Abstract
Hyperhomocysteinemia (HHcy) increases permeability of the blood-brain barrier, but the mechanisms are undetermined. Homocysteine (Hcy) is an agonist of the neuronal N-methyl-D-aspartate receptor (NMDAr). We tested the hypothesis that HHcy disrupts the blood-brain barrier by an NMDAr-dependent mechanism in endothelium. In brain microvascular endothelial cells, there was no change in expression of the adherens junction protein VE-cadherin with Hcy treatment, but there was a significant decrease in the amount of β-catenin at the membrane. Moreover, Hcy caused nuclear translocation of β-catenin and attachment to the promoter for the tight junction protein claudin-5, with concomitant reduction in claudin-5 expression. Using a murine model of HHcy (cbs+/−), treatment for 2 weeks with an NMDAr antagonist (memantine) rescued cerebrovascular expression of claudin-5 and blood-brain barrier permeability to both exogenous sodium fluorescein and endogenous IgG. Memantine had no effect on these parameters in wild-type littermates. The same results were obtained using an in vitro model with brain microvascular endothelial cells. These data provide the first evidence that the NMDAr is required for Hcy-mediated increases in blood-brain barrier permeability. Modulating cerebral microvascular NMDAr activity may present a novel therapeutic target in diseases associated with opening of the blood-brain barrier in HHcy, such as stroke and dementia.
Introduction
Microvessels are responsible for facilitating the exchange of nutrients between blood and tissue while maintaining a barrier against the undesired movement of other molecules. This barrier is most finely tuned and structurally competent at the interface between the blood and the brain, the blood-brain barrier (BBB). The BBB is formed by intercellular junctions that limit paracellular diffusion combined with a low-flux transcellular system that selectively transports molecules either into or out of the brain parenchyma. Increasing evidence implicates chronic BBB permeability in the onset of neurologic and neurovascular diseases (eg, Alzheimer disease, dementia, small-vessel disease, stroke).1 Although the BBB prevents the flux of harmful substances into the brain, it can also exclude the delivery of therapeutic drugs. Understanding the molecular regulation of the BBB is therefore important for the understanding of disease etiology and treatment.
Paracellular diffusion of molecules at the BBB is minimized by endothelial cell-cell (EC-EC) junctions. Chief among these are the adherens junctions and tight junctions, of which vascular-endothelial cadherin (VE-cadherin; VEC) and claudin-5 are well-known members, respectively. Although VEC is endothelial specific, the claudin family of proteins is found in many epithelial tissues. However, claudin-5 is specific to tight junctions of ECs and is required to block paracellular diffusion of small molecules (< ∼ 800 Da) in the microvasculature of the brain.2 In particular, claudin-5 is now recognized as the predominant claudin isoform in tight junctions of the BBB. Although claudins 1, 10, 11, and 12 (at least) are also expressed in brain endothelium, claudin-5 mRNA is nearly 600-fold higher than the other claudins expressed in microvascular ECs freshly isolated from the brain.3 On electron microscopy, plasma membranes of adjacent cells associate close enough to merge without apparent intercellular space at tight junctions.4
Homocysteine (Hcy) is an intermediate aminothiol derived from methionine catabolism, and its elevation in plasma, known as hyperhomocysteinemia (HHcy), is a chronic condition associated with cerebral small-vessel disease, stroke, and dementia (Alzheimer and vascular).5,6 The list of Hcy-mediated vasculopathies is still growing but includes endothelial dysfunction, vessel wall malformations, loss of extracellular matrix collagen, and loosening of the BBB in rodents and humans.5,6 Mounting evidence suggests that an increase in BBB permeability is an important factor in initiating cerebral small-vessel disease and lacunar stroke.7,8 Hence, identifying the mechanisms by which HHcy opens the BBB is of great clinical interest. We reported that treating human umbilical vein ECs with Hcy increases EC monolayer permeability and decreases expression of claudin-5.9 However, the mechanism by which Hcy triggers these events has not been elucidated, and the intracellular mechanisms for decreased expression of claudin-5 in HHcy are unknown.
Recently, a novel mechanism for regulation of claudin-5 expression by the VEC/β-catenin complex was reported.10 In this model, β-catenin is quenched by incorporation into a VEC complex at the EC membrane. If this quenching is impaired, β-catenin accumulates in the nucleus where it stabilizes forkhead box O1 (FOXO1) at position −2906 to −2871 on the claudin-5 promoter and represses its transcription.10 Although several receptors are hypothesized to modulate the VEC/β-catenin complex (eg, vascular endothelial growth factor receptors), the sensor for Hcy remains unknown.
A fundamental gap in knowledge of the mechanisms by which Hcy affects endothelium is whether there is a receptor to mediate intracellular signal transduction in response to increases in Hcy concentration in the blood. A survey of the literature identifies the ionotropic glutamate receptor, N-methyl-D-aspartic acid receptor (NMDAr), as a receptor for Hcy in neurons.11 NMDAr is expressed in cerebral endothelium and involved in glutamate-induced damage to EC integrity by disrupting tight junctions at EC-EC contacts and increasing permeability.12,13 Memantine is an NMDAr antagonist prescribed for moderate to severe cognitive impairment that blocks the NMDAr from overexcitation. Memantine rescued the increase in BBB permeability in an experimental model of multiple sclerosis.14 Building on this knowledge-base, the NMDAr is a rational target for therapeutic intervention in HHcy in the context of BBB disruption, but data exploring this approach are not available.
The purpose of these experiments was to determine the NMDAr dependence of Hcy-mediated increases in paracellular permeability at the BBB. In the first set of experiments, we tested the hypothesis that Hcy uncouples the VEC/β-catenin complex and causes nuclear accumulation of β-catenin where it binds the FOXO1 repression domain of the claudin-5 promoter, resulting in decreased claudin-5 protein expression. In the second set of experiments, we used in vitro and in vivo models to test the hypothesis that memantine-sensitive mechanisms are responsible for the reduction in claudin-5 expression and opening of the BBB by Hcy.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Idaho State University. All reagents used were purchased from Fisher Scientific or Sigma-Aldrich unless indicated otherwise. For all experiments, the Hcy used was D/L-Hcy purchased from Sigma-Aldrich.
Brain microvascular EC culture
An immortalized murine cell line of cerebral microvascular ECs (bEnd.3)15 was purchased from ATCC, aliquoted, and stored as recommended. The bEnd.3 cells were cultured at 37°C in a humidified incubator with 5% CO2 balance room air in DMEM high glucose (DMEM-H; Invitrogen), 10% BCS (Hyclone; Fisher Scientific), and 1× gentamicin/amphotericin B (Invitrogen) in plastic culture flasks. All experiments were performed with cells from the third to sixth passages.
Brain microvascular EC treatments
All cells were studied 3 days after confluence; culture media was changed to 5% BCS for 24 hours and then serum-free for 2 hours before treatment. D, L-Hcy (Sigma-Aldrich), N-methyl-D-aspartic acid (NMDA; Tocris), 3,5-dimethyl-tricyclo[3.3.1.13,7]decan-1-amine hydrochloride (memantine; Tocris), and (+)-5-methyl-10,11- dihydro-5H-dibenzo[a, d]cyclohepten-5,10-imine maleate (MK-801; Tocris) were dissolved in PBS as stock solutions and diluted in DMEM-H on treatment. An equal volume of PBS in DMEM-H was used as vehicle controls for experiments.
Western blot analysis of brain homogenates and bEnd.3 lysates
bEnd.3 cells were harvested in PBS with 1% Triton X-100, protease inhibitors (Complete Mini; Roche Diagnostics), and 1mM sodium vanadate. Samples were denatured in 5× Laemmli sample buffer with 10% β-mercaptoethanol, separated by SDS-PAGE, blotted to polyvinylidene difluoride membrane, and probed using polyclonal goat anti-VEC (C-19; Santa Cruz Biotechnology), monoclonal mouse antiphosphotyrosine (pY99; Santa Cruz Biotechnology), or monoclonal mouse anti-NMDA R1 (436487A; Invitrogen). Horseradish peroxidase-conjugated monoclonal anti–β-actin (C4; Santa Cruz Biotechnology) antibody was incubated at 1:1000 overnight at 4°C. Host species appropriate and alkaline phosphatase-conjugated secondary antibodies were applied for 1 hour, and immunopositive bands were photographed (Versadoc; Bio-Rad) after chemiluminescent detection (Lumiphos, Pierce Chemical). Band densities were quantified using QuantityOne 4.6.9 (Bio-Rad) or ImageJ 1.435 software. For immunoprecipitation experiments, samples were incubated with Protein G Dynabeads (Invitrogen) charged with anti-VEC antibodies; precipitates were washed and eluted, and samples were handled as for Western blotting.
Flow cytometry
bEnd.3 cells were blocked in PBS with 3% BSA for 10 minutes at room temperature and then stained with monoclonal mouse anti-NMDA R1 (436487A; Invitrogen). Secondary antibody was anti–rabbit IgG conjugated to FITC (Invitrogen); control experiments were performed using secondary antibody only. Stained cells were washed with cold PBS and analyzed immediately or fixed with 1% paraformaldehyde overnight. Staining was analyzed on a BD Biosciences FACSCalibur flow cytometer, with gates set on live cells stained with secondary antibody alone. Data analysis was performed using CellQuest 3.3 software (BD Biosciences).
Whole cell ELISA and immunochemical analyses
After respective treatments, bEnd.3 cells were washed twice in Tris-buffered saline (TBS) with 2mM CaCl2, 2mM MgCl2, and 1mM sodium vanadate. TBS was completely removed and cells were incubated in 4% paraformaldehyde for 15 minutes, rinsed twice in TBS, permeabilized with 1% Triton X-100 in TBS, and blocked with 3% BSA in TBS with 0.01% Tween-20 for 2 hours at room temperature. Samples were then incubated with primary antibodies diluted 1:500 in TBS with 0.01% Tween-20 with 3% BSA at 4°C overnight. Samples were then washed 3 times for 10 minutes each using TBS with 0.1% Tween-20. For whole-cell enzyme-linked immunosorbent assay (ELISA) experiments, samples were incubated with alkaline phosphatase-conjugated goat anti–rabbit IgG for 30 minutes, washed again, and detected using a colorimetric ELISA substrate (BluePhos; KPL). Phosphorylation of β-catenin was quantified by ELISA using phospho-specific antibodies (pY142: CP1081–3, pY86: CP11191–2, pY489: CP2961–1; ECM Biosciences). Absorbance at 610 nm was measured using a plate reader (Synergy HT, BioTek). To quantify expression of NMDAr at the membrane of bEnd.3 cells after Hcy treatment, the permeabilization step was omitted and detergents were omitted from all solutions to ensure only cell surface expression was quantified. For immunocytochemistry experiments, primary antibodies were goat anti-VEC (C-19; Santa Cruz Biotechnology) and rabbit anti–β-catenin (CP1061; ECM Biosciences). Secondary antibodies conjugated to AlexaFluor-594 (anti-VEC) or AlexaFluor-488 (anti–β-catenin) were used for detection. Images were acquired by fluorescence microscopy (Leica, DMLFS) with digital camera (MicroPublisher 3.3 RTV; QImaging; QCapture 5.1 software) using a 40×/NA 0.55 objective.
Chromatin immunoprecipitation assay
Cells were cross-linked with 1% paraformaldehyde for 20 minutes at 21°C. Cells were scraped and resuspended in sonication buffer (20mM Tris, pH 8.1, 1% SDS, 1% Triton X-100, 10mM EDTA) and sonicated (Cell Disruptor W-10; Heat Systems) at power 6 for 15 seconds on, 45 seconds off for 15 minutes. DNA fragments were coimmunoprecipitated using β-catenin antibody (ECM Biosciences; CP1061) preconjugated to protein G Plus agarose beads (Santa Cruz Biotechnology; sc-2002), then rinsed in high- and low-salt buffers (20mM Tris, pH 8.1, 0.1% SDS, 1% Triton X-100, 2mM EDTA, 500mM NaCl, and 20mM Tris, pH 8.1, 0.1% SDS, 1% Triton X-100, 2mM EDTA, 150mM NaCl, respectively) and eluted with 1% SDS plus 100mM NaHCO3. Eluates were incubated with proteinase K at 45°C for 1 hour and then at 65°C for 4 hours. DNA was precipitated with 70% ethanol at −20°C and resuspended in TE (10mM Tris, 1mM EDTA, pH 8.1). PCR was performed at: 95°C for 15 minutes (94°C for 30 seconds; 54°C for 30 seconds, 72°C for 1 minute) for 35 cycles; 72°C for 7 minutes using: 5′-CTGCTGAACTTGGGGAAGAC-3′ and 5′- AAGGGAGTGAGGGAAGGAAA-3′ at 1mM final concentration in 25 μL of total reaction mixture. This region was recently identified as the primary regulator of claudin-5 expression under the control of β-catenin.10 Total PCR product/volume was loaded into ethidium bromide gels and run following standard procedures. Bands were imaged and density quantified as for Western blots.
EC monolayer permeability
Confluent monolayers on ThinCert membranes (Greiner Bio-One; 0.4-μm pores) were treated with Hcy (20μM), Hcy plus memantine (100μM), memantine only, or vehicle (saline, control). To ensure relatively constant dose, media was changed every 12 hours. After 3 days of treatment, sodium fluorescein (∼ 400 Da) was added to upper chambers. A total of 50 μL was collected from lower chambers at 15, 30, 60, and 120 minutes. The rate of permeability was quantified as slope of the fluorescence-time plot for each well.
BBB permeability assay
BBB permeability was assessed using adult mice heterozygous for the cystathionine β-synthase gene (cbs+/−; a murine model of HHcy) and wild-type (WT) littermates, which were divided into 4 groups: WT, WT with memantine, cbs+/−, and cbs+/− with memantine. Both male and female mice were used and divided evenly among the groups. Founders for our colony were provided by Dr Steven Lentz, University of Iowa, and have been backcrossed at least 12 generations on the C57Bl/6 background. Animals were housed individually on a 12/12 hour light/dark cycle with food and water ad libitum. For memantine treatments, water was supplemented for 2 weeks with 10 mg memantine per 33 mL water based on a pilot study of each animal's drinking pattern. After the 2 weeks of memantine treatment, BBB permeability was assessed as described previously by Gulati et al with minor modifications.16 Briefly, 10 mg sodium fluorescein (NaFl) in 0.1 mL sterile saline was injected intraperitoneally, and animals were returned to their cages. Two hours later, mice were anesthetized with isoflurane; blood was collected from the left ventricle into serum separator tubes (BD Biosciences) followed by transcardial perfusion (1% BSA in PBS + heparin + sodium nitroprusside 10−4) to flush remaining blood from the cerebrovasculature. Brains were rapidly harvested, weighed, and homogenized on ice with a Dounce homogenizer (∼ 6 or 7 strokes) in 1 mL ice-cold PBS containing protease inhibitors (Complete Mini; Roche Diagnostics) followed by centrifugation at 12 000g for 15 minutes at 4°C. Supernatants and pellets were separated, frozen in liquid nitrogen, and stored at −80°C. Samples were used for Western blot of claudin-5 processed for BBB permeability. Protein was precipitated from brain and serum samples with trichloroacetic acid (TCA) to remove potential background fluorescence. To prevent precipitation of NaFl in serum, the samples were diluted 1:10 in sterile PBS before an additional 1:10 dilution in 20% TCA. Supernatants from brain homogenates were diluted 1:10 in 20% TCA. All samples were incubated at 4°C for 24 hours. Samples were centrifuged at 10 000g for 15 minutes to remove precipitated protein. The supernatant was removed and diluted with equal volumes of borate buffer (0.05M, pH 10), resulting in a final concentration of 10% TCA and 0.025M borate buffer to neutralize pH. Brain/serum fluorescence of NaFl was quantified as an indicator of BBB permeability using a fluorescence plate reader (excitation 480/20 nm, emission 538/20 nm; Synergy HT, BioTek) and expressed as relative units (RU) of fluorescence per gram of brain tissue normalized to plasma fluorescence: brain/serum fluorescence = RU brain fluorescence/RU plasma fluorescence/brain weight (g). NaFl can be endocytosed into endothelium; to ensure that the NaFl measurements quantified brain concentrations and not compartmentalization in the vasculature, the original supernatants and pellets from the brain homogenates were tested for the presence of the pan-endothelial marker VEC by Western blotting, which demonstrated that the fluorescence measurements were from EC-depleted fractions. Extravasated, endogenous (mouse) IgG was quantified by Western blot.
Quantification of Hcy concentration
Total Hcy concentrations were measured in serum samples collected for BBB permeability experiments of each mouse using a commercial enzyme immunoassay kit, Homocysteine-EIA kit (Bio-Rad), according to the manufacturer's directions.
Statistics
For in vivo experiments, n = 8 or 9; for in vitro experiments, n = 12 to 36. Pair-wise comparisons were made with Student t test, and group-wise data were compared by analysis of variance with Tukey or Dunnett post-hoc analyses; α was set at 0.05 a priori for statistical significance.
Results
Experiment 1
Hcy uncouples VEC/β-catenin association at the plasma membrane.
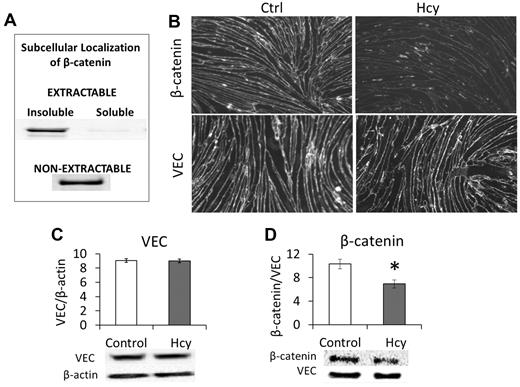
We found that β-catenin was located only in nonionic detergent-insoluble fractions of the cell. When cells were lysed with a nonionic detergent (Triton X-100 or NP-40), β-catenin was only found in a pellet fraction of what washed off of the cells; we call this the “extractable, insoluble” fraction (Figure 1A). However, the majority of β-catenin in the cell remained in the “nonextractable” fraction and could only be solubilized from the culture flask with 1% SDS (Figure 1A). This is consistent with the role of β-catenin in coupling VEC to the cytoskeleton. Treating bEnd.3 cells with 20μM Hcy for 3 days significantly reduced the overall cellular expression of β-catenin (Figure 1B-D). Subsequent immunohistochemistry demonstrated a substantial loss of β-catenin from the cell membrane/cytoskeleton (Figure 1B). However, there was no significant change in the expression of VEC (Figure 1B), although this does not preclude the loss of VEC-VEC coupling of adjacent cells.
β-catenin dissociates from VEC in response to Hcy. Brain microvascular ECs were cultured and treated beginning 3 days after confluence. Cells were treated with Hcy (20μM) or vehicle control (PBS) for 3 days to mimic a chronic, mild elevation in Hcy. (A) β-catenin associates with the detergent-insoluble, cytoskeletal fraction. To determine the subcellular localization of β-catenin, it was necessary to run pilot experiments to show prevalence of β-catenin in different fractions obtained when applying Nonidet P-40 lysis. Cells were grown to confluence and maintained for 3 days; then a 1% Nonidet P-40 lysis buffer was applied. The portion obtained from this solution was called “extractable” and was centrifuged at 19 000g for 2 hours and then further categorized into “soluble” (supernatant) and “insoluble” (pellet). The fraction of cellular material that remained adherent to the culture plate was solubilized with an ionic detergent lysis buffer (SDS and deoxycholic acid) and called the “non-extractable” fraction. Blots were probed for β-catenin (1:1000, CP1061; ECM Biosciences). (B) Immunohistochemistry demonstrates localization of VEC and β-catenin at cell borders. Hcy had little effect on VEC expression but substantially reduced expression of β-catenin. Scale bar represents 30 μm. (C) Western blot confirms lack of change in VEC but (D) significant loss of β-catenin after Hcy treatment.
β-catenin dissociates from VEC in response to Hcy. Brain microvascular ECs were cultured and treated beginning 3 days after confluence. Cells were treated with Hcy (20μM) or vehicle control (PBS) for 3 days to mimic a chronic, mild elevation in Hcy. (A) β-catenin associates with the detergent-insoluble, cytoskeletal fraction. To determine the subcellular localization of β-catenin, it was necessary to run pilot experiments to show prevalence of β-catenin in different fractions obtained when applying Nonidet P-40 lysis. Cells were grown to confluence and maintained for 3 days; then a 1% Nonidet P-40 lysis buffer was applied. The portion obtained from this solution was called “extractable” and was centrifuged at 19 000g for 2 hours and then further categorized into “soluble” (supernatant) and “insoluble” (pellet). The fraction of cellular material that remained adherent to the culture plate was solubilized with an ionic detergent lysis buffer (SDS and deoxycholic acid) and called the “non-extractable” fraction. Blots were probed for β-catenin (1:1000, CP1061; ECM Biosciences). (B) Immunohistochemistry demonstrates localization of VEC and β-catenin at cell borders. Hcy had little effect on VEC expression but substantially reduced expression of β-catenin. Scale bar represents 30 μm. (C) Western blot confirms lack of change in VEC but (D) significant loss of β-catenin after Hcy treatment.
Hcy leads to phosphorylation of β-catenin at Y86.
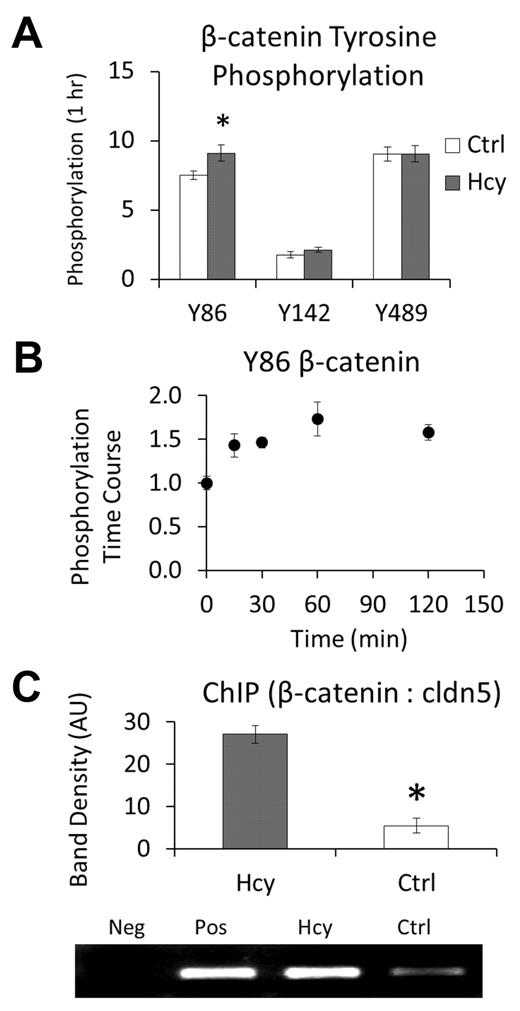
These findings led us to question whether β-catenin is modified in a manner that may target it for the nucleus. As an initial screening tool, we treated cells with a high dose of Hcy (100μM) and quantified phosphorylation of β-catenin at 3 regulatory sites: Y86, Y142, and Y489. There was a significant increase in pY86 within 1 hour of treatment (Figure 2A). Scaling back to a lower dose of Hcy (20μM), we further demonstrated a time course for pY86 that plateaus beyond approximately 1 hour (Figure 2B). Hence, Hcy treatment not only dissociates β-catenin from the VEC and cytoskeletal complex but phosphorylates at a site that is associated with nuclear accumulation and transcriptional regulation.17
Hcy leads to phosphorylation, nuclear localization, and binding of β-catenin to the claudin-5 promoter. (A-B) Data were obtained by performing whole-cell ELISAs using the respective site-specific antiphosphotyrosine antibodies. (A) Initial screening of modification sites on β-catenin after 1-hour treatment with Hcy (100μM). (B) Time course for phosphorylation of β-catenin at Y86, a modification that has been associated with nuclear accumulation, in response to Hcy (20μM). (C) Chromatin immunoprecipitation assay performed by immunoprecipitation of β-catenin and PCR for the repression segment of the claudin-5 promoter region. Assay was run after 3 days of treatment with: Hcy (20μM), Ctrl (vehicle, PBS), Pos (positive control, whole cell lysate DNA), or Neg (negative control, primers only, no DNA).
Hcy leads to phosphorylation, nuclear localization, and binding of β-catenin to the claudin-5 promoter. (A-B) Data were obtained by performing whole-cell ELISAs using the respective site-specific antiphosphotyrosine antibodies. (A) Initial screening of modification sites on β-catenin after 1-hour treatment with Hcy (100μM). (B) Time course for phosphorylation of β-catenin at Y86, a modification that has been associated with nuclear accumulation, in response to Hcy (20μM). (C) Chromatin immunoprecipitation assay performed by immunoprecipitation of β-catenin and PCR for the repression segment of the claudin-5 promoter region. Assay was run after 3 days of treatment with: Hcy (20μM), Ctrl (vehicle, PBS), Pos (positive control, whole cell lysate DNA), or Neg (negative control, primers only, no DNA).
Hcy induces β-catenin nuclear localization, interaction with the claudin-5 promoter, and decreased claudin-5 expression.
Chromatin immunoprecipitation assays were performed to determine whether β-catenin binds to the promoter region of claudin-5 known to repress its expression.10 After 3 days of treatment with 20μM Hcy, the amount of PCR product for this region that immunoprecipitated with β-catenin was increased approximately 6-fold (Figure 2C), demonstrating that Hcy does cause β-catenin accumulation in the nucleus with subsequent and specific binding to the repression region for the expression of claudin-5. Consistent with the role in regulation of claudin-5 expression by nuclear β-catenin, Hcy decreased expression of claudin-5 at cell borders (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Experiment 2
Brain ECs express NMDAr and increase expression in response to Hcy treatment.
A search for known Hcy “sensors” or receptors and cross-reference with those that are known to induce nuclear localization of β-catenin revealed the NMDAr (eg, Hcy activates the NMDAr11 ) and NMDAr activation induces nuclear accumulation of β-catenin.18 Given findings from experiment 1, we determined whether brain ECs express the NMDAr, Hcy has any effect on its expression in these cells, and selective pharmacology modulates NMDAr activity. Using flow cytometry (Figure 3A) and Western blotting (Figure 3B), we confirmed that NMDAr is expressed on brain ECs, consistent with previous reports in vitro and in vivo.19-21 After 3 days of treatment with 20μM Hcy in vitro, cell surface ELISA demonstrated a significant increase in the expression of NMDAr at the plasma membrane (Figure 3C). We used the selective NMDAr antagonist MK-801 (25μM) and agonist NMDA (25μM) to establish the specific role of NMDAr on claudin-5 expression using whole-cell ELISA. Hcy and NMDA decreased claudin-5 expression after 3 days of treatment compared with control; there was no change in claudin-5 expression with Hcy plus MK-801 or MK-801 alone (Figure 3D).
NMDA expression on brain microvascular ECs. (A) Flow cytometry and (B) Western blotting independently confirm expression of NMDAr subunit 1. Antibody control in panel A is fluorescent secondary antibody only. (C) In response to chronic Hcy treatment (3 days, 20μM), NMDAr subunit 1 expression was significantly induced on the luminal surface of EC membranes. (D) Chronic treatment with Hcy (3 days, 20μM) and NMDA (3 days, 25μM) decreased expression of claudin-5 using whole-cell ELISA. Cotreatment of Hcy plus MK-801 (3 days, 20μM + 25μM) rescued the Hcy-mediated decrease in claudin-5 expression, which was not different from Ctrl or MK-801 alone.
NMDA expression on brain microvascular ECs. (A) Flow cytometry and (B) Western blotting independently confirm expression of NMDAr subunit 1. Antibody control in panel A is fluorescent secondary antibody only. (C) In response to chronic Hcy treatment (3 days, 20μM), NMDAr subunit 1 expression was significantly induced on the luminal surface of EC membranes. (D) Chronic treatment with Hcy (3 days, 20μM) and NMDA (3 days, 25μM) decreased expression of claudin-5 using whole-cell ELISA. Cotreatment of Hcy plus MK-801 (3 days, 20μM + 25μM) rescued the Hcy-mediated decrease in claudin-5 expression, which was not different from Ctrl or MK-801 alone.
In the next sets of studies, we explored these relations in vivo using 4 groups of mice: cbs+/− ([Hcy] 11.5 ± 2μM), cbs+/− with memantine ([Hcy] 9.8 ± 0.3μM), WT littermates, (cbs+/+; [Hcy] 4.6 ± 0.6μM), and WT littermates with memantine ([Hcy] 4.6 ± 0.7μM). Hcy levels for WT mice were significantly lower than for cbs+/− mice (P < .05), whereas memantine treatment had no effect (P > .05). Complementary experiments in vitro were performed using brain microvascular ECs. Ages, body mass, and brain mass are given in Table 1. Memantine intake was 34 ± 2 mg/kg per day, appropriate for a clinically relevant plasma level of approximately 1μM.22 We used both male and female mice in our studies; although the experiments were not designed to specifically address potential sex differences, we did not find any differences between the sexes for any measurements in these studies.
Descriptive data for animals used
| . | Age, mo . | Body mass, g . | Brain mass, g . | Relative brain mass, % . |
|---|---|---|---|---|
| CBS+/− (n = 17) | 6.5 ± 0.5 | 27.5 ± 1.1 | 0.329 ± 0.010 | 1.23 ± 0.06 |
| CBS+/+ (n = 16) | 6.9 ± 0.6 | 27.3 ± 0.9 | 0.332 ± 0.008 | 1.24 ± 0.05 |
| . | Age, mo . | Body mass, g . | Brain mass, g . | Relative brain mass, % . |
|---|---|---|---|---|
| CBS+/− (n = 17) | 6.5 ± 0.5 | 27.5 ± 1.1 | 0.329 ± 0.010 | 1.23 ± 0.06 |
| CBS+/+ (n = 16) | 6.9 ± 0.6 | 27.3 ± 0.9 | 0.332 ± 0.008 | 1.24 ± 0.05 |
There were no significant differences.
Hcy reduces claudin-5 expression in vivo and in vitro via NMDAr activation.
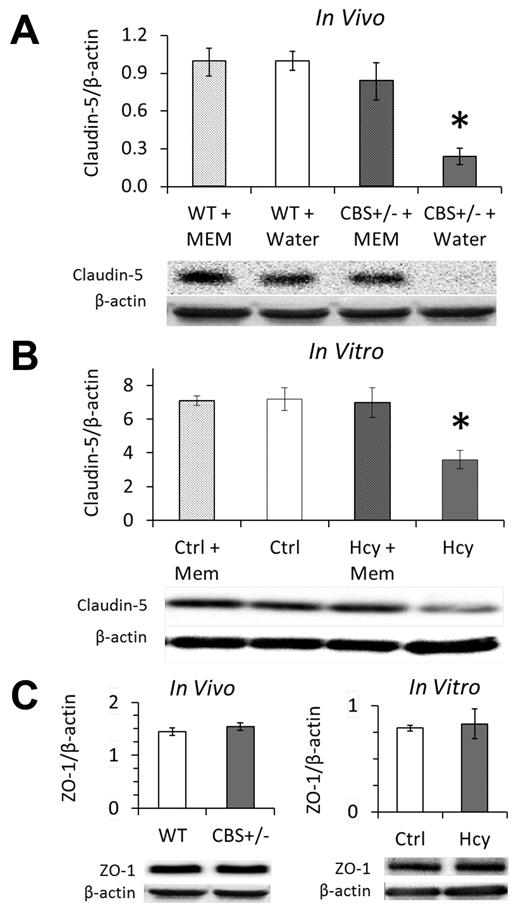
Immunohistochemistry localized claudin-5 expression to EC borders in vivo and in vitro (Supplemental data). Brain homogenates from HHcy cbs+/− mice had significantly less claudin-5 expression; expression was rescued by supplementing drinking water with memantine for 2 weeks (Figure 4A). Memantine in drinking water of WT mice had no effect on claudin-5 expression compared with control (water only). Similarly, 3 days of treatment with 20μM Hcy significantly decreased claudin-5 expression, which was restored to baseline in the presence of memantine (Figure 4B). We did not find an effect of Hcy on the expression of ZO-1, a tight junction associated intracellular protein, either in vivo or in vitro (Figure 4C).
Elevated Hcy reduces claudin-5 expression of cerebral microvascular endothelium, which is rescued by NMDAr inhibition with memantine. Western blotting in vivo (A) and in vitro (B) demonstrates significant loss of claudin-5 expression in the mouse model (cbs+/−) and cell culture model (bEnd.3 cells, 20μM) of elevated Hcy. This loss was completely recovered by 2 weeks of treatment with the NMDAr antagonist memantine in vivo (A) and fully inhibited by concomitant memantine treatment in vitro (B). (C) Expression of the tight junction-associated protein ZO-1 was not affected by HHcy. Brains of hyperhomocysteinemic mice (cbs+/−) showed the same expression levels of ZO-1 as WT littermates (WT; cbs+/+), and brain microvascular ECs (bEnd.3) expressed the same amount of ZO-1 whether treated for 3 days with Hcy (20μM) or vehicle control (Ctrl, PBS).
Elevated Hcy reduces claudin-5 expression of cerebral microvascular endothelium, which is rescued by NMDAr inhibition with memantine. Western blotting in vivo (A) and in vitro (B) demonstrates significant loss of claudin-5 expression in the mouse model (cbs+/−) and cell culture model (bEnd.3 cells, 20μM) of elevated Hcy. This loss was completely recovered by 2 weeks of treatment with the NMDAr antagonist memantine in vivo (A) and fully inhibited by concomitant memantine treatment in vitro (B). (C) Expression of the tight junction-associated protein ZO-1 was not affected by HHcy. Brains of hyperhomocysteinemic mice (cbs+/−) showed the same expression levels of ZO-1 as WT littermates (WT; cbs+/+), and brain microvascular ECs (bEnd.3) expressed the same amount of ZO-1 whether treated for 3 days with Hcy (20μM) or vehicle control (Ctrl, PBS).
Hcy induces BBB permeability in vivo and in vitro via NMDAr activation.
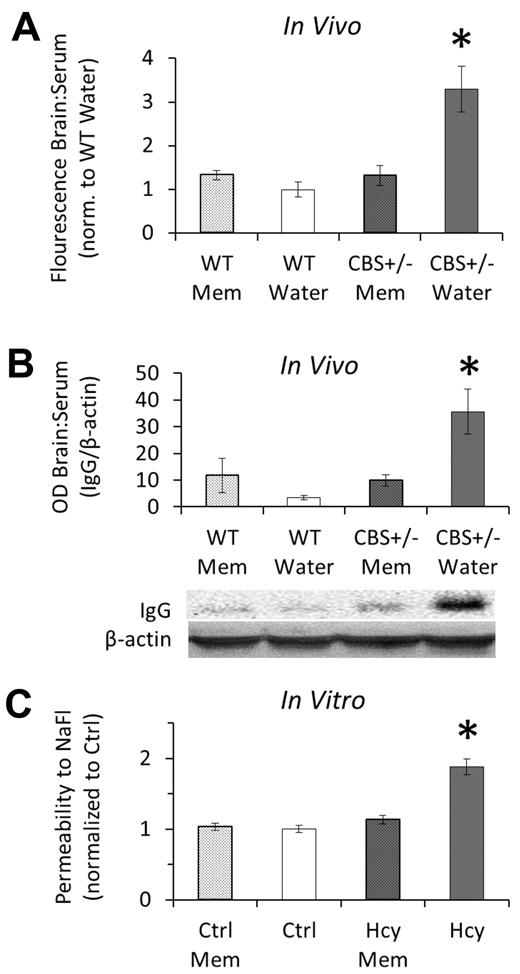
Brain homogenates from cbs+/− mice had significantly greater BBB permeability (brain/serum fluorescence per gram of brain) to both exogenous NaFl (Figure 5A) and endogenous IgG (Figure 5B) compared with WT littermates; permeability was rescued by supplementing drinking water with memantine for 2 weeks. Supernatants from the brain homogenates that were used for measurements of NaFl and IgG did not label for VEC compared with the pellets, which did positively label for VEC (supplemental Figure 2). These results confirm that the measurements reflect plasma leak into the brain compartment rather than uptake into the endothelium. Memantine in drinking water of WT mice had no effect on permeability of the intact BBB. Repeating these experiments in vitro demonstrated that the effects are endothelial-cell specific. Three days of treatment with 20μM Hcy significantly increased endothelial monolayer permeability (flux of NaFl across the monolayer over 2 hours), which was restored to baseline in the presence of memantine (Figure 5C).
Elevated Hcy increases cerebral microvascular permeability, which is rescued by NMDAr inhibition with memantine. (A) Brain/serum distribution ratio of tracer (NaFl) injected into the peritoneum 2 hours before flushing blood from the vasculature and brain collection. Values were calculated as relative units of brain NaFl to serum NaFl per gram brain mass (“BBB permeability assay”) and are shown here normalized to the basal condition (WT drinking water). (B) Endogenous IgG was quantified by Western blot in brain homogenates after flushing of the vasculature. Brains of hyperhomocysteinemic mice (cbs+/−) were significantly more permeable to IgG, which was rescued with 2 weeks of NMDAr inhibition using memantine (Mem). (C) In vitro, brain microvascular ECs (bEnd.3 cells) were significantly more permeable (flux of NaFl over 2 hours) after Hcy treatment (20μM) for 3 days, which was inhibited with concomitant antagonism of the NMDAr using memantine (Mem).
Elevated Hcy increases cerebral microvascular permeability, which is rescued by NMDAr inhibition with memantine. (A) Brain/serum distribution ratio of tracer (NaFl) injected into the peritoneum 2 hours before flushing blood from the vasculature and brain collection. Values were calculated as relative units of brain NaFl to serum NaFl per gram brain mass (“BBB permeability assay”) and are shown here normalized to the basal condition (WT drinking water). (B) Endogenous IgG was quantified by Western blot in brain homogenates after flushing of the vasculature. Brains of hyperhomocysteinemic mice (cbs+/−) were significantly more permeable to IgG, which was rescued with 2 weeks of NMDAr inhibition using memantine (Mem). (C) In vitro, brain microvascular ECs (bEnd.3 cells) were significantly more permeable (flux of NaFl over 2 hours) after Hcy treatment (20μM) for 3 days, which was inhibited with concomitant antagonism of the NMDAr using memantine (Mem).
Discussion
These data clarify several previously undetermined effects of Hcy on cerebral vascular endothelium. First, Hcy dissociated β-catenin, but not VEC, from the plasma membrane. Second, β-catenin localized to the cell nucleus where it bound to a repression region for expression of the tight junction protein claudin-5. Third, claudin-5 expression and endothelial barrier integrity in vitro and in vivo were controlled by Hcy through an NMDAr-dependent process. Data from cell signaling, targeted pharmacologic intervention, and our in vivo model show that a receptor-dependent signaling cascade is responsible for barrier disruption in cerebral microvasculature caused by Hcy and support our idea that the NMDAr antagonist memantine may be useful in patients with Hcy-mediated cerebral microvascular barrier dysfunction. To our knowledge, these data are the first to couple regulation of tight junction composition and function with adherens junction signaling in the context of the human microvascular disease HHcy.
Phosphorylation of β-catenin at Y86, which occurred in response to Hcy treatment (Figure 2A-B), is associated with nuclear targeting and transcriptional regulation.17 Conversely, sequestering β-catenin in plasma membrane complexes is required for claudin family expression and stability at brain EC-EC junctions.23 We found most of the VEC/β-catenin complex in the nonionic detergent nonextractable (cytoskeleton associated) fraction (Figure 1A), which is consistent with the function of this complex in coupling cytoskeletal elements between adjacent ECs through VEC complexes. In our brain microvascular EC line (bEnd.3), the intercellular barrier continues to “tighten” for several days after confluency. The cells narrow, lengthen, and swirl in groups that may be described as having the appearance of “crop circles.” We study our cells after at least 3 days after confluency and only after they have adopted this morphology. This morphology is consistent with primary brain microvascular ECs but differs from that of ECs from peripheral vascular beds (eg, human umbilical vein ECs), which generally have a “fried egg” morphology.
Claudin-5 is highly expressed in cerebral ECs, and claudin-5 knockout mice (cldn5−/−) experience a size-selective increase in BBB permeability for molecules < 800 Da.2 We used NaFl for our in vivo studies, which is approximately 400 Da. Thus, increased permeability to NaFl in HHcy mice is consistent with the observed loss of claudin-5 expression. It has been proposed that claudins, through homotypic and heterotypic association, form paracellular channels that regulate size and/or charge-selective ion permeability and define aqueous pores that may facilitate concomitant osmosis.24 These findings are clinically significant as it has been suggested that chronic permeability to small molecules (eg, sucrose, 342 Da), such as occurs in diabetes mellitus, may be an important factor in the etiology of cognitive decline.25 Although this study has focused on pathologic opening of the BBB by Hcy, opening of the BBB is not always problematic. Indeed, the BBB presents significant challenges for pharmacologic therapies by limiting or inhibiting drug delivery into the central nervous system. By elucidating a mechanism for rescuing the effects of Hcy, this work also identifies a potential therapeutic strategy for transient opening of the BBB for selected pharmacologic intervention with drugs that may otherwise be excluded from the brain.
The NMDAr is well known for its involvement in brain trauma and neurodegenerative disorders.26,27 However, most previous studies have focused on neuronal and glial damage. The idea of NMDAr in EC dysfunction is relatively new. NMDAr modulates barrier properties of cerebral endothelium in vitro28 and may play a critical role in monocyte transmigration across the BBB in vivo.21 Memantine, an NMDAr antagonist and Food and Drug Administration–approved treatment for Alzheimer disease, rescues BBB integrity after cerebral ischemia-reperfusion injury29 and during experimental allergic encephalomyelitis.14 MK-801 is a noncompetitive antagonist of NMDAr that functions by blocking ion flux through the channel. In Figure 3D, we demonstrate the definitive role of NMDAr in Hcy-mediated decrease in expression of claudin-5 using both MK-801 and NMDA. However, complete blockade of the NMDAr with a drug, such as MK-801, is not a viable therapeutic option in humans because of severe and unacceptable side effects.30 Use of memantine, which allows normal functionality of the channel but inhibits overexcitation,31 is a strength of our study because of the potential for clinical application. Although memantine is approved for use in treating Alzheimer disease, recommendations differ among organizations. Among groups that do not recommend its use, the prevailing justification is a lack of evidence supporting efficacy. In light of the present findings, it may be informative for future trials of memantine in dementia to stratify patients based on Hcy levels and on vascular contributions (or lack thereof) to the dementia.
We also tested brain homogenates for the presence of endogenous IgG; there was a strong IgG signal in the samples from cbs+/− mice but little or no signal in the other 3 groups (Figure 5B). Given the small size selectivity of claudin-5 junctions and the substantial quantity of IgG in the brains of cbs+/− mice, HHcy must disrupt additional components of barrier integrity. VEC is involved in regulating paracellular permeability throughout the vascular system, and loss of β-catenin from the complex increases permeability independent of total expression.32 Hence, loss of VEC intercellular adhesion may contribute to the opening of the BBB to molecules as large as IgG in these mice. ZO-1 expression was not altered in our pilot studies (Figure 4C), so we did not pursue this protein further in these experiments. Nitta et al2 found that strong ZO-1 labeling remained at EC-EC borders in the cldn-5−/− mouse, which suggests that claudin-5 expression is not a corequisite for ZO-1 membrane expression. In the experimental autoimmune encephalomyelitis model of multiple sclerosis, release of vascular endothelial growth factor A was associated with down-regulation of both claudin-5 and occludin.33 Expression of recombinant claudin-5 rescued the increase in permeability, whereas expression of recombinant occludin did not. Therefore, the claudins seem to play the principle role in forming tight junction strands that restrict the paracellular diffusion of substances at the BBB. Although we did not find a change in occludin expression in pilot experiments, it is important to recognize that occludin moves away from the membrane and is diffusely expressed in ECs in vitro, making any changes in expression with Hcy treatment difficult to interpret as changes would not necessarily reflect structural modifications at tight junctions.
The cerebral endothelium expresses functional NMDAr in vitro and in vivo as shown by several groups.19-21 The system is also responsive to physiologic stimuli. For example, oxidative stress increases NMDAr expression.34 We found that Hcy treatment of brain microvascular ECs also increases NMDAr expression (Figure 3C), consistent with a similar response in rat aortic ECs.35 It is presently unknown whether an increase in NMDAr expression exacerbates the effects of a given level of Hcy by making the cell more sensitive. Such a mechanism might help to explain why vitamin therapy is successful at lowering Hcy by up to approximately 25% but does not seem to lower disease risk: that is, if the cells are more sensitive, then 25% lowering of Hcy levels may be insufficient to prevent the cellular dysfunction. The importance of altered NMDAr expression in the degree of BBB dysfunction is unknown. Consistent with the present findings, Sharp et al found that glutamate decreases EC barrier integrity by activating NMDAr in vitro.36
HHcy is a risk factor for cerebral small-vessel disease via endothelial dysfunction37 and is an independent risk factor for silent brain infarcts and white matter lesions as found in the Rotterdam Scan Study,38 the Northern Manhattan Study,39 and the Framingham Offspring Study.40 Several laboratories have uncovered a specific association between HHcy and the lacunar subtype of stroke.41-43 Disruption of the BBB in rodents44,45 and humans46 is among the most striking homeostatic disturbances caused by Hcy (vascular complications of HHcy5,6 ). There is a growing recognition that lacunar strokes may result from reduced BBB integrity.47 It appears that this relation is unique to the lacunar subtype of stroke because the BBB is “leaky” throughout the white matter of patients with lacunar stroke but not those with cortical strokes.7 Thus, Hcy is a significant factor in a spectrum of structural and functional cerebrovascular pathologies that are associated through a chronic increase in BBB permeability, subcortical damage, stroke subtypes, and progressive dementia. The volume of white matter lesions in cerebral small-vessel disease is directly related to the level of Hcy40 and predicts an increased risk of stroke, dementia, and death.48 Plasma levels of Hcy in our mice are approximately 10μM, which is below the clinical cutoff for diagnosis of mild HHcy (15μM). The Lentz and Faraci groups have described several important microvasculopathies in the brains of these mice, particularly altered extracellular matrix composition and impaired vasoreactivity, which may further contribute to the clinical disease etiology.49,50 The cerebral microcirculation is therefore particularly sensitive to chronic HHcy.
In conclusion, we provide evidence that Hcy modulates both adherens junctions (VEC/β-catenin) and tight junctions (claudin-5). There is growing recognition of an intimate relation among Hcy, cerebral small-vessel disease, stroke, and BBB disruption. Our data provide a mechanistic basis for the relation between Hcy and BBB disruption. Current therapies for HHcy are limited to vitamin supplements, which serve as cofactors in Hcy metabolism but may not provide the therapeutic efficacy required for disease prevention. We have demonstrated that the Hcy-mediated BBB dysfunction is reversible in a murine model by directed pharmacology with an Food and Drug Administration-approved and well-tolerated NMDAr antagonist. Future work to further elucidate the role of the NMDAr in Hcy-mediated BBB permeability as well as other Hcy-induced neurovascular pathologies presents novel possibilities for future treatment of patients with HHcy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Idaho State University (FRC4019 and UF1012) and the National Institutes of Health (P20 RR-016454).
National Institutes of Health
Authorship
Contribution: R.S.B., J.J.R., and S.E.B. designed experiments, collected and analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shawn E. Bearden, Department of Biological Sciences, Idaho State University, 921 S 8th Ave, Stop 8007, Pocatello, ID 83209-8007; e-mail: bearshaw@isu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal