Congenital erythropoietic porphyria is caused by a deficiency in uroporphrynogen III synthase, the fourth enzyme in the heme biosynthetic pathway. In this issue of Blood, To-Figueras and colleagues present convincing genetic and biochemical evidence that the clinical phenotype of the rare disorder is markedly affected by the coinheritance of an activating mutation in the erythroid-specific isoform of δ-aminolevulinic acid synthase.1
Most physicians look on the porphyrias with a jaundiced eye. These disorders are rare, the clinical phenotypes are complex and highly variable, the laboratory evaluation is expensive and difficult to access, and the results are usually negative or inconclusive. Like jaundice, the porphyrias are in the province of both the gastroenterologist and the hematologist. The classification scheme currently in use helps to demystify this group of arcane and complex disorders. Those of most interest to the hematologist are the erythropoietic porphyrias: congenital erythropoietic porphyria (CEP) and congenital erythropoietic protoporphyria.
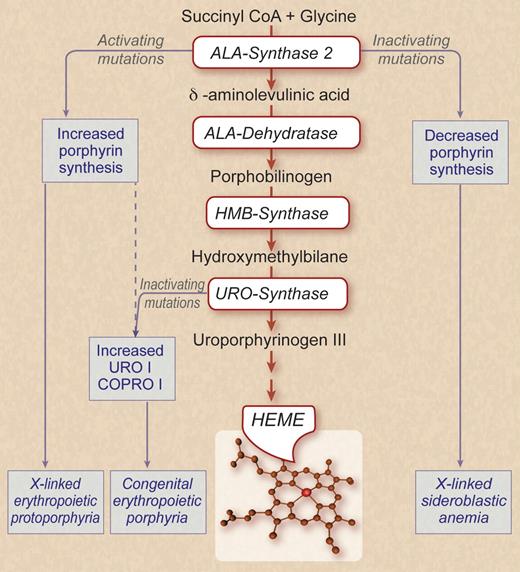
Because of the huge demand imposed on erythroid cells to produce hemoglobin, there is coordinated up-regulation of the expression of both globin and the enzymes required for the synthesis of heme. The figure depicts a simplified scheme of the heme biosynthetic pathway. The first 4 enzymatic steps, aminolevulinic acid synthase (ALAS), ALA dehydratase, hydroxymethylbilane synthase, and uroporphrynogen synthase (UROS), all use erythroid-specific isoforms for high-level heme production. Erythroid ALAS (ALAS-2) is encoded on a gene different from the housekeeping ALAS-1. The other 3 genes all have erythroid-specific promoters generated by alternate splicing. These promoters respond in a robust manner to erythroid-specific transcription factors.2
Patients with CEP are either homozygotes or, more often, compound heterozygotes with inactivating mutations in UROS. Enzymatic activity is low but never absent. The homozygous null mouse is embryonic lethal,3 and thus total lack of activity in humans is likely to be incompatible with life. As shown in the figure, UROS has a dual function, converting the linear tetrapyrole hydroxymethylbilane into the closed (cyclic) porphrynogen form and orienting the pyroles into the III isomer essential for the asymmetrical heme structure. When UROS is deficient there is not only a partial block in heme synthesis but, more importantly, markedly enhanced production of URO I and its derivatives. Further metabolism of URO I and other type I “phrynogens” cannot occur because a subsequent enzyme in the pathway, coproporphrynogen (COPRO) oxidase, is stereospecific for the III isomer. The accumulation of these toxic compounds is responsible for the clinical phenotype of CEP.
The clinical severity of CEP is only roughly proportional to the degree of UROS deficiency.2 Patients with relatively modest deficiencies usually have late onset of cutaneous photosensitivity with secondary inflammation. At lower levels of UROS the disease appears earlier in life with more extensive skin lesions and hemolytic anemia. Patients with the most severe phenotype have severe anemia and splenomegaly and are transfusion-dependent because of marked hemolysis and ineffective erythropoiesis. Their skin lesions are aggravated by excoriation and accompanied by hypertrichosis. They have pigmenturia along with red-brown fluorescent teeth. Their skin lesions are greatly aggravated by exposure to sunlight. There is probably some substance to the lore that so-called “werewolves” were victims of severe CEP and recluses from society, emerging only at night and shunning contact with others because of their bizarre physical deformities and grotesque appearance.4
Abundant synthesis of heme during erythropoiesis is enabled by erythroid-specific genes or mRNA isoforms for the first 4 enzymatic steps in heme biosynthesis. Congenital erythropoietic porphyria (CEP) is caused by inactivating mutations in URO-synthase, the enzyme responsible for the synthesis of uroporphrynogen III. Activating mutations in ALA synthase-2 (ALAS-2) can cause X-linked erythropoietic protoporphyria or, as To-Figueras et al now show, can aggravate the clinical and laboratory phenotype of CEP. Inactivating mutations of ALAS-2 cause hereditary X-linked sideroblastic anemia. ALA indicates aminolevulinic acid; HMB, hydroxymethylbilane; URO I, uroporphrynogen I; and COPRO I, coproporphrynogen I. Professional illustration by Debra T. Dartez.
Abundant synthesis of heme during erythropoiesis is enabled by erythroid-specific genes or mRNA isoforms for the first 4 enzymatic steps in heme biosynthesis. Congenital erythropoietic porphyria (CEP) is caused by inactivating mutations in URO-synthase, the enzyme responsible for the synthesis of uroporphrynogen III. Activating mutations in ALA synthase-2 (ALAS-2) can cause X-linked erythropoietic protoporphyria or, as To-Figueras et al now show, can aggravate the clinical and laboratory phenotype of CEP. Inactivating mutations of ALAS-2 cause hereditary X-linked sideroblastic anemia. ALA indicates aminolevulinic acid; HMB, hydroxymethylbilane; URO I, uroporphrynogen I; and COPRO I, coproporphrynogen I. Professional illustration by Debra T. Dartez.
Is the clinical variability of CEP because of nurture or nature? As mentioned, exposure to sunlight is the primary environmental irritant. Other extrinsic factors such as drugs could also affect the severity of CEP by impacting on the activity of ALAS-2, the rate-limiting step in heme biosynthesis. In pondering the contribution of nature to the phenotypic variability of CEP, To-Figueras et al wondered whether there might be concurrent mutations in other genes that participate in the pathway of heme biosynthesis. In 2008 several of their coauthors reported 8 families harboring 1 of 2 deletions in the 3′ end of the coding region of the erythroid-specific ALAS-2 gene that is located on the X chromosome.5 As indicated in the figure, the truncation of the ALAS-2 protein results in enhanced enzymatic activity resulting in the biochemical and clinical phenotype of erythropoietic protoporphyria, a disorder that was previously thought due only to inactivating mutations in ferrochelatase, the last enzymatic step in heme biosynthesis. Considering the extreme rarity of both CEP and X-linked erythropoietic protoporphyria, it is rather astounding that To-Figueras et al identified 1 of 4 unrelated CEP patients with the same UROS genotype to also have an ALAS-2 activating mutation. They then showed that the patient with the 2 mutant enzymes differed markedly from the other 3 in having more severe skin findings, splenomegaly, hemolytic anemia, and much more elevated urine and erythrocyte porphyrin levels. Clearly, in this patient the hyperactive ALAS-2 was fanning the fire, greatly increasing the substrate available to the defective UROS and causing enhanced levels of URO I and coproporphrynogen I (COPRO 1). The remarkable concurrence of these mutations raises the possibility that activating mutations of ALAS-2 may be more common than initially suspected, but brought to clinical awareness only through environmental stresses or in tandem with another mutation in a gene in the heme biosynthetic pathway.
This report should prompt clinicians to be on the lookout for other instances in which a disorder that is assumed to be monogenetic may be either exacerbated or ameliorated by the concurrence of another mutation within the same pathway or system. An example of the former are concurrent mutations in proteins affecting ciliary function resulting in variants of the Bardet-Biedl syndrome.6 An example of the latter are observations that patients with a given β-thalassemia genotype have a milder clinical and laboratory phenotype if they have also inherited 1 or more α-thalassemia genes.7,8
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal