Abstract
The Notch signaling pathway plays a fundamental role during blood vessel development. Notch signaling regulates blood vessel morphogenesis by promoting arterial endothelial differentiation and pro-viding spatial and temporal control over “tip cell” phenotype during angiogenic sprouting. Components of the Notch signaling pathway have emerged as potential regulators of lymphatic development, joining the increasing examples of blood vessel regulators that are also involved in lymphatic development. However, in mammals a role for the Notch signaling pathway during lymphatic development remains to be demonstrated. In this report, we show that blockade of Notch1 and Dll4, with specific function-blocking antibodies, results in defective postnatal lymphatic development in mice. Mechanistically, Notch1-Dll4 blockade is associated with down-regulation of EphrinB2 expression, been shown to be critically involved in VEGFR3/VEGFC signaling, resulting in reduced lymphangiogenic sprouting. In addition, Notch1-Dll4 blockade leads to compromised expression of distinct lymphatic markers and to dilation of collecting lymphatic vessels with reduced and disorganized mural cell coverage. Finally, Dll4-blockade impairs wound closure and severely affects lymphangiogenesis during the wound healing in adult mouse skin. Thus, our study demonstrates for the first time in a mammalian system that Notch1-Dll4 signaling pathway regulates postnatal lymphatic development and pathologic lymphangiogenesis.
Introduction
The lymphatic vasculature is critically required for survival of an organism and is responsible for many important functions including the regulation of interstitial fluid homeostasis, immune surveillance, and absorption of lipids (reviewed in Alitalo et al1 ). Lymphatic development is initiated when endothelial cells residing in the venous vasculature are induced to differentiate into the first lymphatic endothelial cells, which occurs at embryonic day 9 in the mouse.2,3 The signaling cascade that initiates lymphatic differentiation starts with expression of the transcription factors Sox18, followed by Prox1 within endothelial cells of the dorsal cardinal vein.2,3 The Sox18/Prox1-positive cells then bud off the vein in response to vascular endothelial growth factor (VEGF) receptor 3-VEGFC/D migration and proliferation cues, and they give rise to the lymphatic sacs.4,5 Through the process of lymphangiogenesis and recruitment of support cells, a functional lymphatic network is established. The mature lymphatic network is comprised of vessels of different function and caliber. Lymphatic capillaries are a dense network of blunt-ended vessels with loose endothelial cells connections and specialize in collecting fluid, macromolecules, and immune cells. The lymphatic capillaries drain into a network of lymphatic collecting vessels that specialize in fluid transport, have structural support cells, and have a system of one-way valves to direct fluid flow.
The signaling pathways involved in lymphatic endothelial cell differentiation, lymphangiogenesis, maturation, and survival are rapidly being identified. Despite the structural and functional differences between the blood vasculature and lymphatic vasculature, common signaling pathways, such as angiopoietein-Tie2, EphrinB2, activin receptor-like kinase 1 (ALK1), hepatocyte growth factor, platelet-derived growth factor B, and VEGFR3 are used for both systems.6-13
The mammalian Notch signaling pathway consists of 4 receptors (Notch1-4) and 5 ligands (Dll1/3/4 and Jagged1/2). Receptor-ligand interaction between neighboring cells results in proteolytic cleavage of the receptor, the release of the receptor intracellular domain, and its subsequent translocation to the nucleus where it regulates gene transcription through binding of transcriptional regulators. The Notch signaling pathway is critically required for specification of arterial endothelial cell fate and for providing spatial and temporal control over “tip cell” phenotype during angiogenic sprouting.14-18 Although the role of Notch signaling pathway during blood vessel development is well established,19-21 its function during lymphatic development have just started to emerge.22,23 Notch receptors have been found to be expressed in the lymphatics of both normal and tumor tissues.22,24 The Notch pathway has been shown to regulate expression of several important lymphatic genes such as Prox1, VEGFR3, and LYVE1 in vitro.25 Recently, it was shown in zebrafish that the Notch pathway regulates lymphatic development.23 In zebrafish partial silencing of Dll4 or its receptors resulted in defective lymphatic development associated with decreased lymphatic endothelial cell fate specification, and a reduction in migration/sprouting of lymphatic endothelial cells.23 However, it remains to be determined whether the findings in zebrafish can be extended to mice and other mammals. In mice, genetic inactivation of many Notch pathway components, including Notch1 and Dll4, are embryonic lethal before the onset of lymphatic vascular development, complicating the investigation of their role during this process.26,27 In this report, we take advantage of function-blocking antibodies targeting individual Notch receptors and ligands to investigate their roles in postnatal lymphatic development as well as lymphangiogenesis during cutaneous wound healing in mice.
Methods
Reagents
Notch1, Notch2, Dll4, and VEGF-A neutralizing antibodies were characterized previously and purified after transfection into mammalian cells.18,28,29 Human anti–mouse Jagged1/2 and Dll1 neutralizing antibodies are phage-derived antibodies and were purified after transfection into mammalian cells (unpublished reagents). Human anti-EphrinB2, human Dll4-ECD-Fc, and human VEGFR3-Fc were purified after transfection into mammalian cells. VEGFC-C137S was purified after transfection into mammalian cells, and its properties have been characterized previously.30 Goat anti-LYVE1 and goat anti-VEGFR3 were purchased from R&D Systems. Cy3–anti-smooth muscle actin and FITC-isolectin-B4 were purchased from Sigma-Aldrich. Rat anti-CD31 was purchased from BD Biosciences Pharmingen. Hamster anti-podoplanin and rabbit anti-LYVE1 were purchased from Cell Sciences. All secondary antibodies were chicken anti-(species on interest) Alexa-594, Alexa-488, or Alexa-647 purchased from Invitrogen. FITC-lectin was purchased from Vector Laboratories. HUVECs and adult human microvascular dermal lymphatic endothelial cells were purchased from Lonza. HUVECs were cultured in EGM-2 (Lonza), and adult human microvascular dermal lymphatic endothelial cells were cultured in EGM-2MV (Lonza) supplemented with 200 ng/mL VEGFC-C137S.
Tissue harvest
Mouse tail dermis and ear tissue were collected and stained as described previously.11 Retinas were collected and stained as described previously.18 Lymphatic drainage assays were conducted; 30 minutes before harvesting of the upper leg skin, 5 μL of FITC-lectin was injected subcutaneously into the footpad. The upper leg skin was then removed from the muscle and bone and immediately fixed in 4% paraformaldehyde. Lymphatic collecting vessels were stained for VEGFR3 and smooth muscle actin using same protocol as the tail dermis.
RNA extraction and real-time quantitative RT-PCR
For the Notch signaling stimulation assay, 10 000 HUVECs and lymphatic endothelial cells in 96-well plates were stimulated with human IgG or Dll4-ECD-Fc conjugated to BioMag goat anti–human magnetic beads (QIAGEN) for 3 hours. For the Notch signaling inhibition assay, 6000 lymphatic endothelial cells were plated into 96-well plate. The following day, the cells were transfected with OnTarget-Plus smart pool small-interfering RNA (siRNA) targeting control, Notch1, or Dll4 sequences using Dharmafect#1 (Thermo Fisher Scientific) according to manufacturer's recommendations. The following day, the media were changed and 100nM dibenzazepine (DBZ) was added to siControl wells; 48 hours later, samples were collected. RNA isolation, DNase treatment, and cDNA synthesis were performed using TaqMan Gene Expression Cells-to-CT kit (Applied Biosystems) according to manufacturer's protocol. Gene expression was analyzed using TaqMan Gene Expression Master Mix- (Applied Biosystems), GAPDH- (Hs00266705_g1), Hey2- (Hs00232622_m1), podoplanin- (Hs00366764_m1), VEGFR3- (Hs00176607_m1), EphrinB2- (Hs00970627_m1), and LYVE1 (Hs00272659_m1)-specific TaqMan Gene Expression assays (Applied Biosystems) and analyzed on a 7500 RT-PCR system (Applied Biosystems). Statistical significance was analyzed using a Student t test.
Transwell migration assay
Lymphatic endothelial cells were grown under confluent conditions for 48 hours in vehicle control or 100nM DBZ. Cells were resuspended in EBM-2 (Lonza) + 0.2% FBS. Then, 30 000 cells were added to the upper chamber of an 8-μm pore transwell (Falcon; BD Biosciences Discovery Labware) coated with fibronectin (Sigma-Aldrich). EBM2 + 0.2% FBS ± 300 ng/mL VEGFC-C137S was added to the lower well. After 16 hours, the cells on the bottom of the chamber were fixed, stained with SYTOX Green (Invitrogen), and the number of cells in 3 fields per well were counted. Four technical replicates were performed per experiment, and a minimum of 3 experimental replicates was used to generate represented data. Statistical significance was analyzed using a Student t test.
Wound-healing assay
Eight-week-old female C57BL/6 mice were used in this study. Circular wounds of 6 mm in diameter were generated at the scapular region of anesthetized females by excising skin and the subcutaneous fat and muscle panniculus carnosus using a punch (Stiefel, Olfenbach, Germany). αVEGF-A (5 mg/kg) or αDll4 (10 mg/kg) was administered intraperitoneally at day 2 and then every other day until day 8. Each group consisted of 5 mice. Macroscopic wound closure was determined by measuring the wound area until day 12.
Animal studies
Animal treatment schedule and treatment amount is listed in the figure legends. For each treatment, a minimum of 3 CD-1 pups were assessed in each of 3 litters. In all animal experiments, group differences were evaluated by Student t test. P values < .05 were considered significant. All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (publication 85-23, revised 1985). The Genentech Institutional Animal Care and Use Committee approved all animal protocols.
Image acquisition
Images were captured in Figures 1A through E, 3A and 4B using a 10×/0.45NA objective, in Figure 1B using a 40×/1.2NA water objective, in Figure 5B using a 5×/0.25NS, and in Figure 3B using a 25×/0.8NA objective on a Zeiss LSM510 confocal microscope. Images in Figures 1D and 2A were captured using a 10×/0.3NA objective on a Zeiss LSM510 Meta inverted confocal microscope. Images in Figure 2B were captured using a 5×/0.16NA objective and in Figure 2G using a 63×/1.2NA water objective on a Zeiss Axio Imager.Z2 with a Phototmetrics CoolSNAP HQ2 camera and Slidebook 5.0 software. Tissue was mounted in Fluromount-G purchased from Southern Biotech.
Results
Inhibition of Notch1-Dll4 signaling impairs lymphatic development in the neonatal mouse
To circumvent the early embryonic lethality observed with genetic inactivation of Notch pathway components, we used function-blocking antibodies targeting Notch1, Notch2, Jagged1/2, Dll1, and Dll4 to interrogate their role during postnatal lymphatic development.26,27 All antibodies have been demonstrated previously to specifically block the corresponding targets.18,28 In addition, their activities were confirmed in this study by assessing their activity on the retinal vascular development in neonatal mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article; data not shown).
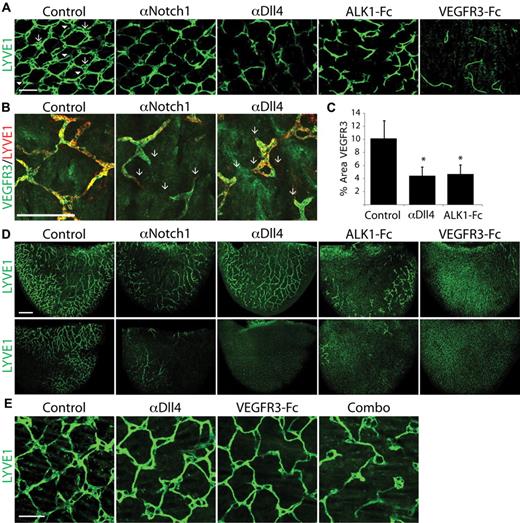
The neonatal mouse is a robust system that allows the in vivo investigation of multiple aspects of lymphatic development, including sprouting and remodeling of capillary lymphatic vessels and maturation of collecting vessels. Lymphatic development in the tail dermis is characterized by a stereotypic hexagonal “honeycomb” lattice of lymphatic capillaries.11 The honeycomb pattern develops after birth and by 8 days after birth (postnatal day [P]8) is established throughout the dermis but continues to undergo further remodeling. When the tail dermis was analyzed at P7 in pups treated with αNotch1 or αDll4, there was a marked defect in lymphatic development (Figure 1A-B). In control tissue, the lymphatic network has the stereotypic honeycomb morphology with a multiringed structure (arrows) at the connection points between individual honeycomb units that are interconnected by 1 or 2 (arrowhead) lymphatic vessels (Figure 1A-B). In animals treated with αNotch1 or αDll4, the ringed structures were mostly absent, and many of the honeycomb units were disconnected or only loosely connected by a single vessel (Figure 1A-B). Quantification of lymphatic vessel density, based on VEGFR3 staining, in the tail dermis confirmed αDll4 treatment decreases overall lymphatic density (Figure 1C). We demonstrated previously that ALK1, a type-I receptor of the TGFβ superfamily, is involved in lymphatic development.11 Blockade of ALK1 signaling with ALK1-Fc also resulted in defects in lymphatic development in the tail dermis (Figure 1A). In many experiments throughout our study, the effect of ALK1-Fc is compared with Notch blockade during lymphatic development. As expected, blocking VEGFC/D signaling with VEGFR3-Fc dramatically impairs lymphatic development (Figure 1A). Treatment with αNotch2, αDll1, or αJagged1/2 neutralizing antibodies had no discernible effect on the lymphatic development (supplemental Figure 2), suggesting Notch1-Dll4 interaction is uniquely required during lymphatic development in mouse. In addition to tail dermis, blocking Notch1 or Dll4 caused lymphatic defects in ear (discussed below) and intestine (data not shown), suggesting Notch1-Dll4 has a general role in postnatal lymphatic development.
Inhibition of Notch1 and Dll4 signaling impairs lymphatic development. (A) Lymphatic network in the P7 tail dermis isolated from neonates treated with PBS (control), αNotch1, αDll4, ALK1-Fc, and VEGFR3-Fc. In all treatments, LYVE1 staining was imaged at saturation to highlight the entire lymphatic network. The arrow indicates the multiringed structures that are interconnected by 1 or 2 (arrowhead) lymphatic vessels that are inhibited by Notch1/Dll4 blockade. Scale bar represents 200 μm. (B) LYVE1 and VEGFR3 costaining of the P7 tail dermis isolated from neonates treated with PBS (control), αNotch1, and αDll4. The arrows indicate the failed connection multiringed structures in αNotch1- or αDll4-treated pups, observed by both LYVE1 and VEGFR3 expression. Scale bar represents 200 μm. (C) Quantification of VEGFR3 staining in the tail dermis isolated from P7 neonates treated with PBS (control), αDll4, and ALK1Fc. n = 3, *P < .05. (D) LYVE1 staining of P7 ear tissue from neonates treated with PBS (control), αNotch1, αDll4, ALK1-Fc, and VEGFR3-Fc. Top panels are the inner surface and the lower panels are the outer surface of the ear. Scale bar represents 500 μm. (E) LYVE1 staining of P8 tail dermis isolated from neonates treated with PBS (control), αDll4, VEGFR3-Fc, or combination of αDll4 and VEGFR3-Fc treatments. Scale bar represents 200 μm. In panels A, B, and D, pups were injected with 10 mg/kg proteins on P2 and P5. In panel E. pups were injected with 10 mg/kg αDll4 on P3 and P5, VEGFR3-Fc on P7, or combination of both treatments.
Inhibition of Notch1 and Dll4 signaling impairs lymphatic development. (A) Lymphatic network in the P7 tail dermis isolated from neonates treated with PBS (control), αNotch1, αDll4, ALK1-Fc, and VEGFR3-Fc. In all treatments, LYVE1 staining was imaged at saturation to highlight the entire lymphatic network. The arrow indicates the multiringed structures that are interconnected by 1 or 2 (arrowhead) lymphatic vessels that are inhibited by Notch1/Dll4 blockade. Scale bar represents 200 μm. (B) LYVE1 and VEGFR3 costaining of the P7 tail dermis isolated from neonates treated with PBS (control), αNotch1, and αDll4. The arrows indicate the failed connection multiringed structures in αNotch1- or αDll4-treated pups, observed by both LYVE1 and VEGFR3 expression. Scale bar represents 200 μm. (C) Quantification of VEGFR3 staining in the tail dermis isolated from P7 neonates treated with PBS (control), αDll4, and ALK1Fc. n = 3, *P < .05. (D) LYVE1 staining of P7 ear tissue from neonates treated with PBS (control), αNotch1, αDll4, ALK1-Fc, and VEGFR3-Fc. Top panels are the inner surface and the lower panels are the outer surface of the ear. Scale bar represents 500 μm. (E) LYVE1 staining of P8 tail dermis isolated from neonates treated with PBS (control), αDll4, VEGFR3-Fc, or combination of αDll4 and VEGFR3-Fc treatments. Scale bar represents 200 μm. In panels A, B, and D, pups were injected with 10 mg/kg proteins on P2 and P5. In panel E. pups were injected with 10 mg/kg αDll4 on P3 and P5, VEGFR3-Fc on P7, or combination of both treatments.
The ear of early postnatal mouse is another useful model system to study lymphatic development. It has an inner layer of preexisting lymphatic network that undergoes further postnatal growth and branching and an outer layer of lymphatic vessels that develops after birth (supplemental Figure 3). αNotch1 or αDll4 treatment significantly decreased the lymphatic density in the developing ear, with the most dramatic effect on the lymphatic vessels in the outer surface of the ear (Figure 1D). In addition to the lymphatic vessels in the tail dermis and ear, there is an extensive network of blood vessels. Although the vascular density and caliber of blood capillaries in these neonatal tissues were moderately increased by the treatment of αNotch1 or αDll4, the overall vascular network remains intact (supplemental Figure 4; data not shown). Furthermore, cardiac injection of FITC-lectin into anesthetized control and αDll4-treated pups demonstrated perfusion of the tail capillary network after 3 minutes of circulation, suggesting the blood vasculature function is not significantly altered by αDll4 treatment (supplemental Figure 4B). These data suggest that the effect of αNotch1 and αDll4 on lymphatic development is direct and not caused by a general disruption of blood vascular function in these tissues.
The VEGFR3-VEGFC pathway is critically required for lymphatic development, and postnatal blocking of VEGFC prevents further lymphatic development and even causes regression of less mature lymphatic vessels.5,31 We next sought to explore whether Notch1 or Dll4 blockade sensitizes lymphatic vessels to VEGFC depletion. When the start of αDll4 and VEGFR3Fc treatment was delayed, the resulting lymphatic defect was less severe (Figure 1E), compared with early start (Figure 1A). However, the combined treatment of αDll4 and VEGFR3Fc had a much more dramatic effect (Figure 1E). Together, these findings clearly demonstrate a fundamental role of Notch1-Dll4 signaling pathway in early postnatal lymphatic development in mouse.
Blocking Notch1-Dll4 inhibits lymphatic vessel sprouting
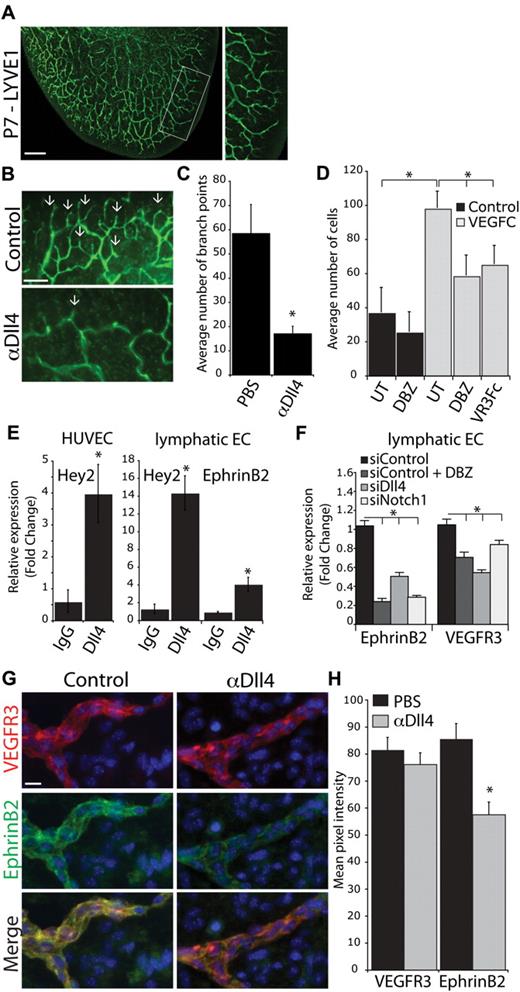
At P7, the lymphatic vasculature at the edge of the ear is undergoing dramatic sprouting, with multiple new branch points (Figure 2A). A detailed analysis of branching in the ear was undertaken to investigate the role of Notch signaling during this process. αDll4 treatment markedly reduced the number of branch points, and the remaining lymphatic vessels had a “smooth” appearance (Figure 2B arrow). Quantification of the branch points confirmed a defect in lymphatic sprouting and branching in αDll4-treated animals (Figure 2C).
Notch1-Dll4 inhibition blocks sprouting and EphrinB2 expression in lymphatic vessels. (A) LYVE1 staining of the inner lymphatic network of a P7 ear. Box represents the area used for quantification of lymphatic branching. Scale bar represents 500 μm. (B) LYVE1 staining of the ear in control and αDll4 (10 mg/kg on P2/4) treated pups at P7. Arrows indicate sites of lymphatic sprouting. Scale bar represents 200 μm. (C) Quantification of the number of lymphatic branch points in PBS and αDll4 neonates (n = 5; P < .05). (D) LECs transwell migration assay toward VEGFC (300 ng/mL). Cells were either untreated (UT) or pretreated for 48 hours with 100nM DBZ before transwell assay. VEGFR3-Fc (10 μg/mL, VR3Fc) effectively inhibition of directed LEC migration toward VEGFC (n = 4; *P < .05). (E) qPCR analysis for Hey2 and EphrinB2 expression in lymphatic endothelial cells and HUVECs stimulated with human IgG or hDll4-Fc for 3 hours. (F) qPCR analysis for EphrinB2 and VEGFR3 expression in lymphatic endothelial cells treated with 100nM DBZ or transfected with siRNA targeting Dll4 or Notch1 (n = 3; *P < .05). (G) VEGFR3 and EphrinB2 costaining in P7 tail dermis isolated from neonates treated with PBS (control) or αDll4 (10 mg/kg on P2/5). Scale bar represents 10 μm. (H) Quantification of VEGFR3 and EphrinB2 expression in control and αDll4 treated pups (n = 3; *P < .01).
Notch1-Dll4 inhibition blocks sprouting and EphrinB2 expression in lymphatic vessels. (A) LYVE1 staining of the inner lymphatic network of a P7 ear. Box represents the area used for quantification of lymphatic branching. Scale bar represents 500 μm. (B) LYVE1 staining of the ear in control and αDll4 (10 mg/kg on P2/4) treated pups at P7. Arrows indicate sites of lymphatic sprouting. Scale bar represents 200 μm. (C) Quantification of the number of lymphatic branch points in PBS and αDll4 neonates (n = 5; P < .05). (D) LECs transwell migration assay toward VEGFC (300 ng/mL). Cells were either untreated (UT) or pretreated for 48 hours with 100nM DBZ before transwell assay. VEGFR3-Fc (10 μg/mL, VR3Fc) effectively inhibition of directed LEC migration toward VEGFC (n = 4; *P < .05). (E) qPCR analysis for Hey2 and EphrinB2 expression in lymphatic endothelial cells and HUVECs stimulated with human IgG or hDll4-Fc for 3 hours. (F) qPCR analysis for EphrinB2 and VEGFR3 expression in lymphatic endothelial cells treated with 100nM DBZ or transfected with siRNA targeting Dll4 or Notch1 (n = 3; *P < .05). (G) VEGFR3 and EphrinB2 costaining in P7 tail dermis isolated from neonates treated with PBS (control) or αDll4 (10 mg/kg on P2/5). Scale bar represents 10 μm. (H) Quantification of VEGFR3 and EphrinB2 expression in control and αDll4 treated pups (n = 3; *P < .01).
Because VEGFR3-VEGFC signaling regulates the lymphatic sprouting and lymphatic endothelial cell (LEC) migration, we investigated the impact of Notch inhibition on LEC migration toward VEGFC. We found that pretreatment of LECs with the γ-secretase inhibitor DBZ, a pan-Notch inhibitor, resulted in significantly reduced LEC migration in response to VEGFC. This result suggests a potential crosstalk between Notch signaling pathway and VEGFC signaling, and raises the possibility that the defect in lymphatic sprouting on αDll4 treatment is because of impaired response to VEGFC.
Notch1-Dll4 regulates EphrinB2 expression in lymphatic vessels
The Notch pathway has been demonstrated previously to directly regulate VEGFR3 expression in both vascular and lymphatic endothelial cells in vitro.22,25 Therefore, it is possible that decreased VEGFR3 expression after Notch1-Dll4 blockade might contribute to the observed in vivo lymphatic defects. To investigate regulation of VEGFR3 expression in vitro and in vivo, we examined its expression when the Notch signaling is inhibited. Hey2, a known downstream target gene of Notch1, was up-regulated in HUVECs stimulated with immobilized extracellular domain of Dll4 (Figure 2E). Similarly, Hey2 expression was up-regulated in LECs stimulated with Dll4, demonstrating the Notch pathway is intact in these cells. However, VEGFR3 expression was only modestly down-regulated by DBZ treatment or gene silencing of Notch1 or Dll4 (Figure 2F). In addition, we examined the expression of VEGFR3 in vivo in tissues collected from neonatal mice treated with αDLL4. Immunostaining and quantification of VEGFR3 expression revealed no significant difference between control and αDll4-treated pups (Figure 2G-H). These data suggest that it is unlikely that the lymphatic defects observed after Notch1-Dll4 blockade are driven by altered VEGFR3 expression.
In a recent a study, EphrinB2 was demonstrated to play a critical role in regulating lymphatic endothelial response to VEGFR3-VEGFC signaling.32 EphrinB2 is a transmembrane ligand for the receptor tyrosine kinase EphB4 and similar to Notch1 and DLL4, is expressed by arterial endothelial cells.9 It is well established that EphrinB2-EphB4 interaction provides repulsive cues at the arterial-venous boundaries to prevent intermixing of arterial- and venous-fated endothelial cells.9 It is thought that EphrinB2-EphB4 interaction leads to EphB4-mediated forward signaling as well as EphrinB2-mediated reverse signaling. In addition to the well established role of EphrinB2-EphB4 signaling in blood vessel development, EphrinB2 also was demonstrated to be required for lymphatic vessel sprouting and remodeling.10 In LECs, EphrinB2 expression was shown to be required for the internalization of VEGFR3 on VEGFC binding, to ensure sustained VEGFR3 signaling.32 Given that Notch signaling regulates EphrinB2 expression during blood vessel development, we reason that the down-regulation of EphrinB2 in the lymphatic endothelium might contribute to the observed lymphatic development defect resulting from Notch1/Dll4 blockage.33 We found that in cultured LECs, activation of Notch signaling by immobilized Dll4 up-regulated EphrinB2 expression (Figure 2E). In addition, the inhibition of Notch signaling by treatment with the γ-secretase inhibitor DBZ or gene silencing of Notch1 or Dll4 markedly down-regulated EphrinB2 expression (Figure 2F). Importantly, EphrinB2 is expressed in the lymphatic vasculature in the tissues where we observed lymphatic defects in vivo, although at a much lower level than in the blood vessels (Figure 2G; supplemental Figure 7). Quantification of EphrinB2 immunostaining demonstrated a significant reduction in expression in lymphatic vessels on αDll4 treatment (Figure 2G-H). Together, our data suggest that the Notch1/Dll4 signaling pathway modulates the response of LECs to VEGFC by regulating EphrinB2 expression.
Notch inhibition leads to lymphatic collecting vessel dilation and decreased coverage of mural cells
During vascular development, the Notch pathway regulates remodeling of the vascular plexus into a functional network of arteries, capillaries, and veins.18,34 Enforced Notch activation is sufficient to induce arterialization of the venous vasculature,35 whereas the deficiency of Notch1 or Dll4 results in the up-regulation of venous markers and failed arterial specification.18,34 EphrinB2 has been demonstrated to play an important role downstream of Notch signaling during segregation of arterial endothelial cells from venous endothelial cells and recruitment of support cells in the blood vasculature.36,37 During lymphatic development, loss of EphrinB2-reverse signaling results in dilation of the lymphatic collecting vessels, irregular recruitment of support cells, and the absence of lymphatic valves.10 We next investigated these additional aspects of lymphatic development when the Notch pathway was inhibited.
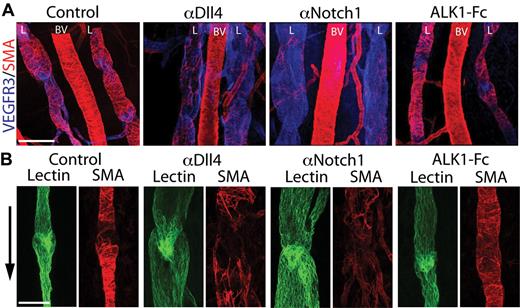
The skin of the upper leg contains a network of lymphatic collecting vessels of different caliber, including 2 large lymphatic collecting vessels that drain into the popliteal lymph node. Lymphatic drainage through the skin of the lower leg can be visualized by injection of FITC-lectin into the footpad. Defects of lymphatic collecting vessels can then be assessed by the presence or absence of FITC-lectin in the lymph node and the overall structural appearance. When mouse neonates were treated with αDll4, FITC-lectin drainage to the lymph nodes or thoracic duct was largely unaffected, suggesting the overall lymphatic function was not severely affected (supplemental Figure 5). However, Dll4-blockade was associated with a significant decrease in mural cell coverage of both large- and small-caliber lymphatic collecting vessels (Figure 3A-B; supplemental Figure 5). By comparison, when the ALK1 pathway is inhibited by ALK1-Fc treatment, the collecting lymphatic vessels had normal mural coverage and caliber (Figure 3A-B).
Notch inhibition causes lymphatic collecting vessel dilation and decreased coverage of mural cells. (A) VEGFR3 (blue) and SMA (red) costaining of the upper leg skin in P6 neonate treated with PBS (control), αDll4, αNotch1, and ALK1-Fc (10 mg/kg on P2/5). Images show the lymphatic vessels (L) that drain into the popliteal lymph node on either side of a blood vessel (BV). Scale bar represent 200 μm. The smooth muscle actin (SMA) staining in αNotch sample was imaged at a higher exposure setting due the reduction of mural cell coverage of the lymphatic vessels. (B) FITC-lectin (green) and SMA (red) staining of a lymphatic valve in a P6 neonate treated with PBS (control), αDll4, αNotch1, and ALK1-Fc (10 mg/kg on P2/5). Arrow indicates the direction of lymph flow, and scale bar represents 100 μm.
Notch inhibition causes lymphatic collecting vessel dilation and decreased coverage of mural cells. (A) VEGFR3 (blue) and SMA (red) costaining of the upper leg skin in P6 neonate treated with PBS (control), αDll4, αNotch1, and ALK1-Fc (10 mg/kg on P2/5). Images show the lymphatic vessels (L) that drain into the popliteal lymph node on either side of a blood vessel (BV). Scale bar represent 200 μm. The smooth muscle actin (SMA) staining in αNotch sample was imaged at a higher exposure setting due the reduction of mural cell coverage of the lymphatic vessels. (B) FITC-lectin (green) and SMA (red) staining of a lymphatic valve in a P6 neonate treated with PBS (control), αDll4, αNotch1, and ALK1-Fc (10 mg/kg on P2/5). Arrow indicates the direction of lymph flow, and scale bar represents 100 μm.
Defects in lymphatic valve development are commonly associated with dilation of the collecting vessels.10 The lymphatic valves have a characteristic V-shape and extend into the lumen of the collecting vessel. However, the lymphatic valve structures seem normal in αDll4-treated pups, which is consistent with the apparently normal lymphatic drainage function in the treated animals (Figure 3B). These data suggest that postnatal Notch blockade does not significantly affect the structure and function of lymphatic valves.
Regulation of lymphatic differentiation by the Notch pathway
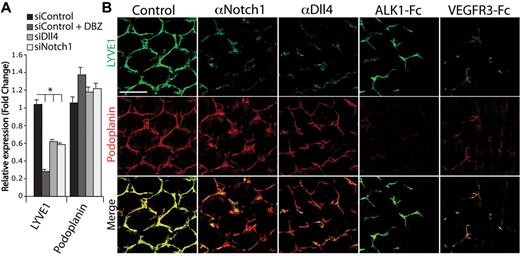
To gain additional insights into the lymphatic defect caused by Notch1-Dll4 inhibition, we investigated the expression of genes involved in lymphatic development. We found that LYVE1 was significantly down-regulated in cultured LECs treated with the Notch inhibitor DBZ or transfected with siRNA targeting Notch1 or Dll4 (Figure 4A). The down-regulation of LYVE1 expression also was observed in the tail dermis of pups treated with αDll4 or αNotch1 (Figure 4B). These findings are consistent with recently published reports where constitutive activation of Notch1 up-regulated LYVE1 expression.25 In contrast, the expression of podoplanin, another lymphatic maker, was not affected by Notch blockade both in vitro and in vivo (Figure 4A-B). No significant change in expression was observed for several other lymphatic genes when Notch signaling was inhibited (supplemental Figure 6). Interestingly, in comparison to αDll4 or αNotch1, ALK1-Fc treatment had the opposite effect on podoplanin and LYVE1 expression, where podoplanin expression was decreased and LYVE1 expression was unchanged (Figure 4B; previous report11 ). These data suggest Notch1-Dll4 regulates a distinct set of lymphatic markers.
Regulation of lymphatic differentiation by the Notch pathway. (A) qPCR analysis for LYVE1 and podoplanin expression in lymphatic endothelial cells treated with 100nM DBZ or transfected with siRNA targeting Dll4 or Notch1 (n = 3; *P < .05). (B) LYVE1 (green) and podoplanin (red) staining of P7 tail dermis isolated from neonates treated with PBS (control), αNotch1, αDll4, ALK1-Fc, and VEGFR3-Fc (10 mg/kg on P2/5). Scale bar represents 200 μm.
Regulation of lymphatic differentiation by the Notch pathway. (A) qPCR analysis for LYVE1 and podoplanin expression in lymphatic endothelial cells treated with 100nM DBZ or transfected with siRNA targeting Dll4 or Notch1 (n = 3; *P < .05). (B) LYVE1 (green) and podoplanin (red) staining of P7 tail dermis isolated from neonates treated with PBS (control), αNotch1, αDll4, ALK1-Fc, and VEGFR3-Fc (10 mg/kg on P2/5). Scale bar represents 200 μm.
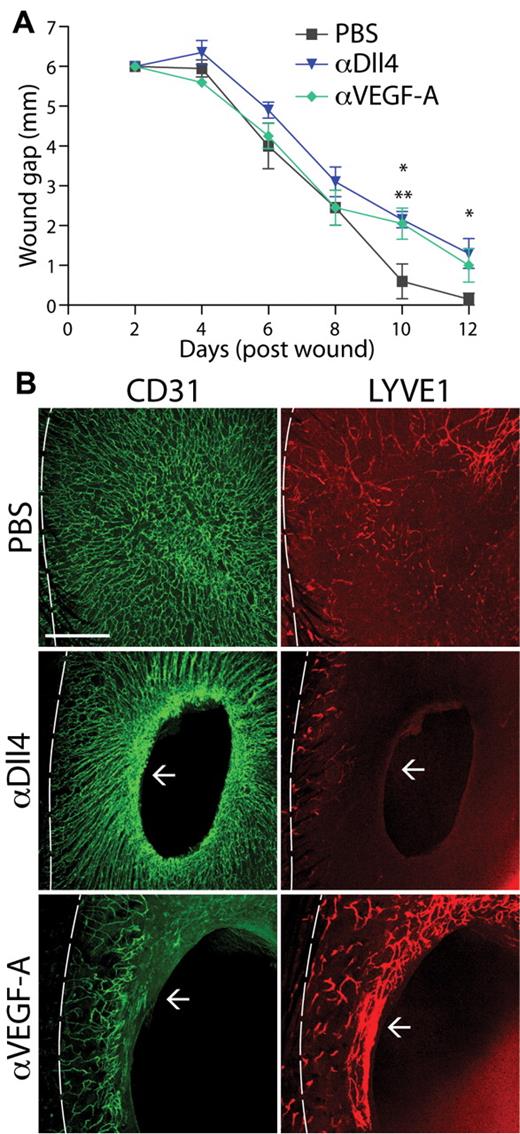
Dll4 blockade results in defective lymphangiogenesis in wound healing
Cutaneous wound healing requires the precise control of many cells types during the process of ingrowth of granulation tissue and re-epithelialization of a wound. In response to inflammatory cytokines and growth factors, angiogenesis and lymphangiogenesis are initiated to aid in the wound closure and healing processes. γ-Secretase inhibition of Notch signaling was shown to delay the wound healing process.38 However, Notch1 is expressed by skin keratinocytes and could account for the delayed wound healing.38 We used a full thickness skin biopsy assay in adult mice to study the role of Dll4 during wound healing. In this assay, a 6-mm-diameter full-thickness skin wound is made on the back of the mouse. The rate of wound closure was measured, and the effect on angiogenesis and lymphangiogenesis was monitored by CD31 and LYVE1 staining, respectively. In control mice, wound closure was associated with a robust angiogenic and lymphangiogenic response (Figure 5B). When the mice were treated with αDll4, there was a significant delay in wound closure (Figure 5A). Consistent with the previously reported observation in tumor angiogenesis, blockade of Dll4 caused excessive angiogenic sprouting and resulted in a dramatic increase in blood vessel density at the wound edge (Figure 5B arrow). However, there was almost an absence of LYVE1-positive lymphatic vessels in the wounds of αDll4-treated animals (Figure 5B). These results are consisted with our findings that αDll4 prevents lymphangiogenesis while promoting nonfunctional angiogenesis in the neonate mouse (Figure 1; supplemental Figure 1). There was a similar delay in the rate of wound closure in αVEGF-treated mice (Figure 5A) and as expected αVEGF caused a significant decrease in angiogenesis (Figure 5B). Interestingly, there seemed to be a more robust lymphangiogenesis response in αVEGF-treated animals at the edge of the wound; however, the underlying mechanism for the increased lymphangiogenesis requires further investigation (Figure 5 arrow). Wound healing is a complex process that involves coordinated activity and integrated function of multiple cell types. Although in the wound healing process Notch signaling might exert potent effects on multiple targets, including blood endothelial cells14-18 and inflammatory cells,39 our data show that disruption of Notch signaling has a marked impact on lymphangiogenesis. Collectively, our data demonstrate that Notch1-Dll4 signaling pathway is fundamentally important not only during postnatal lymphatic development but also in pathologic lymphangiogenesis.
Notch blockade results in defective lymphangiogenesis during wound healing. (A) Wound gap size in control (PBS), αDll4, and αVEGF-A treated mice for 12 days after punch biopsy (n = 5; *αDll4 treatment, P < .05; **αVEGF-A treatment, P < .05). (B) Images of CD31 (green) and LYVE1 (red) costaining of the skin dermis in PBS- (control), αDll4-, or αVEGF-A–treated mice 12 days after a full thickness cutaneous wound. Dotted line represents the approximate skin/wound margin and arrow represents the edge of wound closure. Scale bar represents 500 μm.
Notch blockade results in defective lymphangiogenesis during wound healing. (A) Wound gap size in control (PBS), αDll4, and αVEGF-A treated mice for 12 days after punch biopsy (n = 5; *αDll4 treatment, P < .05; **αVEGF-A treatment, P < .05). (B) Images of CD31 (green) and LYVE1 (red) costaining of the skin dermis in PBS- (control), αDll4-, or αVEGF-A–treated mice 12 days after a full thickness cutaneous wound. Dotted line represents the approximate skin/wound margin and arrow represents the edge of wound closure. Scale bar represents 500 μm.
Discussion
The evolutionarily conserved Notch signaling pathway is critically required for proper blood vessel development. Our current study demonstrates an important role of Notch signaling in regulating postnatal lymphatic development and pathologic lymphangiogenesis in mice. Our findings are consistent with a recent report demonstrating a role of Notch signaling in the formation and wiring of lymphatic network in zebrafish.23
In mammals, Notch signaling pathway consists of 4 receptors (Notch1-4) and 5 ligands (Dll1/3/4 and Jagged1/2). Targeted deficiency of Notch signaling components results in early embryonic lethality before the onset of lymphangiogenesis because of defective cardiovascular development, making it difficult to investigate the role of Notch signaling in lymphatic development and function.26,27 In this study, we took advantage of specific function-blocking antibodies against Notch1, Notch2, Dll1, Dll4, and Jagged1/2. We found that blocking Notch1 and Dll4, but not other Notch components, had profound effect on lymphatic development in neonatal mice. This finding is reminiscent of what has been established in blood vascular development where Notch1-Dll4 interaction seems to be the major Notch pathway in regulating blood vascular development.15,28
Notch signaling regulates the remodeling of vascular plexus into a functional network of veins, capillaries, and arteries.34 Mechanistically, Notch signaling promotes arterial endothelial differentiation. In venous endothelial cells, however, Notch activity is repressed by the COUP-TFII orphan nuclear receptor to maintain the vein identity.40 In mice, lymphatic differentiation starts with induction of the transcription factors Sox18, followed by the expression of Prox1, a homeobox transcription factor, within endothelial cells of the dorsal cardinal vein.2,3 Prox-1 regulates the expression of lymphatic markers including podoplanin and LYVE1. In our study, we show that Notch1-Dll4 signaling is involved in regulating the differentiation of lymphatic endothelial cells. We found that blocking of Notch1-Dll4 signaling down-regulated LYVE1 and EphrinB2 both in vitro and in vivo. These findings are consistent with a previous report demonstrating that constitutive activation of Notch1 up-regulates LYVE1 and EphrinB2 in vitro.25 It remains to be determined how Notch signaling interacts with other transcriptional programs in lymphatic endothelial cells to induce and maintain the lymphatic cell fate. Fundamentally, it is still an open question how Notch signaling pathway regulates 2 apparently competing cell-fate decisions, venous to arterial endothelial specification as opposed to venous to lymphatic endothelial differentiation.
One important aspect of Notch1-Dll4 signaling during blood vessel development is to constrain the angiogenic response to VEGF by negatively regulating the expression of VEGFR2.17,18 Notch-blockade results in up-regulation of VEGFR2 and allows the blood endothelial cells to adapt an explorative migrating “tip cell” behavior, leading to excessive angiogenic sprouting and increased vascular density. In contrast, our data demonstrate that during postnatal lymphatic development, Notch inhibition decreases lymphatic density, sprouting, and tip-cell morphology. This apparent difference is probably attributable to how VEGFR3/VEGFC signaling, the major driver of lymphangiogenic sprouting, is differentially regulated by Notch signaling. It was reported previously that Notch activation leads to the up-regulation of VEGFR3 expression and the down-regulation of VEGFR2 expression in vitro.22 In our study, we investigated the regulation of VEGFR3 expression in vitro and in vivo when the Notch signaling is inhibited. In vitro we found that Notch1-DLL4 blockade causes modest reduction of VEGFR3 expression. However, we failed to detect significant change in VEGFR3 expression in vivo after Notch1-Dll4 inhibition. This apparent discrepancy suggests a more complex regulation of VEGFR3 in lymphatic endothelial cells and requires further investigation.
Recently, it was shown that EphrinB2 plays a critical role in VEGFR3 signaling. In LECs, it is required for the effective internalization of VEGFR3 on VEGFC binding to ensure sustained VEGFR3 signaling.32 In addition, it is well established that Notch signaling regulates EphrinB2 expression during blood vessel development. These findings prompted us to examine the potential involvement of EphrinB2 in the lymphatic defects resulting from Notch1-DLL4 inhibition. We found that in cultured LECs the expression of EphrinB2 is tightly associated with the status of Notch activation. More importantly, EphrinB2 expression in lymphatic vessels is markedly reduced on αDll4 treatment. Together, these findings suggest that the Notch1-DLL4 pathway regulates lymphatic development by modulating VEGFR3 signaling via control over EphrinB2 expression.
Another important aspect of Notch1-Dll4 signaling during blood vessel development is to promote the remodeling of the primary capillary plexus into a network of arteries, veins, and capillaries.34 Enforced Notch activation is sufficient to induce arterialization of the venous vasculature,35 whereas the deficiency of Notch1 or Dll4 results in the up-regulation of venous markers and failed arterial specification.18,34 EphrinB2 has been demonstrated to play an important role downstream of Notch signaling during segregation of arterial endothelial cells from venous endothelial cells and recruitment of support cells in the blood vasculature.36,37 In the current study, we demonstrate that Notch1/Dll4 inhibition resulted in decreased EphrinB2 expression in the lymphatic vasculature, decreased recruitment of mural cells to the collecting vessels, and dilation of the lymphatic collecting vessels. Together, these findings suggest a shared mechanism between the 2 vascular systems with regard to mural cell and endothelial cell interaction regulated by Notch signaling.
Tumor metastases are responsible for the majority of patient deaths caused by solid tumors.41 The presence of tumor cells in regional lymph nodes in many tumor types suggests that the lymphatic vasculature is an important route for the dissemination of human cancers. Recent findings from experimental models of lymphatic metastasis and clinicopathologic analyses suggest that lymphangiogenesis induced by tumor cells can promote tumor cell spread to distant sites. Therefore, targeting tumor lymphangiogenesis may be a potential therapeutic strategy to restrict cancer cell metastasis via tumor lymphatics. VEGFC is a key factor of lymphangiogenesis and promotes metastasis in many tumor models. Inhibition of VEGFC axis is considered to be a promising approach to limiting tumor dissemination. In the current study, we show that blocking Notch-Dll4 attenuates the response of lymphatic endothelial cells to VEGFC and enhances the effect of VEGFC depletion on lymphatic development. Furthermore, we demonstrate that αDll4 inhibits lymphangiogenesis during wound healing in adult mouse skin. These findings raise an important possibility that targeting Dll4 may negatively impact metastasis by inhibiting tumor lymphangiogenesis. In preclinical tumor model studies, Dll4 blockade is associated with an increase in tumor vessel density. However, these tumor vessels are poorly perfused, resulting in an inhibition of tumor growth.18,42-44 Therefore, targeting Notch1-Dll4 signaling pathway may provide additional benefits in cancer therapy by simultaneous disrupting both tumor angiogenesis and lymphangiogenesis. Future studies are required to explore whether blocking of Notch1-Dll4 can indeed lead to a reduction in functional tumor lymphatics and prevent tumor cells from exiting the primary tumor mass.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors kindly thank Drs Greg Plowman and Weilan Ye for helpful discussions.
National Institutes of Health
Authorship
Contribution: K.N. and M.Y. designed the experiments; K.N., G.Z., J.B.R., and H.C. performed the experiments; G.K. performed the wound healing assay; antibody characterization was performed by C.W.S.; K.N. and M.Y. wrote the paper; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Minhong Yan, Genentech Inc, Department of Molecular Biology, 1 DNA Way, MS 93B, South San Francisco, CA 94080; e-mail: minhong@gene.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal