Abstract
GABP is an ets transcription factor that regulates genes that are required for myeloid differentiation. The tetrameric GABP complex includes GABPα, which binds DNA via its ets domain, and GABPβ, which contains the transcription activation domain. To examine the role of GABP in myeloid differentiation, we generated mice in which Gabpa can be conditionally deleted in hematopoietic tissues. Gabpa knockout mice rapidly lost myeloid cells, and residual myeloid cells were dysplastic and immunophenotypically abnormal. Bone marrow transplantation demonstrated that Gabpα null cells could not contribute to the myeloid compartment because of cell intrinsic defects. Disruption of Gabpa was associated with a marked reduction in myeloid progenitor cells, and Gabpα null myeloid cells express reduced levels of the transcriptional repressor, Gfi-1. Gabp bound and activated the Gfi1 promoter, and transduction of Gabpa knockout bone marrow with Gfi1 partially rescued defects in myeloid colony formation and myeloid differentiation. We conclude that Gabp is required for myeloid differentiation due, in part, to its regulation of the tran-scriptional repressor Gfi-1.
Introduction
Differentiation of granulocytes and monocytes from bone marrow myeloid progenitors is dependent on the orderly expression of key transcription factors. The transcription factors PU.1 and C/EBPα are required for myeloid differentiation, and abnormalities in their expression are associated clinically with some cases of human acute myelogenous leukemia (AML). Similarly, retinoic acid receptor-α is required for myeloid differentiation, and acute promyelocytic leukemia is invariably associated with chromosomal rearrangements that generate retinoic acid receptor-α fusion proteins. C/EBPϵ and the transcriptional repressor Gfi-1 regulate late stages of granulocytic differentiation, and abnormalities of each are associated with neutropenia. Thus, leukemia and neutrophil defects are associated with disordered expression of transcription factors that are required for normal myeloid differentiation.1,2
GABP is an ets-related transcription factor, and it is the only obligate multimer among more than 2 dozen mammalian ets factors. It consists of 2 molecules of GABPα, which binds DNA through its ets domain, and 2 molecules of GABPβ, which contains the transcriptional activation domain. GABP regulates genes that are important in myeloid differentiation, including CD18 (β2 integrin), neutrophil elastase (ELANE), α4 integrin, and others.3 GABP is an essential component of a retinoic acid-dependent enhanceosome that includes retinoid receptors and the transcriptional coactivator p300; expression of dominant negative forms of GABPα or p300 physically disrupts this complex and prevents retinoic acid-associated transcriptional activation.4
Because GABPα is the only ets factor that can recruit its partner GABPβ to DNA,3 we reasoned that disruption of the encoding gene would abrogate GABP function. Disruption of mouse Gabpa causes early embryonic lethality, thereby preventing analysis of its role in hematopoiesis.5,6 Gabp is required for cell-cycle entry and cell proliferation in mouse embryonic fibroblasts.6 However, hematopoietic cells express several ets factors, and it has been unclear whether Gabp has an essential and nonredundant role in myeloid differentiation.
We created mice in which Gabpa can be conditionally deleted in hematopoietic cells. Gabpa knockout (KO) mice rapidly lost myeloid cells, and the remaining myeloid cells exhibited dysplastic morphology, aberrant immunophenotype, and abnormal gene expression. Gabpa disruption markedly reduced myeloid progenitor cells and reduced expression of the transcriptional repressor Gfi-1. We found that Gfi-1 is a direct target of Gabp and that Gfi-1 partially reversed the aberrant growth and differentiation of Gabpα null cells. In summary, GABP is required for normal myeloid differentiation due, in part, to its previously unrecognized role in regulating the transcriptional repressor Gfi-1.
Methods
Mice and bone marrow transplantation
Generation of Gabpafl/fl mice and genotyping of genomic DNA were described previously.6 Gabpafl/fl mice were bred to mice that carry the Mx1 Cre transgene (The Jackson Laboratory) to generate Gabpafl/fl-Mx1 Cre mice. Six- to 12-week-old mice were used for all studies. Where indicated, Gabpafl/fl mice and the Gabpafl/fl Mx1 Cre mice were treated every other day with 3 intraperitoneal injections of polyinosine-polycytosine at 10 mg/kg (pIC; GE Healthcare). Bone marrow transplantation was performed as described previously.7 All animal studies were approved by the University of Massachusetts Institutional Animal Care and Utilization Committee.
Immunoblotting
Analysis of peripheral blood and mouse tissues
Bone marrow cell counts were determined by flushing marrow from femora and pelves of individual mice; cells were counted by hemocytometer, and blood cell subset counts were determined by multiplying cell counts by percentage of individual leukocyte subsets. Peripheral blood was obtained from mouse tail. Cytospin of bone marrow and peripheral blood cells was performed with Shandon Cytospin II centrifuge (Thermo Fisher Scientific). Cells were fixed with methanol, stained with Wright-Giemsa (EM Scientific), mounted with toluene solution (Permount; Sigma-Aldrich), and examined with a microscope (Nikon).
Image acquisition
The bright field microscope used to examine the prepared tissue slides is an Eclipse E600 (Nikon), with an objective lens of 40×/0.75 NA room air. The total magnification is 400×. Images were acquired with a DS-QilMc digital camera and NIS-Elements BR Version 3.10 software (both from Nikon) at 25°C in room air. Acquired images were processed with Microsoft Office Picture Manager SP1 MSO (Microsoft).
Flow cytometry, cell sorting, and cell-cycle analysis
Staining of bone marrow, spleen, thymus, and peripheral blood cells was performed with fluorescent conjugated antibodies against Gr1, CD11b, B220, CD3e, F4/80, and Mac3 (eBiosciences). Other antibodies used for flow cytometry included M-CSF-receptor (R), Ly6C, c-kit, CD33, Sca-1, CD34, FcγII/III, and annexin V (eBiosciences). LSRII and MoFlow cell sorters (BD Biosciences) were used for flow cytometry and cell sorting.
Reverse transcription and real-time Q-PCR
Reverse transcription was performed with a DyNAmo cDNA synthesis kit (New England BioLabs) and a thermal cycler (Applied Biosystems). Real-time Q-PCR was performed with a Q-PCR master kit (QIAGEN) and a thermal cycler (Eppendorf).
In vitro colony-forming assays
In vitro colony-forming assays (StemCell Technologies) used cytokines from R&D Systems.
ChIP and luciferase assays
Chromatin was immunoprecipitated with GABPα antibody and protein G-Sepharose beads (Pierce Chemical). Luciferase assays used Promega protocol.
Cell culture and retroviral gene transfer
NIH/3T3 and HEK293 (ATCC) cells were maintained in DMEM (Invitrogen) with 10% FBS. Bone marrow cells were grown in RPMI 1640/10% FBS with indicated cytokines. Bone marrow cells were transduced with retroviruses packaged in helper-free Phoenix cells (ATCC) for 48 hours and grown in 2.5 μg/mL puromycin (Sigma-Aldrich) for 3 to 5 days.
Plasmids
Site-directed mutagenesis (GGAA to ACTA) by Phusion DNA polymerase of pGL3 Basic (Promega) was confirmed by DNA sequencing.
Statistical analysis
Kaplan-Meier survival curves were generated with Prism 5 (GraphPad Software). Flow cytometry data were analyzed with Diva (BD Biosciences) and FlowJo Version 7.5.5 software (TreeStar).
Results
Genetic disruption of mouse Gabpa
We created mice with loxP sites that flank ets domain exons of mouse Gabpa (Gabpafl/fl or floxed)6 (Figure 1A). We bred Gabpafl/fl mice to mice that express the Mx1 Cre transgene,9 to generate the Gabpafl/fl-Mx1 Cre mice (Figure 1B); these mice undergo rearrangement of Gabpa after pIC treatment. As controls, Gabpaf/fl mice that lack Mx1 Cre also were treated with pIC.
Conditional disruption of mouse Gabpa. (A) Schematic representation of relevant regions of the wild-type (+), floxed (loxP-flanked, or fl), and the recombined (deleted, or del) mouse Gabpa genomic loci. Rectangles indicate exons and numbered arrows refer to primers used to amplify the floxed (primers 1 and 2) or the deleted (primers 1 and 3) alleles. (B) Breeding schema used to generate mice that undergo conditional Gabpa deletion in response to pIC induction of Cre recombinase from the Mx1 Cre locus. Pairs of triangles indicate loxP sites of the floxed allele, single triangles indicate the deleted Gabpa locus; and rectangle indicates Cre recombinase. (C) PCR of genomic DNA from tail (Ta), liver (Li), bone marrow (BM), spleen (Sp), and thymus (Th) of control and KO mice for the fl and del Gabpa alleles and Cre recombinase. (D) Immunoblot of muscle (Mu), BM, S, and Th for Gabpα and β-actin.
Conditional disruption of mouse Gabpa. (A) Schematic representation of relevant regions of the wild-type (+), floxed (loxP-flanked, or fl), and the recombined (deleted, or del) mouse Gabpa genomic loci. Rectangles indicate exons and numbered arrows refer to primers used to amplify the floxed (primers 1 and 2) or the deleted (primers 1 and 3) alleles. (B) Breeding schema used to generate mice that undergo conditional Gabpa deletion in response to pIC induction of Cre recombinase from the Mx1 Cre locus. Pairs of triangles indicate loxP sites of the floxed allele, single triangles indicate the deleted Gabpa locus; and rectangle indicates Cre recombinase. (C) PCR of genomic DNA from tail (Ta), liver (Li), bone marrow (BM), spleen (Sp), and thymus (Th) of control and KO mice for the fl and del Gabpa alleles and Cre recombinase. (D) Immunoblot of muscle (Mu), BM, S, and Th for Gabpα and β-actin.
Rearrangement of Gabpa in response to pIC was examined by PCR of genomic DNA (Figure 1C). As expected, unrearranged floxed Gabpa is detected in all tissues in the Gabpafl/fl control mice; unrearranged Gabpa also was seen in DNA from nonhematopoietic tissue (eg, tail) of the Gabpafl/fl-Mx1 Cre mice but not in their liver, bone marrow, spleen, or thymus. In contrast, the deleted Gabpa allele was detected in hematopoietic tissues (liver, bone marrow, spleen, and thymus) but not the tail of Gabpafl/fl-Mx1 Cre mice nor in any tissues of control mice. Immunoblotting confirmed the absence of Gabpα protein in bone marrow, spleen, and thymus of the Gabpafl/fl-Mx1 Cre mice, but Gabpα was present in muscle, and in all tissues of the control mice (Figure 1D). Thus, Gabpafl/fl-Mx1 Cre mice efficiently rearrange floxed Gabpa and their hematopoietic tissues lack Gabpα protein expression; these pIC-treated mice are hereinafter referred to as KO mice.
Bone marrow of Gabpa KO mice
Overall bone marrow cellularity was comparable between control and KO mice (Table 1), but total bone marrow leukocytes were reduced 3-fold in KO mice (Table 1; P < .05). The percentage of mature Ter119+CD71− red blood cells was increased in KO bone marrow (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article; P < .05), whereas the percentage of less mature Ter119+CD71+ erythroid cells was unaffected. Thus, disruption of Gabpa did not impair erythroid differentiation.
Bone marrow cell counts
| Bone marrow . | Control . | KO . |
|---|---|---|
| Total cell count | 72.0 + 15.0 | 71.0 + 5.5 |
| Leukocyte cell count | 20.8 + 3.3 | 6.5 + 4.9* |
| B220+ B lymphocytes (%) | 16.4 + 5.1 | 44.2 + 8.6* |
| B220+ B-lymphocyte count | 3.4 + 0.5 | 2.9 + 2.8 |
| B220+IgM− lymphocytes (%) | 82.3 + 17.8 | 51.7 + 18.3 |
| B220+IgM− lymphocyte count | 2.8 + 0.4 | 1.5 + 1.4 |
| pro-B (B220+CD43+IgM−) lymphocytes (%) | 4.6 + 0.7 | 2.4 + 1.1* |
| pro-B (B220+CD43+IgM−) lymphocyte count | 0.13 + 0.02 | 0.04 + 0.02* |
| Myeloid (CD11b+) cells (%) | 54.9 + 4.1 | 22.8 + 7.3* |
| Myeloid (CD11b+) count | 11.4 + 1.8 | 1.5 + 1.1* |
| Granulocytes (CD11bhi Gr1hi; %) | 35.2 + 5.0 | 1.3 + 0.9* |
| Granulocytes (CD11bhi Gr1hi) count | 7.3 + 1.2 | 0.1 + 0.1* |
| Immature myeloid (CD11bhi Gr1lo; %) | 18.1 + 4.6 | 21.7 + 12.9 |
| Immature myeloid (CD11bhi Gr1lo) count | 6.0 + 1.0 | 1.4 + 0.9* |
| CD11b− Gr1lo (%) | 3.7 + 1.3 | 13.6 + 3.1* |
| CD11b− Gr1lo count | 0.8 + 0.3 | 0.9 + 0.2 |
| Bone marrow . | Control . | KO . |
|---|---|---|
| Total cell count | 72.0 + 15.0 | 71.0 + 5.5 |
| Leukocyte cell count | 20.8 + 3.3 | 6.5 + 4.9* |
| B220+ B lymphocytes (%) | 16.4 + 5.1 | 44.2 + 8.6* |
| B220+ B-lymphocyte count | 3.4 + 0.5 | 2.9 + 2.8 |
| B220+IgM− lymphocytes (%) | 82.3 + 17.8 | 51.7 + 18.3 |
| B220+IgM− lymphocyte count | 2.8 + 0.4 | 1.5 + 1.4 |
| pro-B (B220+CD43+IgM−) lymphocytes (%) | 4.6 + 0.7 | 2.4 + 1.1* |
| pro-B (B220+CD43+IgM−) lymphocyte count | 0.13 + 0.02 | 0.04 + 0.02* |
| Myeloid (CD11b+) cells (%) | 54.9 + 4.1 | 22.8 + 7.3* |
| Myeloid (CD11b+) count | 11.4 + 1.8 | 1.5 + 1.1* |
| Granulocytes (CD11bhi Gr1hi; %) | 35.2 + 5.0 | 1.3 + 0.9* |
| Granulocytes (CD11bhi Gr1hi) count | 7.3 + 1.2 | 0.1 + 0.1* |
| Immature myeloid (CD11bhi Gr1lo; %) | 18.1 + 4.6 | 21.7 + 12.9 |
| Immature myeloid (CD11bhi Gr1lo) count | 6.0 + 1.0 | 1.4 + 0.9* |
| CD11b− Gr1lo (%) | 3.7 + 1.3 | 13.6 + 3.1* |
| CD11b− Gr1lo count | 0.8 + 0.3 | 0.9 + 0.2 |
Cell counts of total bone marrow cells and leukocyte subsets (determined after lysis of red blood cells; × 106/animal), percentages of the indicated immunophenotypic subsets, and calculated numbers of leukocyte subsets (× 106/animal) in at least 3 different control and KO mice.
P < .05 by Student t test.
Decreased immature B and T lymphocytes in Gabpa KO mice
Bone marrow leukocytes are composed primarily of B220+ B lymphocytes and CD11b+ myeloid cells. Although the percentage of B220+ B lymphocytes increased, the overall reduction in leukocytes indicates that there was no significant change in the total B lymphocytes in KO bone marrow (Table 1). Despite a modest reduction in total splenocytes, the percentage and total number of B220+ lymphocytes was unchanged in KO mice (supplemental Table 1). GABP plays important roles in lymphocytic gene expression and development,7,10 so we examined B- and T-lymphocyte development. As B lymphocytes mature, B220+ cells sequentially acquire expression of CD24+ and BP1+. These B220+CD24+BP1+ pro-B cells (IgM−B220+CD43+) progress to pre-B cells (IgM−B220+CD43−) and later express cell surface IgM or IgD. The percentage of IgM+ or IgD+ splenic B lymphocytes did not differ between KO and control mice (supplemental Figure 2A-B). Total bone marrow B220+ B lymphocytes did not differ between control and KO mice (Figure 2A; Table 1). Although the percentage of pre-B cells in bone marrow was increased in KO mice (supplemental Figure 2C), the reduction in total bone marrow B lymphocytes indicates that the number of pre-B cells was unaffected by loss of Gabpα (Table 1). The percentage and number of pro-B cells was decreased in KO bone marrow (supplemental Figure 2C; Table 1), but development of pro-B cells from BP1−CD24− to BP1−CD24+ to BP1+CD24+ was unaffected by Gabpa deletion (supplemental Figure 2D). In summary, Gabpa deletion was associated with a modest reduction in bone marrow pro-B cells.
Mature granulocytes are reduced in bone marrow and peripheral blood after Gabpa deletion. Flow cytometry for B220 and CD11b, forward scatter (FSC) and side scatter (SSC) of bone marrow cells (A; circles indicate cells with a high degree of SSC) and CD11b and Gr1 in bone marrow cells of control and KO mice (B); numbers indicate percentage of cells in individual quadrants or boxes. (C) Wright-Giemsa stained cytospin preparations of the following sorted bone marrow cell populations: control CD11bhi Gr1lo cells (open arrows indicate immature granulocytes, and thin arrows indicate monocytic cells), control CD11bhi Gr1hi cells, and KO CD11bint Gr1lo cells, in left, center, and right panels, respectively. Images shown at ×400 original magnification. All findings include data from at least 3 independent biologic specimens.
Mature granulocytes are reduced in bone marrow and peripheral blood after Gabpa deletion. Flow cytometry for B220 and CD11b, forward scatter (FSC) and side scatter (SSC) of bone marrow cells (A; circles indicate cells with a high degree of SSC) and CD11b and Gr1 in bone marrow cells of control and KO mice (B); numbers indicate percentage of cells in individual quadrants or boxes. (C) Wright-Giemsa stained cytospin preparations of the following sorted bone marrow cell populations: control CD11bhi Gr1lo cells (open arrows indicate immature granulocytes, and thin arrows indicate monocytic cells), control CD11bhi Gr1hi cells, and KO CD11bint Gr1lo cells, in left, center, and right panels, respectively. Images shown at ×400 original magnification. All findings include data from at least 3 independent biologic specimens.
Immature T lymphocytes in the thymus express both CD4 and CD8 and as they mature, they express either CD4 or CD8 and migrate to the spleen. The percentage and total number of CD4+ or CD8+ mature T lymphocytes was unaffected in KO spleen and thymus (supplemental Figure 3; supplemental Tables 1-2), but immature CD4+CD8+ T lymphocytes were decreased in KO thymus (supplemental Figure 3; supplemental Tables 1-2). Thus, consistent with previous reports,7,10 disruption of Gabpa in adult mice modestly reduced immature B and T lymphocytes in bone marrow, spleen, and thymus.
Myeloid cells in Gabpa KO mice exhibit aberrant differentiation
More than half of the nucleated bone marrow cells in control mice were CD11b+ myeloid cells, whereas fewer than one-quarter of KO bone marrow cells were CD11b+ (Figure 2A). Coupled with the reduction in total leukocytes, this indicates a nearly 8-fold decrease in total myeloid cells in KO bone marrow (Table 1). Bone marrow from control mice includes cells that express high levels of both CD11b and Gr1 (CD11bhiGr1hi), and a second population that expresses high levels of CD11b but lower levels of Gr1 (CD11bhiGr1lo; Figure 2B). Peripheral blood and spleen from control mice contain similar populations of myeloid cells (supplemental Figure 4). Morphologically, the CD11bhiGr1lo bone marrow cells consist primarily of cells with thick, ring-shaped nuclei with little central clearing that is characteristic of immature granulocytes (bands), and a smaller population of monocytes with characteristic reniform nuclei (Figure 2C). In contrast, CD11bhiGr1hi cells have more condensed nuclei with larger central clearing that are characteristic of mature mouse granulocytes. Maturation of mouse granulocytes is associated with accumulation of cytoplasmic granules, as demonstrated by a high degree of side scatter (Figure 2A).
Myeloid cells from KO mice are distinctly different. There is a near absence of CD11bhiGr1hi cells in KO bone marrow (Figure 2B), peripheral blood and spleen (supplemental Figure 4). The mean fluorescence of CD11b+ KO bone marrow myeloid cells (6100 + 900) is approximately half that of control CD11bhi cells (11 000 + 1500); we refer to these KO cells that express intermediate levels of CD11b as CD11bintGr1lo. Microscopic evaluation of KO CD11bintGr1lo cells reveals few mature granulocytes; rather, these cells display an intermediate degree of nuclear condensation and central nuclear clearing, compared with immature and mature control granulocytes (Figure 2C). Their cytoplasm has few cells with a high degree of side scatter, characteristic of granule-laden mature granulocytes (Figure 2A). Differential counts of bone marrow cells confirmed a marked loss of mature granulocytes in the KO CD11bintGr1lo population (Table 2). Instead, the KO CD11bintGr1lo population has a large percentage of these aberrant cells. Based on their abnormal expression of myeloid antigens, lack of cytoplasmic granules, and aberrant morphology, we conclude that the CD11bintGr1lo cells of KO mice resemble neither immature nor mature granulocytes.
Myeloid cell differential counts
| . | Blast . | Meta . | Band . | Gran . | Lymph . | Mono . | Aberrant . |
|---|---|---|---|---|---|---|---|
| Control: CD11bhiGr1lo | 0 | 1 | 18 | 23 | 3 | 54 | 0 |
| Control: CD11bhiGr1hi | 0 | 0 | 6 | 86 | 3 | 5 | 0 |
| KO: CD11bintGr1lo | 2 | 0 | 17 | 14 | 5 | 22 | 42 |
| . | Blast . | Meta . | Band . | Gran . | Lymph . | Mono . | Aberrant . |
|---|---|---|---|---|---|---|---|
| Control: CD11bhiGr1lo | 0 | 1 | 18 | 23 | 3 | 54 | 0 |
| Control: CD11bhiGr1hi | 0 | 0 | 6 | 86 | 3 | 5 | 0 |
| KO: CD11bintGr1lo | 2 | 0 | 17 | 14 | 5 | 22 | 42 |
Differential counts of the indicated bone marrow populations from control and KO mice expressed as percentage of at least 300 cells. Cells are classified as follows: blast (myeloblasts), meta (metamyelocytes), band (immature granulocytes), gran (mature granulocytes), lymph (lymphocytes), mono (monocytes), and aberrant myeloid cells.
The percentage of CD11b−Gr1lo cells is increased in KO bone marrow, peripheral blood, and spleen (Figure 2B; Table 1; supplemental Figure 4). However, their absolute numbers remain unchanged because of the reduction of total leukocytes in KO mice (Table 1). Evaluation of the CD11b−Gr1lo cells in control bone marrow reveals a small number of Ly6C+ immature myeloid cells (supplemental Figure 5A). Morphologically, this population includes immature granulocytes and monocytes (supplemental Figure 5B, a and b). The CD11b−Gr1lo cells in KO mice include a higher percentage of Ly6C+ immature myeloid cells, apoptotic granulocytes, and B lymphocytes (supplemental Figure 5A and B, c, d, and e).
Maturing granulocytes sequentially express primary granule protein genes (myeloperoxidase [MPO] and ELANE), secondary granule protein genes (lactoferrin [LF]), and tertiary granule protein genes (neutrophil collagenase [NC] and neutrophil gelatinase [NG]). As granulocytes mature beyond the promyelocyte stage, they express less MPO and ELANE and more secondary and tertiary granule protein genes. As monocytes mature, they lose expression of markers of immaturity (c-kit and Ly6C) and increase expression of Mac3, F4/80, and M-CSF-R antigens and cathepsin B, fibronectin 1, and phospholipid transfer protein genes.
We examined granulocytic gene expression in control and KO bone marrow by quantitative RT-PCR. In Figure 3A, granulocyte gene expression is presented relative to levels in control CD11bhiGr1lo immature granulocytes. As expected, mature granulocytes (CD11bhiGr1hi cells) from control mice express lower levels of primary granule protein genes and higher levels of tertiary granule protein genes than immature CD11bhiGr1lo cells (P < .05); expression of the secondary granule protein gene LF did not differ significantly between the immature and mature granulocytes of control mice. Compared with control CD11bhiGr1lo myeloid cells, KO CD11bintGr1lo cells express lower levels of ELANE and LF (P < .05) and similar levels of MPO, NC, and NG. Compared with CD11bhiGr1hi control granulocytes, KO myeloid cells express less tertiary granule protein genes (P < .05), but levels of MPO, ELANE, and LF were not significantly altered. The disordered granulocyte gene expression by KO CD11bintGr1lo cells does not correspond to either immature or mature granulocytes and indicates that disordered KO myeloid cells do not manifest a simple block in granulocytic differentiation.
Expression of granulocyte and monocyte/macrophage genes by myeloid cells. (A) Quantitative RT-PCR from the indicated cell populations, normalized to ActinB and expressed relative to control CD11bhi Gr1locells for the following (A) neutrophil genes: myeloperoxidase (MPO), neutrophil elastase (ELANE), lactoferrin (LF), neutrophil collagenase (NC), and neutrophil gelatinase (NG), and (B) monocyte genes: calpactin (Cal), cathepsin B (CaB), fibronectin 1 (FN1), Legumain (LEG), and phospholipid transfer protein (PTP). Experiments were performed 3 times each on 3 different biologic specimens. *P < .05 between KO and control CD11bhi Gr1lo cells; **P < .05 between KO and control CD11bhi Gr1hi cells.
Expression of granulocyte and monocyte/macrophage genes by myeloid cells. (A) Quantitative RT-PCR from the indicated cell populations, normalized to ActinB and expressed relative to control CD11bhi Gr1locells for the following (A) neutrophil genes: myeloperoxidase (MPO), neutrophil elastase (ELANE), lactoferrin (LF), neutrophil collagenase (NC), and neutrophil gelatinase (NG), and (B) monocyte genes: calpactin (Cal), cathepsin B (CaB), fibronectin 1 (FN1), Legumain (LEG), and phospholipid transfer protein (PTP). Experiments were performed 3 times each on 3 different biologic specimens. *P < .05 between KO and control CD11bhi Gr1lo cells; **P < .05 between KO and control CD11bhi Gr1hi cells.
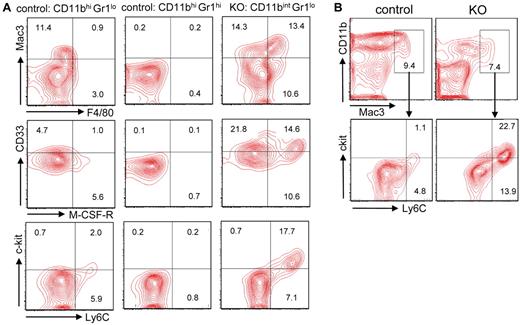
Monocytes are present in the control CD11bhiGr1lo cells, and as expected they express Mac3, F4/80, M-CSF-R, and Ly6C (Figure 4A; supplemental Figure 6A). None of these markers is expressed by control CD11bhiGr1hi cells. However, KO CD11bintGr1lo cells express significantly higher levels of Mac3 and F4/80 antigens (Figure 4A; supplemental Figure 6A) and higher levels of the monocytic genes CaB, FN1, and PTP than control CD11bhiGr1lo cells (Figure 3B). KO CD11bintGr1lo cells also express high levels of M-CSF-R, c-kit, and Ly6C, which are expressed by immature monocytes (Figure 4A). The KO CD11bintGr1lo cells might be interpreted as containing a dual population of mature monocytes and immature granulocytes, but it is the KO Mac3+ monocytes that overexpress c-kit and Ly6C (Figure 4B; supplemental Figure 6B). This disordered monocytic maturation emphasizes that KO myeloid do not resemble either immature or mature granulocytes or monocytes, or a simple block in the differentiation of either lineage.
Aberrant expression of monocytic antigens by Gabpa KO myeloid cells. (A-B) Flow cytometric analysis of indicated populations of control and Gabpa KO cells for expression of Mac3, F4/80, CD33, M-CSF-R, c-kit, and Ly6C; numbers indicate percentage of cells in individual quadrants or boxes. All findings include data from at least 3 independent biologic specimens.
Aberrant expression of monocytic antigens by Gabpa KO myeloid cells. (A-B) Flow cytometric analysis of indicated populations of control and Gabpa KO cells for expression of Mac3, F4/80, CD33, M-CSF-R, c-kit, and Ly6C; numbers indicate percentage of cells in individual quadrants or boxes. All findings include data from at least 3 independent biologic specimens.
Gabpa KO cells contribute poorly to the myeloid compartment
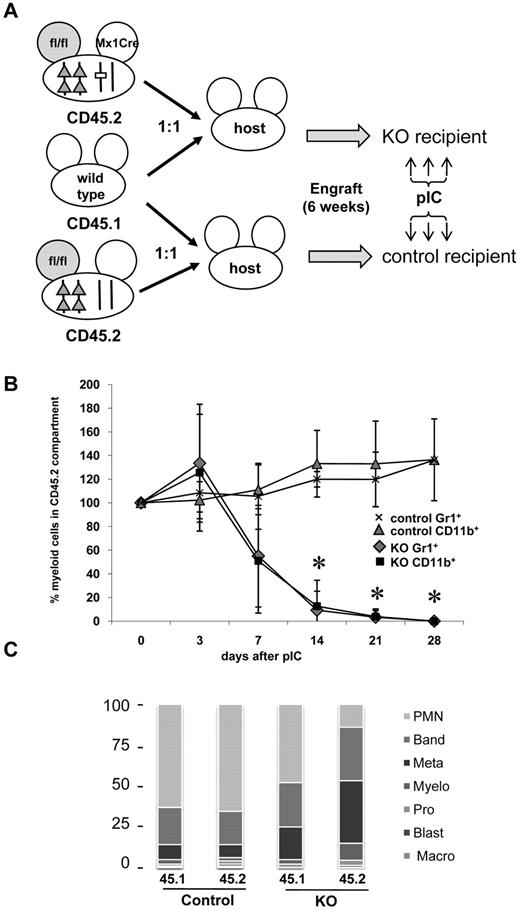
We sought to determine whether Gabpα null cells can contribute to the myeloid compartment in vivo. We used a competitive transplantation strategy that distinguishes 2 different sources of donor bone marrow cells by distinct CD45 isoforms. Irradiated C57BL/6J mice were injected with equal numbers of bone marrow cells from (1) CD45.2-expressing Gabpafl/fl-Mx1 Cre mice and (2) wild-type donors that express CD45.1; these host mice are referred to as KO recipients (Figure 5A). Control recipient mice were injected with equal numbers of bone marrow cells from (1) CD45.2-expressing Gabpafl/fl mice and (2) CD45.1-expressing wild-type donor mice. Donor mice were not injected with pIC before bone marrow harvest, so all donor cells should express normal levels of Gabpα.
Competitive transplantation with wild-type and Gabpa KO cells. (A) Schematic representation of competitive bone marrow transplantation. Irradiated C57BL/6J KO recipient mice were infused with equal numbers of bone marrow cells from CD45.1+ wild-type donor mice and CD45.2+Gabpafl/fl-Mx1 Cre donor mice. Irradiated C57BL/6J control recipient mice were infused with equal numbers of bone marrow cells from CD45.1+ wild-type donor mice and CD45.2+Gabpafl/fl donor mice. Engraftment of both donor populations was examined 4 weeks later by flow cytometry of peripheral blood for CD45 isoforms. Both groups of recipient mice were injected with pIC 6 weeks after initial transplantation. (B) CD45.2+ cells were examined by flow cytometry for expression of CD11b and Gr1 at the indicated times. Sequential percentage values ± SEM of CD11b+ or Gr1+ cells in the CD45.2+ compartment are normalized to each mouse's values immediately before pIC injection. All findings from at least 3 independent biologic specimens. (C) Differential cell counts of bone marrow sorted for the indicated CD45 isoforms, from recipient control or recipient KO mice, presented as percentage from > 300 cell count.
Competitive transplantation with wild-type and Gabpa KO cells. (A) Schematic representation of competitive bone marrow transplantation. Irradiated C57BL/6J KO recipient mice were infused with equal numbers of bone marrow cells from CD45.1+ wild-type donor mice and CD45.2+Gabpafl/fl-Mx1 Cre donor mice. Irradiated C57BL/6J control recipient mice were infused with equal numbers of bone marrow cells from CD45.1+ wild-type donor mice and CD45.2+Gabpafl/fl donor mice. Engraftment of both donor populations was examined 4 weeks later by flow cytometry of peripheral blood for CD45 isoforms. Both groups of recipient mice were injected with pIC 6 weeks after initial transplantation. (B) CD45.2+ cells were examined by flow cytometry for expression of CD11b and Gr1 at the indicated times. Sequential percentage values ± SEM of CD11b+ or Gr1+ cells in the CD45.2+ compartment are normalized to each mouse's values immediately before pIC injection. All findings from at least 3 independent biologic specimens. (C) Differential cell counts of bone marrow sorted for the indicated CD45 isoforms, from recipient control or recipient KO mice, presented as percentage from > 300 cell count.
All donor cells engrafted within 4 weeks, based on peripheral blood CD45 isoform expression, but individual mice engrafted with distinct ratios of donor cells (range, 30%-70% CD45.2+ cells). Six weeks after engraftment, recipient mice were injected with pIC. To reduce the confounding effect of the initial engraftment ratio, the CD45.2% of myeloid cells immediately before pIC injection was set to 100% for each mouse, and subsequent values were normalized to that baseline value.
Peripheral blood was examined weekly for expression of CD45 isoforms Gr1 and CD11b. In the control recipient mice, the percentage of CD45.2+ peripheral blood myeloid cells in control recipient mice did not change significantly after pIC injection (Figure 5B). More than 80% of both wild-type CD45.1+ and Gabpafl/fl CD45.2+ bone marrow donor cells were mature granulocytes or band forms (Figure 5C). In KO recipient mice, the percentage of CD45.2+Gabpafl/fl-Mx1 Cre myeloid cells declined dramatically within 14 days (Figure 5B; P < .01). In KO recipient mice, wild-type CD45.1+ myeloid cells were predominantly mature (similar to control recipients), but there was shift toward immature myeloid forms among CD45.2+Gabpafl/fl-Mx1 Cre donor cells. We conclude that Gabpα null cells do not generate normal mature granulocytes and that their failure to contribute to the myeloid compartment is a cell intrinsic defect.
Decreased myeloid progenitor cells in Gabpa KO mice
We examined the ability of KO bone marrow to form myeloid colonies in vitro. After injection of control and KO mice with pIC, bone marrow was cultured in vitro in the presence of cytokines, and colonies were examined 7 days later. Compared with control bone marrow, KO colony formation decreased markedly in the presence of GM-CSF, G-CSF, or M-CSF (Figure 6A; P < .03). KO myeloid cells express M-CSF-R (Figure 4) and other cytokine receptors (data not shown), so their failure to form in vitro colonies is not because of reduced expression of these essential cytokine receptors. Cells grown from control mice in GM-CSF include immature granulocytes and monocytes, but KO colonies consist almost exclusively of large macrophagelike cells with foamy cytoplasm. Thus, cytokine-stimulated KO bone marrow in vitro colony formation is impaired, and their morphology is biased to a macrophagelike appearance.
Gabpa disruption reduces myeloid progenitors and alters transcription factor expression. (A) Number of in vitro colonies ± SEM per 5 × 104 control and KO bone marrow cells, grown in the indicated cytokines. *P < .03. (B) Wright-Giemsa–stained cells extracted from in vitro colonies of control and KO bone marrow grown in GM-CSF, shown at ×400 original magnification. (C) Flow cytometry for FcγII/III and CD34 of control and KO Lin−, Sca1−, c-kit+ bone marrow cells. Numbers represent percentages of the indicated populations: CMP, GMP, and MEP. (D) Expression of indicated transcription factors by quantitative RT-PCR of control and KO GMP cells normalized to ActinB and expressed relative to control GMP cells. Data are ± SEM. *P < .04. (E) Immunoblotting for Gabpα or β-actin of the indicated tissues from control and KO mice. All findings include data from at least 3 independent biologic specimens.
Gabpa disruption reduces myeloid progenitors and alters transcription factor expression. (A) Number of in vitro colonies ± SEM per 5 × 104 control and KO bone marrow cells, grown in the indicated cytokines. *P < .03. (B) Wright-Giemsa–stained cells extracted from in vitro colonies of control and KO bone marrow grown in GM-CSF, shown at ×400 original magnification. (C) Flow cytometry for FcγII/III and CD34 of control and KO Lin−, Sca1−, c-kit+ bone marrow cells. Numbers represent percentages of the indicated populations: CMP, GMP, and MEP. (D) Expression of indicated transcription factors by quantitative RT-PCR of control and KO GMP cells normalized to ActinB and expressed relative to control GMP cells. Data are ± SEM. *P < .04. (E) Immunoblotting for Gabpα or β-actin of the indicated tissues from control and KO mice. All findings include data from at least 3 independent biologic specimens.
We examined hematopoietic stem cells (HSCs) and myeloid progenitor cells in control and KO bone marrow by flow cytometry. Lineage-negative (Lin−) Sca1+ c-kit+ cells (LSK) are enriched for HSCs. Expression of FcγII/III (CD16/32) and CD34 by Lin−, Sca1−, c-kit+ bone marrow cells distinguishes common myeloid progenitors, megakaryocyte-erythrocyte progenitors, and granulocyte-monocyte progenitors. The increase in the percentage of LSK cells in KO bone marrow (Figure 6C top) was more than offset by the reduced number of total Lin− cells and indicates that there was no significant change in overall HSCs in KO bone marrow (supplemental Table 3). The percentage and total Lin−Sca1−c-kit+ progenitor cells were greatly reduced in KO mice (Figure 6C; supplemental Table 3), but we observed no alteration in the ratios of individual myeloid progenitor subsets. Proliferation in GMP (supplemental Figure 7) and other progenitors (data not shown) was decreased in KO mice. In summary, we observed a marked reduction of proliferating myeloid progenitors in KO bone marrow by in vitro colony formation, immunophenotyping, and cell-cycle analysis.
We examined the differentiation and function of GMP myeloid progenitors in vitro. Isolated GMPs from control and KO bone marrow were incubated with GM-CSF in liquid culture. Five days later, there were fewer mature and immature granulocytes by morphology and immunophenotype (CD11bhiGr1hi and Ly6C+Gr1hi) in KO bone marrow, compared with control bone marrow (supplemental Figure 8A-B). Myeloid cells derived from KO GMPs performed phagocytosis and oxidized dihydrorhodamine-123 normally (supplemental Figure 8C-D). CD11bhiGr1lo or Ly6C+Gr1lo monocytes were reduced in KO bone marrow after in vitro culture. These in vitro findings confirm the myeloid differentiation defects that we observed in vivo.
Gabpa disruption is associated with decreased expression of Gfi-1 in myeloid cells
We sought to determine whether the abnormal morphology and aberrant gene expression of Gabpα null cells is associated with disordered expression of other myeloid transcription factors. Although there were modest changes in the expression of several transcription factors in KO GMP, only the 3-fold reduction of Gfi-1 reached statistical significance (Figure 6D; P < .01).
Because the dysplastic morphology and aberrant gene expression of Gabpa KO myeloid cells resemble those of Gfi1−/− mice,10,11 we examined Gfi-1 expression in Gabpa KO mice. Gfi-1 protein was abundant in control bone marrow, spleen, thymus, and CD11b+ bone marrow cells (Figure 6E) but nearly absent in KO bone marrow, spleen, and CD11b+ cells. Interestingly, KO thymus expressed Gfi-1 at a level comparable with that of control mice, despite the complete deletion of Gabpa in KO thymus (Figure 1C-D).
Gabp regulates expression of Gfi-1
We pursued Gfi-1 as a biologically relevant target of Gabp in myeloid cells. The Gfi1 promoter includes a cluster of 3 GGAA Gabp consensus sequences (ets1,2,3) 1.5 kb upstream of the transcriptional site, and another ets site near the transcriptional start site (ets4). We cloned the mouse Gfi1 promoter into the pGL3 luciferase reporter construct and transfected it into 293 fibroblast cells along with Gabpa and Gabpb expression vectors. Transfection with both Gabpa and Gabpb activated the Gfi1 promoter, but empty vector, or either Gabpa and Gabpb alone did not activate it (Figure 7A); the transfected cells express endogenous Gabp proteins, and although the activation is modest, this represents a statistically significant increase (P < .01). Next, we used chromatin immunoprecipitation to determine whether Gabp binds to the endogenous Gfi1 promoter in vivo. Figure 7B demonstrates that the region of the Gfi1 promoter that contains the upstream ets1,2,3 cluster is precipitated by antibody against Gabpα, as is the ets4 region, but an irrelevant region of the Gfi1 gene is not bound by Gabp. Thus, the Gfi1 promoter is a direct target of Gabp in vivo.
Gabp activates and binds Gfi-1 promoter and Gfi-1 expression partially rescues Gabpa KO phenotype. (A) Expression from Gfi1 promoter when cotransfected with Gabpa alone, Gabpb alone, or Gabpa and Gabpb together, expressed as normalized relative light units (RLU). *P < .01 by ANOVA. (B) ChIP of indicated regions of mouse Gfi1 gene performed with no input DNA (H2O); input DNA not subjected to ChIP (Input); and ChIP performed with no antibody (no Ab), control IgG, and anti-Gabpα antibody (GABP); ets 1,2,3 and ets 4 correspond to Gfi-1 promoter regions shown in panel C. (C) Transfection into 293 cells of indicated Gfi1 promoter constructs alone, described relative to transcriptional start site; mut refers to mutation of the indicated ets sites (left). Promoter activity expressed as normalized RLU, ± SEM (right). *P < .01 by Student t test. (D) In vitro colony formation per 5 × 104 plated cells ± SEM by Lin− bone marrow cells from control or KO mice, transduced with MSCV empty virus or MSCV–Gfi-1 retrovirus, selected in puromycin, and grown in G-CSF and GM-CSF. *P < .01. (E) Statistical summary of CD11b and Gr1 expression by Lin− control or KO bone marrow cells transduced with control MSCV or MSCV–Gfi-1 virus as shown in supplemental Figure 9. *P < .05 between the corresponding control and KO cells. All findings include data from at least 3 independent biologic specimens.
Gabp activates and binds Gfi-1 promoter and Gfi-1 expression partially rescues Gabpa KO phenotype. (A) Expression from Gfi1 promoter when cotransfected with Gabpa alone, Gabpb alone, or Gabpa and Gabpb together, expressed as normalized relative light units (RLU). *P < .01 by ANOVA. (B) ChIP of indicated regions of mouse Gfi1 gene performed with no input DNA (H2O); input DNA not subjected to ChIP (Input); and ChIP performed with no antibody (no Ab), control IgG, and anti-Gabpα antibody (GABP); ets 1,2,3 and ets 4 correspond to Gfi-1 promoter regions shown in panel C. (C) Transfection into 293 cells of indicated Gfi1 promoter constructs alone, described relative to transcriptional start site; mut refers to mutation of the indicated ets sites (left). Promoter activity expressed as normalized RLU, ± SEM (right). *P < .01 by Student t test. (D) In vitro colony formation per 5 × 104 plated cells ± SEM by Lin− bone marrow cells from control or KO mice, transduced with MSCV empty virus or MSCV–Gfi-1 retrovirus, selected in puromycin, and grown in G-CSF and GM-CSF. *P < .01. (E) Statistical summary of CD11b and Gr1 expression by Lin− control or KO bone marrow cells transduced with control MSCV or MSCV–Gfi-1 virus as shown in supplemental Figure 9. *P < .05 between the corresponding control and KO cells. All findings include data from at least 3 independent biologic specimens.
To determine whether these Gabp-bound regions regulate Gfi1 gene expression, we generated a nested series of deletions and mutations of the Gfi1 promoter (Figure 7C). Reporter activity of construct −1575, which lacks the ets1,2,3 cluster, was reduced by half compared with the full-length −2251 construct (Figure 7C; P < 0.04). Mutation of any of the individual upstream ets sites also reduced reporter activity compared with −2251, but mutation of the proximal ets4 site (−732 mut4) affected promoter activity only modestly. Thus, full activity of the Gfi1 promoter depends on integrity of a cluster of 3 upstream ets sites that are bound by Gabp.
Gfi-1 partially restores the phenotype of Gabpα null myeloid cells
We sought to determine whether expression of Gfi-1 in Gabpα null cells could restore normal myeloid differentiation. We transduced control and KO bone marrow with murine stem cell virus (MSCV)–Gfi-1 or empty virus. Because both viruses encode puromycin resistance, we selected transduced cells with puromycin and plated them in semisolid medium with G-CSF and GM-CSF. Transduction of control cells with MSCV–Gfi-1 caused a modest, but not statistically significant, increase in myeloid colony formation (Figure 7D). As expected, colony formation was markedly reduced Gabpa KO bone marrow transduced with empty vector, compared with controls (P < 4 × 10−6). Transduction of Gabpα null cells with MSCV–Gfi-1 increased the plating efficiency of Gabpα null cells more than 4-fold (P < .0002). Nevertheless, colony formation by MSCV-Gfi-1–transduced Gabpα null cells remained less than half of controls (P < .0003). Thus, Gfi-1 partially restored the reduced in vitro myeloid colony formation after Gabpa disruption.
We sought to determine whether Gfi-1 could rescue the aberrant immunophenotype of Gabpα null myeloid cells. We transduced control and KO cells with MSCV-Gfi-1 or MSCV, selected transduced cells with puromycin, and grew them in liquid culture with G-CSF and GM-CSF for 5 days. Transduction of Gabpα null cells with MSCV–Gfi-1 restored Gfi1 mRNA expression to levels comparable with those of control cells. Transduction of control cells with Gfi1 did not significantly alter the percentage of CD11bhiGr1hi cells (Figure 7E; supplemental Figure 9; Table 3). As expected, disruption of Gabpa is associated with a reduction of CD11bhiGr1hi cells (P < .001), but transduction of Gabpα null cells with Gfi1 increased the percentage of CD11bhiGr1hi cells by 60% (P < .001) and doubled the level of CD11b expression (Table 3). Transduction of Gabpα null cells with Gfi-1 partially reversed the overexpression of Mac3 and F4/80 (Table 3). Thus, transduction with Gfi1 partially rescued the aberrant immunophenotype of Gabpα null cells. We conclude that Gfi1 is a direct transcriptional target of Gabp and that its re-expression partially rescues the proliferative defect and aberrant phenotype of Gabpα null bone marrow cells.
Summary of Gfi-1 rescue
| . | Gfi-1 mRNA (%) . | CD11b+Gr1+ (%) . | CD11b GeoMean . | F4/80+Mac3+ (%) . |
|---|---|---|---|---|
| Control: MSCV-puro | 100 | 40.0 + 2.8 | 10 200 + 6800 | 4.1 + 3.3 |
| Control: MSCV-Gfi1 | 161 + 27 | 36.0 + 6.0 | 8900 + 3100 | 4.4 + 4.1 |
| KO: MSCV-puro | 34 + 5* | 16.5 + 2.7* | 2300 + 1500* | 25.9 + 6.0* |
| KO: MSCV-Gfi1 | 115 + 40 | 26.4 + 1.9 | 4700 + 2200 | 8.1 + 7.0 |
| . | Gfi-1 mRNA (%) . | CD11b+Gr1+ (%) . | CD11b GeoMean . | F4/80+Mac3+ (%) . |
|---|---|---|---|---|
| Control: MSCV-puro | 100 | 40.0 + 2.8 | 10 200 + 6800 | 4.1 + 3.3 |
| Control: MSCV-Gfi1 | 161 + 27 | 36.0 + 6.0 | 8900 + 3100 | 4.4 + 4.1 |
| KO: MSCV-puro | 34 + 5* | 16.5 + 2.7* | 2300 + 1500* | 25.9 + 6.0* |
| KO: MSCV-Gfi1 | 115 + 40 | 26.4 + 1.9 | 4700 + 2200 | 8.1 + 7.0 |
Summary of rescue of KO bone marrow cells in 4 distinct experiments, with MSCV–Gfi-1 or MSCV empty vector, subjected to flow cytometry for CD11b, Gr1, F4/80, and Mac3, presented as percentage of positive cells and geometric mean fluorescence.
P < 0.05 by ANOVA.
Discussion
We disrupted Gabpa in mouse hematopoietic cells to better define the role of the GABP transcription factor in myeloid differentiation. Gabpa KO mice had a profound loss of myeloid cells in blood and bone marrow, and the remaining myeloid cells exhibited aberrant morphology, immunophenotype, and gene expression. Gabpα null cells contributed poorly to the myeloid compartment because of a cell intrinsic defect. Gabpa KO mice had a marked reduction in proliferating myeloid progenitors in their bone marrow, and Gabpα null myeloid cells express reduced levels of the Gfi-1 transcriptional repressor. We showed that Gabp binds and activates the Gfi1 promoter and that transduction of Gfi1 into Gabpα null bone marrow cells partially rescued the differentiation defects of Gabpa KO myeloid cells. We conclude that GABP is required for normal myeloid differentiation, in part, through its previously unrecognized role in regulating expression of Gfi1 in hematopoietic cells.
GABP regulates genes that are required for innate immunity through its functional interactions with the ets factor PU.1, the b-zip factor C/EBPα, and Sp1,3 and it is an essential component of a retinoic acid-responsive myeloid enhanceosome.4 GABP is a member of a large transcription factor family, and it has not been clear whether other ets factors could replace GABP in myeloid differentiation and hematopoiesis. In lymphoid cells, GABP was shown previously to regulate expression of the critical lymphoid gene IL7-Rα.10 Recently, deletion of Gabpa in T lymphocytes by Lck-driven Cre expression was shown to decrease thymic cellularity and reduce immature T lymphocytes (CD4+CD8+) and was associated with a block at the DN3 stage of T-cell development7,11 In the current report, deletion of Gabpa driven by pIC-induced Cre recombinase also was associated with a reduction of immature thymic T lymphocytes. The experimental approaches to Gabpa disruption differ, that is, Lck-driven Cre expression causes Gabpa deletion throughout development, whereas the current approach induces Gabpa disruption in mature mice that possess an intact immune system. Nevertheless, the experimental findings are similar and indicate that GABP is required for normal T-cell maturation. Transplantation of fetal liver with reduced levels of Gabpα (by a gene trap knockdown) into Rag2−/− recipients demonstrated decreased mature pro-B and pre-B cells and implicated GABP in the regulation of the transcription factor Pax-5.7 In the current report, we observed a reduction of pro-B cells, but not pre-B cells, in bone marrow of Gabpα null mice. Our findings confirm the importance of Gabp in the development of B cells and T lymphocytes.
We now demonstrate that GABP has a nonredundant, essential role in normal myeloid cell development, in part, through its control of Gfi1 expression. Gfi-1 is a zinc finger- and SNAG domain-containing transcriptional repressor that is expressed by T and B lymphocytes and granulocytes but not by macrophages.12,13 Gfi-1 is required for terminal granulocytic differentiation12,14 and for preservation of the hematopoietic stem cell population.15,16 Gfi-1 influences lineage commitment of granulocytes and lymphocytes by regulating HoxA9 and PU.1,8,17-19 as well as CSF-1,20 and Id2.21 Gfi-1 acts as a tumor suppressor for hematopoietic cells, because its loss predisposes to myeloid leukemia.8 Clinically, defects in Gfi-1 are implicated in some patients with severe congenital neutropenia,22 reduced levels of Gfi-1 expression are seen in some cases of myelodysplastic syndrome,23 and certain single nucleotide polymorphisms may be associated with the development of AML.24 Thus, Gfi-1 is a critical regulator of normal and malignant myeloid cell development.25
We recognized that many aspects of the aberrant morphology, immunophenotype, and gene expression of Gabpα null myeloid, B, and T cells resembled those properties of cells that lack Gfi-1.12,14,17 This suggested that Gabp might share a regulatory pathway with Gfi-1 in the development of myeloid and lymphoid cells. Indeed, we found a significant reduction of Gfi1 expression by Gabpa KO myeloid cells, we showed that Gfi-1 is a direct transcriptional target of Gabp and that Gfi-1 partially restored the aberrant differentiation of Gabpα null myeloid cells.
Despite the role of Gabp in regulating Gfi1, the latter cannot fully account for the hematopoietic effects of Gabpa disruption. Restoration of Gfi-1 partially restored the abnormal growth and differentiation of myeloid cells but did not fully rescue these defects. More importantly, the phenotypes of Gabpa and Gfi1 KO mice differ in important ways. Disruption of Gabpa caused a rapid loss of myeloid cells, decreased myeloid progenitor cells, and reduced their proliferation. In contrast, Gfi-1 null mice have normal12 or increased14 circulating myeloid cells, increased numbers of myeloid progenitors, and increased cycling hematopoietic stem cells.12,15,16 These phenotypic differences emphasize the observation that Gfi-1 contributes to differentiation defects of Gabpα null myeloid cells but cannot account for their defects in progenitor cells.
Gabp controls expression of Gfi1 in CD11b+ myeloid cells. Interestingly, in thymus Gabpa was fully disrupted (Figure 1), but expression of Gfi-1 was not reduced (Figure 6). Thus, mechanisms other than Gabp transcriptional regulation must control Gfi-1 expression in T lymphocytes. Disruption of the ets factor Mef/Elf4 in HSCs was associated with increased expression of Gfi1 in HSCs, so other ets factors may regulate Gfi-1 in different cellular contexts.26 Similarly, Gfi-1 is a target of p53 in HSCs.27 The identity of the ets factor or other transcription factors that control Gfi-1 expression in T lymphocytes remains to be defined.
Gfi1 expression was markedly reduced in Gabpa KO GMPs, but there were modest changes in other transcription factors, as well. We pursued Gfi-1 as a physiologic target because its disruption caused defects that resemble those in Gabpa KO cells. The modest alterations in the expression of Irf8, PU.1, and other myeloid transcription factors did not rise to the level of statistical significance. Nevertheless, interactions of GABP with retinoid receptors, PU.1, C/EBPα, and other transcription factors may contribute to the defective myeloid differentiation in Gabpa KO mice and may account for the failure of Gfi-1 to fully rescue the myeloid differentiation defects in Gabpα null mice. Because each of these transcription factors is associated with AML, GABP also may contribute to clinical hematologic conditions, including neutropenia, myelodysplasia, and acute leukemia.
Note added in proof: During the final revision of this manuscript, Yu et al described a decrease in myeloid progenitor cells and HSCs after conditional disruption of Gabpa.28
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lee Grimes for MSCV–Gfi-1 and for antibody against Gfi1, and Cong Peng and Shaoguang Li for advice and assistance with virus preparation. They also thank Lee Grimes, Glen Raffel, Shaoguang Li, and Lucio Castilla for thoughtful discussions.
This work was supported by the National Institutes of Health (HL073945, A.G.R.).
National Institutes of Health
Authorship
Contribution: Z.-F.Y., K.D., J.C., J.W., and X.Z. performed experiments; and Z.-F.Y. and A.G.R. designed the research, analyzed results, made the figures, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan G. Rosmarin, MD, University of Massachusetts Medical School, University Hospital, Rm H8–533, 55 Lake Ave, Worcester, MA 01655; e-mail: alan.rosmarin@umassmed.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal