demonstrates an independent and substantial reduction of the leukemic relapse risk after early replicative HCMV infection in a homogeneous population of adult AML patients, which challenge a reassessment of the biologic relevance of HCMV on the outcome of alloSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to the technicians of the Department of Virology at the University Hospital of Essen, Germany, and the Department of Bone Marrow Transplantation for their excellent technical long-term support in the performance of the HCMV pp65-antigenemia assays. They are particularly grateful for the provision and reevaluation of cytogenetic and molecular disease-specific data provided by coworkers at the Munich Leukemia Laboratory (MLL), Germany.
Authorship
Contribution: A.H.E. and D.W.B. conceived and designed the study and provided study materials and patients; A.H.E. provided financial support and administrative support; M.D., T.G., A.H.E., M.K., L.K., R.S.R., N.K.S., Y.H., S.C., and S.S. collected and assembled data; D.W.B., A.H.E., and M.K. analyzed data and interpreted data; D.W.B., A.H.E., and Y.H. wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ahmet H. Elmaagacli, Department of Bone Marrow Transplantation, University Hospital of Essen, Hufelandstr 55, 45122 Essen, Germany; e-mail: ahmet.elmaagacli@uni-duisburg-essen.de.
References
Author notes
A.H.E. and D.W.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal