In this issue of Blood, Patel-Hett et al demonstrate the requirement for spectrin in the appropriate development of the invaginated membrane system, formation of proplatelets and platelet biogenesis from murine megakaryocytes.1
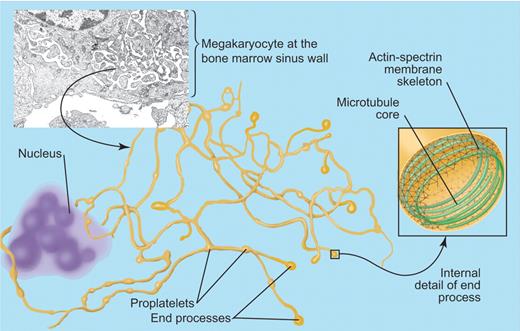
Platelet biogenesis. In vivo, megakaryocytes sit at the bone marrow sinus wall (top left), where they extend proplatelets into the vascular sinus. The membrane required for extension of the proplatelets is derived from the megakaryocyte invaginated membrane system. Proplatelets (shown in yellow) are formed by a microtubule mediated extension but also require reorganization of the actin-spectrin cytoskeleton for their formation. Platelet biogenesis occurs at the terminals of the proplatelets and is associated with the formation of platelet microtubule coils that may be linked to the actin-spectrin membrane skeleton. Electron micrograph by Fern Tablin. Professional illustration by Paulette Dennis.
Platelet biogenesis. In vivo, megakaryocytes sit at the bone marrow sinus wall (top left), where they extend proplatelets into the vascular sinus. The membrane required for extension of the proplatelets is derived from the megakaryocyte invaginated membrane system. Proplatelets (shown in yellow) are formed by a microtubule mediated extension but also require reorganization of the actin-spectrin cytoskeleton for their formation. Platelet biogenesis occurs at the terminals of the proplatelets and is associated with the formation of platelet microtubule coils that may be linked to the actin-spectrin membrane skeleton. Electron micrograph by Fern Tablin. Professional illustration by Paulette Dennis.
Over the past 2 decades considerable efforts have furthered our understanding of the role of the actin and microtubule cytoskeletons in the development of proplatelets and platelet biogenesis. Studies have shown that dynamic microtubule growth, extension, and eventual formation of microtubule coils, as well as filamentous actin assembly and reorganization, are required for these processes.
However, the exact mechanisms by which these 2 cytosketal systems are associated with one another and the plasma membrane have received little attention. Patel-Hett and colleagues define the role of erythroid and nonerythroid spectrins during proplatelet development and platelet formation.1 In erythrocytes, α1 β1 spectrin heterodimers form tetramers by head-to-head association, which confers the elasticity to the membrane skeleton required for withstanding shear forces in circulation. In addition, nonerythroid α2 β2 spectrins have been identified as components of the membrane skeleton of platelets as well as in megakaryocytes. Patel-Hett et al demonstrate the spatial and temporal characteristics of these spectrins using biochemical and morphologic analysis. They show that erythroid spectrins have a punctuate distribution, while nonerythroid spectrins are closely associated with the actin membrane skeleton of the invaginated membrane system (IMS) and plasma membrane. Using a dominant negative α2 spectrin construct, which has previously been shown to disrupt normal α-β tetramer organization, the requirement for spectrin in the formation of the IMS, the elongation of the proplatelet processes and eventual platelet biogenesis was demonstrated. Introduction of the construct before proplatelet elongation greatly diminshed this process, and its introduction subsequent to proplatelet formation resulted in the collapse of cytoskeletal structures and loss of normal architecture.
While the concept of proplatelet formation and platelet release as well as a role for the IMS and the cytoskeleton is not new, these elegant studies have considerably advanced our knowledge of megakaryocyte biology. There is a growing body of literature that has furthered the initial observations of proplatelet formation2 and the roles of the cytoskeleton3 and the IMS (see figure).4 Microtubules and actin play 2 distinctly different roles in proplatelet formation: microtubules have been shown to regulate extension, while actin provides the flexible membrane skeleton required for branching and final platelet formation5 The megakaryocyte IMS was intially proposed to demarcate platelet territories, but has since been shown to provide additional membrane required for the extension of proplatelet processes.6 Actin and actin-binding/severing proteins are associated with the IMS and required for normal IMS organization.7 The present study by Patel-Hett et al not only shows the interaction of spectrin with this membrane system, but convincingly demonstrates, through the use of a dominant negative construct, the absolute requirement of nonerythroid spectrins in the development of the IMS.
Spectrin has been shown to have physical flexibility, which would seem to make it an ideal molecular connection between the plasma membrane and the actin and microtubule cytoskeletons. The spectrin association with the platelet membrane skeleton is well studied, but how might spectrin be linked to the microtubule cytoskeleton during platelet biogenesis? We are informed by the studies of Patel and colleagues8 that indicate a role for the association of dynactin with microtubules during proplatelet formation. Dynactin, a molecular motor cofactor, which binds spectrin, may form one part of the network required for the elements of the cytoskeleton to work in concert during platelet biogenesis. Does spectrin associate directly with the proplatelet plasma membrane or the IMS? It may do so through its affinity for the anionic phospholipids, in particular phosphotidylinositol. Platelet glycoprotein Ib-IX-V has been shown to be present in lipid rafts as well as being the major transmembrane anchor for elements of the membrane skeleton including spectrin.9 It raises the interesting possibility that spectrin might provide an important link between the cytoskeletal elements and lipid rafts. Further examination of spectrin's membrane linkages will help to define additional critical components required for platelet biogenesis and build a comprehensive model of proplatelet and platelet formation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal