Abstract
Plasmodium falciparum relies on anion channels activated in the erythrocyte membrane to ensure the transport of nutrients and waste products necessary for its replication and survival after invasion. The molecular identity of these anion channels, termed “new permeability pathways” is unknown, but their currents correspond to up-regulation of endogenous channels displaying complex gating and kinetics similar to those of ligand-gated channels. This report demonstrates that a peripheral-type benzodiazepine receptor, including the voltage dependent anion channel, is present in the human erythrocyte membrane. This receptor mediates the maxi-anion currents previously described in the erythrocyte membrane. Ligands that block this peripheral-type benzodiazepine receptor reduce membrane transport and conductance in P falciparum-infected erythrocytes. These ligands also inhibit in vitro intraerythrocytic growth of P falciparum. These data support the hypothesis that dormant peripheral-type benzodiazepine receptors become the “new permeability pathways” in infected erythrocytes after up-regulation by P falciparum. These channels are obvious targets for selective inhibition in anti-malarial therapies, as well as potential routes for drug delivery in pharmacologic applications.
Introduction
The most severe form of malaria in humans is caused by parasite Plasmodium falciparum, infecting 225 million people and causing 781 000 deaths in 2009 (World Health Organization, 2010). Erythrocyte invasion by P falciparum provides the parasite access to a plentiful source of nutrients in a locale that is largely shielded from host immune defenses. After invasion, the invading parasite uses a variety of strategies to adapt to the intraerythrocytic environment. To ensure the transport of nutrients and waste products necessary for its replication and survival, P falciparum relies on broad specificity anion channels activated in the erythrocyte membrane after invasion.1 Initially, this transport was attributed to “new” permeability pathways (NPPs)2 exported by the parasite to the host membrane.3 However, later studies revealed that the current is because of up-regulation of endogenous channels4 and that the diversity of anion channel activities recorded in these studies correspond to different kinetic modalities of a unique type of maxi-anion channel.5 This channel displays complex gating and kinetics similar to those of ligand-gated channels.5
Anions are transported through the human erythrocyte membrane by a 2-component system: a large electroneutral exchanger mediated by band 3 and a 4 orders of magnitude smaller electrogenic component estimated at approximately 10 μS/cm2 corresponding presumably to a small number of channels.6 Remarkably, the molecular identification and characterization of this conductive pathway has not yet been achieved. Neither genomic nor proteomic studies have provided meaningful clues to the composition of this pathway.7 Considering the small amount of protein a few hundred channels represent, it is most likely that they remain below the detection limit in current, standard proteomic protocols. However, there is a growing body of information on the electrophysiologic characteristics of this pathway.5
Our previous work using the cell-attached configuration of the patch-clamp technique to this issue demonstrated that a unique type of maxi-anion channel with multiple conductance levels mediates band 3-independent anion conductance across the erythrocyte membrane.5 These channels are dormant under normal physiologic conditions, yet on activation confer a far higher anion conductance to the erythrocyte membrane than the ground leak mediated by band 3. We hypothesized that this anion conductance is mediated by a voltage dependent anion channel (VDAC). VDACs, originally characterized as mitochondrial porins,8 can be expressed in plasma membranes alone,9 or as a component of the peripheral-type benzodiazepine receptor (PBR) complex.10 The PBR complex consists of at least 3 components: a 32-kDa VDAC, a 18-kDa translocator protein (TSPO, also called isoquinoline-binding protein IBP), and a 30-kDa adenine nucleotide transporter (ANT).10 The PBR is characterized by a primary distribution in tissues outside the central nervous system and by nanomolar affinity for the ligands PK 11 195 > Ro5-4864 > diazepam. The TSPO component is considered to be primarily responsible for binding to PK 11 195, while Ro5 4864 and other benzodiazepines may bind to all components of the PBR complex.11 Based on the entropy and enthalpy driven nature of ligand–receptor interactions, PK11195 was classified as an antagonist, whereas Ro5-4864 was classified as an agonist.12
This report demonstrates that the 3 principal components of the peripheral-type benzodiazepine receptor (PBR) are present and functional in the human erythrocyte membrane. Electrophysiologic studies demonstrate that PBR is responsible for the previously described erythrocyte membrane-associated maxi-anion current. PBR ligands reduce membrane transport and conductance in P falciparum–infected erythrocytes and block the intraerythrocytic growth of P falciparum in vitro. These data support the hypothesis that the dormant PBR mediates the band 3-independent anion conductance in normal erythrocytes, and, after up-regulation by P falciparum, become the “new permeability pathways” in P falciparum–infected erythrocytes. These channels are obvious targets for selective inhibition in anti-malarial therapies, as well as potential routes for drug delivery in pharmacologic applications.
Methods
Chemicals
5-Nitro-2-(3-phenylpropylamino)-benzoate (NPPB), Isoquinoline 1-(2-chlorophenyl)-N-methyl-N-(1-methyl-propyl)-3-isoquinolinecarboxamide (PK 11195) and the Benzodiazepine 7-chloro-5-(4-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2–1 (Ro5-4864), diazepam, chloroquine and human serum were purchased from Sigma-Aldrich.
Cells and cell preparation
Venous blood from healthy volunteers on written informed consent was drawn into heparinized vacutainers. Erythrocytes were washed 3 times by centrifugation and resuspension in large volumes of RPMI culture medium (Gibco BRL). The buffy coat was removed by aspiration after each wash. After the last wash, the cells were suspended at 50% hematocrit in RPMI and kept at 4°C.
Ramos cells were obtained from ATCC (CRL-1596) and cultured in RPMI with 10% fetal bovine serum. Human mobilized peripheral blood CD34-selected stem and progenitor cells were obtained from the Yale Center of Excellence in Molecular Hematology Cell Core and cultured in StemSpan SF expansion medium (StemSpan 09650) with estradiol (100 ng/mL), dexamethasone (10 ng/mL), human transferrin (200 ng/mL), insulin (10 ng/mL), Flt3 ligand (100 ng/mL), stem cell factor (100 ng/mL), IL-3 (50 ng/mL), IL-6 (20 ng/mL), insulin-like growth factor 1 (50 ng/mL), and erythropoietin (3 U/mL) for 9 to 14 days.13,14 FACS analysis was used to analyze the cellular expression of CD71 (transferrin receptor) and CD235a (glycophorin A). Magnetic bead selection for CD71 (MACS 130-046-201; Miltenyi Biotech) and CD235a (MACS 130-050-501; Miltenyi Biotech) was used to purify an R3/R4 population of erythroid cells.15
mRNA expression
RNA was prepared from Ramos, CD34+ stem and progenitor cells, and primary cultured erythroid cells (RNeasy mini kit; QIAGEN) and treated with amplification-grade DNase I. cDNA was generated using an iScript cDNA synthesis kit according to manufacturer instructions (BioRad). Quantitative real-time PCR (Q-PCR) was performed on BioRad CFX96 System and analyzed with QbasePLUS software. All samples were assessed for internal RNA integrity. Q-PCR primers were designed using Primer 3 software. For each primer pair, amplification specificity was validated by gradient, standard curve, melting curve, and gel electrophoresis. Q-PCR was performed using iQ SYBR Green Supermix (BioRad). Relative expression was normalized to geometric mean of unvarying genes, OAZ1 (ornithine decarboxylase antizyme 1), HPRT1 (hypoxanthine phosphoribosyltransferase 1), UFM1 (ubiquitin-fold modifier 1), TBP (TATA box binding protein), PUM1 (pumilio homolog 1), and RPS13 (ribosomal protein S13). The changes in specific mRNA levels were calculated using the ΔΔCT method (where CT is threshold cycle), with results presented as means ± SEM. Results were normalized to the gene with the highest expression level in each group.16 This technique provides the most stringent analyses of gene expression comparing multiple genes over multiple cell types.17 Triplicate analyses were performed for each target gene.
Preparation of erythrocyte ghosts
Erythrocyte ghosts were prepared by successive cell washing in 5P8 lysis buffer prepared from 5P8 × 100 containing 0.5M NaH2PO4 (pH 8) and added with a protease inhibitor cocktail (Roche Complete). After each wash the cells were centrifuged at 25 000g for 30 minutes at 4°C until clear supernatant and a white opalescent pellet was obtained.
Immunoblotting
Erythrocyte membranes were lysed in SDSbuffer (SDS 4.5%, NaPi 150mM, EDTA 3mM, DTT 1mM, pH 7,6). Two volumes of membrane extract were mixed with 1 volume of SDS buffer, homogenized for 10 minutes, then sonicated. Proteins were resolved via SDS-PAGE on a 10% acrylamide gel and blotted onto nitrocellulose. Antibodies used were: polyclonal goat anti-TSPO raised against the C terminus of human TSPO (Everest Biotech), polyclonal rabbit anti–VDAC1, -2, -3 (Santa Cruz Biotechnology) and polyclonal rabbit anti-ANT (SantaCruz Biotechnology). Nitrocellulose membranes were incubated in a blocking solution consisting of nonfat milk in Tris buffer saline Tween 20 (TBST) to avoid nonspecific binding. After labeling with rabbit and goat secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology), enhanced chemiluminescence detection (Typhoon; GE Healthcare Life Sciences) was performed.
Immunoprecipitation
Erythrocyte ghosts were lysed in radioimmunoprecipitation assay (RIPA) buffer, sonicated, then centrifugated at 10 000g for 10 minutes. The supernatant was immunoprecipitated for 30 minutes with an anti-VDAC1, then incubated with μMACS protein G microbeads for another 30 minutes at 4°C. The magnetically labeled immune complex was loaded in an M column (Miltenyi Biotec), washed with RIPA buffer and eluted with Laemmli buffer. Samples were subjected to SDS-PAGE on 10% acrylamide gels, stained with colloïdal Coomassie blue, and subjected to Western blot analysis for control and to NanoLC MS/MS for protein identification.
NanoLC MS/MS analysis
Analysis was performed by Innova Proteomics. Mass spectra of VDAC, ANT, TSPO and PBR were obtained using a nanoLC-LTQ-Orbitrap-XL: nanoLC Ultimate 3000 (Dionex) and LTQ-Orbitrap-XL (Thermo Electron). All spectra were processed by the software Proteome Discoverer 1.0 (Thermo Scientific) with combined analysis via Sequest (Thermo Scientific) et Mascot (Matrix Sciences) and Peaks algorithms for protein identification.
Immunostaining
Analyses were carried out on intact RBC according to Campanella and coworkers.18 Cells were washed twice in PBS containing 5mM glucose, and then fixed for 5 minutes in 0.5% acrolein in PBS. They were rinsed 3 times then permeabilized in PBS containing 0.1M glycine (rinsing buffer) plus 0.1% Triton X-100 for 5 minutes and again rinsed 4× in rinsing buffer (including a 30 minutes incubation at room temperature). Then all nonspecific binding was blocked by incubation for > 60 minutes in blocking buffer (PBS containing 0.05mM glycine, 0.2% fish skin gelatin (GE Healthcare). Staining of fixed, permeabilized RBCs was performed using antibodies diluted in blocking buffer. Primary antibodies used were: mouse anti-porin 31HL (Calbiochem), goat anti-TSPO Nter and goat anti-ANT Nter (Santa Cruz Biotechnology). The secondary antibodies were goat anti–mouse and donkey anti–goat both coupled to AlexaFluor 488 nm (Invitrogen). After labeling, resuspended red blood cells were allowed to attach to slides coated with polylysine and mounted using a PBS/glycerol (50/50) solution. Images were acquired on a LEICA SP5 confocal microscope equipped with a 63 × 1.40 oil immersion objective, at the Plate-forme d'Imagerie, Station Biologique de Roscoff.
Controls were performed using a primary antibody generated against the Gardos channel protein known to have similar expression levels (∼100-200 copies/cell). A goat IK1-Cter antibody (Santa Cruz Biotechnology) was used as primary antibody and goat anti–Alexafluor 488 (Molecular Probes) as secondary antibody. Three sets of additional negative controls were carried out. A first series of experiments, where primary antibody was omitted, validated the specificity of the secondary antibody raised against the primary antibody. A second set, where the primary antibody was replaced by the corresponding non immune IgG (goat and mouse) at the same final concentration, showed negative staining, giving evidence for the specificity of the primary antibody. A third set, where the primary antibody was blocked by the corresponding peptide (when commercially available), showed the specificity of the antibody.
Plasmodium growth assays
Plasmodium falciparum 3D7 cultures were synchronized at ring stage by 2 successive exposures to a 5% (wt/vol) sorbitol solution at 48h intervals. Parasitemia was adjusted to 1.5%, and cultures were grown for 72 hours in 96-wells plate in triplicate. Hematocrit was 2% with increasing drug concentrations in the culture media. Half the drug-containing culture media was replaced at 24 and 48 hours. At 72 hours, parasitemia was evaluated using flow cytometry according to the protocol previously described,19 using a Cell Lab Quanta SC (Beckman Coulter) cytometer equipped with a plate reader. Briefly, a Tris-saline solution with 138mM NaCl and 20mM Tris (pH 8.8) was prepared, filtered on 0.22μm membrane. SYBR Green I (Sigma-Aldrich) was added to a final concentration of 1.5×, and solution was distributed to a 96-wells plate (300μL/well). Two minutes before reading, cultures were resuspended, and 15μL of culture was added to each wells of the former plate. Analysis of cell populations (infected /non infected cells) was performed using Cell Lab Quanta SC MPL Analysis software (Beckman Coulter). At least 20 000 cells (infected or non infected) were counted for each well.
Sorbitol hemolysis
For standard semiquantitative hemolysis assays, hemoglobin release was used to estimate lysis time. Culture suspensions (2%-5% parasitemia) were washed 3 times in culture medium without serum and resuspended at 50% hematocrit.
Time courses started with the addition of a 0.4 mL aliquot of cell suspension to 3.6 mL of the sorbitol iso-osmotic solutions (300mM sorbitol, 10mM hepes, 5mM glucose, pH 7.4) to give a cell concentration of approximately 0.5 × 108 cells/mL. Experiments were performed in triplicate. At predetermined intervals (0, 2.5, 5, 10, 15, 30, 60 minutes), 0.5 mL aliquots of the suspension were transferred to microcentrifuge tubes containing 0.5 mL of ice-cold “stopping solution” (400mM sucrose in H2O). Tubes were centrifuged for 30 seconds. Next, 0.2 μL of the supernatant solution was transferred into 96-well plates for spectrophotometric estimation of hemoglobin concentration by absorption at a wavelength of 540 nm (A540). In all experiments, the A540 value corresponding to full hemolysis of trophozoite-infected erythrocytes was estimated from the final A540 value achieved in the supernatant solution from infected cells suspended in an iso-osmotic sorbitol. When drugs were tested, the percentage of inhibition was determined relative to nontreated cells when hemolysis was at maximum. Data analyses were carried out according to Krugliak and Ginsburg.20 The % lysis values at different times were fitted by nonlinear regression using SigmaPlot equation for a sigmoid dependence of y on x. y = a/(1+exp(-(x-x0)/b)) where y is the % lysis, a is the maximal lysis, x is the sampling time, x0 is the t1/2 of lysis, and b is the variability of cells in the population.
Electrophysiology
The whole-cell configuration of the patch-clamp technique was assessed by the development of small capacitance transient and reduction of access resistance. Cation movements across the membrane from the exterior (bath) to the cytoplasmic side is defined as inward current and shown as downward deflection in whole cell recordings. Seal resistances were 4-20 GΩ. Patch pipettes (tip resistance 10-20MΩ) were prepared from borosilicate glass capillaries (GC150 TF-10, Clark Medical Instruments) pulled and polished on a Werner Zeitz DMZ programmable puller (Augsburg). The ruptured patch whole-cell configuration was used to record whole-cell currents. Whole-cell currents were recorded using a RK400 (Biologic) amplifier, with voltage command protocols generated and the currents analyzed using WCP V3.3.3 software by evoking a series of test potentials (VT) from −100 to +100 mV in 10 mV steps for 500 ms from a holding potential (VH) of 0 mV. Data for the construction of I-V curves were the mean current measured between 200 and 400 ms. Because temperature has no influence on whole cell currents in infected erythrocytes (data not shown), all experiments were performed at room temperature.
Statistical analyses
Data are given as mean values ± SEM. Significance was assessed using the Fisher F test and Student t test.
Results
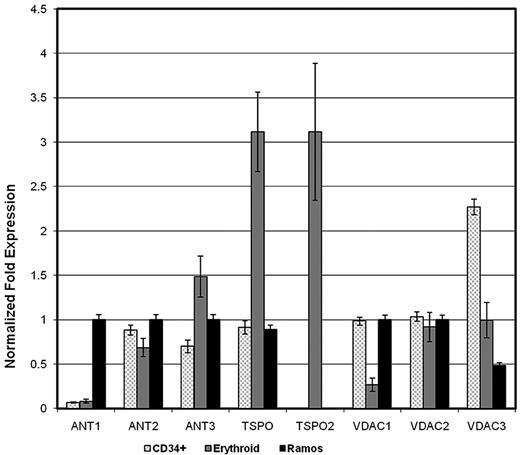
Our previous work demonstrated that a unique type of maxi-anion channel with multiple conductance levels mediates band 3-independent anion conductance across the erythrocyte membrane.5 To address our hypothesis that this anion conductance is mediated by a voltage dependent anion channel (VDAC), we sought molecular and physiologic evidence for a VDAC in the erythrocyte. Because VDACs may exist alone or as part of a PBR complex consisting of VDAC, TSPO, and ANT proteins, we performed quantitative RT-PCR of mRNA isolated from cultured human primary erythroid cells, CD34+ hematopoietic stem and progenitor cells, and Ramos cells, a Burkitt lymphoma cell line, using primers specific for the 3 known VDAC genes (VDAC1, VDAC2, VDAC3), the 2 known TSPO genes (TSPO, TSPO2), and the 3 of the 4 known ANT genes (ANT1, ANT2, ANT3). ANT4 has been shown to be testis-specific. PBR expression has previously been described in hematopoietic cells, particularly lymphoid-derived cells,21 thus CD34+ hematopoietic stem/progenitor cells and Ramos cells (lymphoma cells) were chosen as parallel hematopoietic cell types for study. In the test RNA samples, the OAZ1 gene amplicon demonstrated the least variability, thus all data were normalized to OAZ1. All 3 VDAC genes were expressed in erythroid cells, with expression of VDAC 2 and 3 predominating (Figure 1). Both TSPO genes were highly expressed in erythroid cells, even though TSPO2 was not expressed in stem or lymphoid cells (Figure 1). There were low levels of ANT1 expression and higher levels of ANT2 and ANT3 expression in erythroid cells (Figure 1). There was significant expression of all 3 ANT genes in lymphoid cells.
Quantitative RT-PCR of mRNA isolated from cultured human primary erythroid cells. Q-PCR was performed using iQ SYBR Green Supermix (BioRad). Relative expression was normalized to geometric mean of unvarying, ubiquitously genes, ornithine decarboxylase antizyme 1 (OAZ1), hypoxanthine phosphoribosyltransferase 1(HPRT1), ubiquitin-fold modifier 1 (UFM1), TATA box binding protein (TBP), pumilio homolog 1 (PUM1), and ribosomal protein S13 (RPS13) as controls. The changes in specific mRNA levels were calculated using the ΔΔCT method (where CT is threshold cycle), with results presented as means ± SEM. Results were normalized to the gene with the highest expression level in each group. Triplicate analyses were performed for each target gene.
Quantitative RT-PCR of mRNA isolated from cultured human primary erythroid cells. Q-PCR was performed using iQ SYBR Green Supermix (BioRad). Relative expression was normalized to geometric mean of unvarying, ubiquitously genes, ornithine decarboxylase antizyme 1 (OAZ1), hypoxanthine phosphoribosyltransferase 1(HPRT1), ubiquitin-fold modifier 1 (UFM1), TATA box binding protein (TBP), pumilio homolog 1 (PUM1), and ribosomal protein S13 (RPS13) as controls. The changes in specific mRNA levels were calculated using the ΔΔCT method (where CT is threshold cycle), with results presented as means ± SEM. Results were normalized to the gene with the highest expression level in each group. Triplicate analyses were performed for each target gene.
We next sought evidence of a VDAC protein in the human erythrocyte membrane either alone or interacting with ANT and TSPO proteins to form a PBR complex. The evidence for VDAC being present in the membrane, among the proteins present in the RBC membrane (Figure 2A), is given by 4 bands appearing between 29-30 kDa and 36 kDa on Western blots (Figure 2B). The different isoforms described in the VDAC literature indicate that VDAC isoforms correspond to molecular weights ranging between 30 and 36 kDa which is in keeping with the 4 lower bands visible in Figure 2B. In addition, experiments using the VDAC1 antibody (data not shown) show a faint band at 29-30 kDa and a marked band at 58 kDa indicating that this isoform is predominantly present as dimers. Therefore the 58 kDa band of Figure 2B may reasonably be interpreted as VDAC1 dimers. With regard to trimers, if any, they should be located in a range varying between 90 and 110 kDa. This range is also the range of weight corresponding to the Band 3 protein. In this context, interpretation of the large band appearing between 90 and 120-130 kDa on Figure 2B remains difficult but we cannot exclude that this band corresponds to oligomerization of VDAC proteins22 or to monomers associated with the band 3 protein. Figure 2B also demonstrates the presence of the 2 other components of PBR complex. Polyclonal anti-TSPO antibody raised against the C terminus of human TSPO and polyclonal anti-ANT antibody show that our membrane preparation was positive for both markers at the expected size.
VDAC, ANT and TSPO detection in human red blood cell ghosts. Samples (15 μg of protein) of whole lysates were subjected to SDS-PAGE (10% acrylamide) and stained with Coomassie blue (A) or analyzed by Western blotting using polyclonal anti-ANT (1:1000 dilution), rabbit polyclonal anti VDAC1 -2, -3 (1:100 dilution) or polyclonal goat anti-TSPO raised against the C terminus of human TSPO (1:1000). The positions of molecular weight (kDa) protein standards are indicated by arrows. (B) 4 bands appearing between 29-30 kDa and 36 kDa correspond to different isoforms of VDAC and marked band at 58 kDa correspond to dimers of VDAC1 isoform (29-30 kDa). Multiple bands at higher molecular weights suggest oligomerization of VDAC proteins. ANT and TSPO proteins are also clearly visible. It is to be noted that, according to the supplier assessment (Everest Biotech), the TSPO protein could not be expected at 18 kDa but rather at 36 kDa. These blots are representative of 12 replicates. Immunofluorescence experiments (C) were performed on smears prepared as described in “Immunostaining.” Dilution were 1/5 for primary and 1/20 for secondary antibodies. Scale bars represent 10μm.
VDAC, ANT and TSPO detection in human red blood cell ghosts. Samples (15 μg of protein) of whole lysates were subjected to SDS-PAGE (10% acrylamide) and stained with Coomassie blue (A) or analyzed by Western blotting using polyclonal anti-ANT (1:1000 dilution), rabbit polyclonal anti VDAC1 -2, -3 (1:100 dilution) or polyclonal goat anti-TSPO raised against the C terminus of human TSPO (1:1000). The positions of molecular weight (kDa) protein standards are indicated by arrows. (B) 4 bands appearing between 29-30 kDa and 36 kDa correspond to different isoforms of VDAC and marked band at 58 kDa correspond to dimers of VDAC1 isoform (29-30 kDa). Multiple bands at higher molecular weights suggest oligomerization of VDAC proteins. ANT and TSPO proteins are also clearly visible. It is to be noted that, according to the supplier assessment (Everest Biotech), the TSPO protein could not be expected at 18 kDa but rather at 36 kDa. These blots are representative of 12 replicates. Immunofluorescence experiments (C) were performed on smears prepared as described in “Immunostaining.” Dilution were 1/5 for primary and 1/20 for secondary antibodies. Scale bars represent 10μm.
In another set of experiments, because a few hundred copies of proteins were not detected by mass spectrometry with total extracts, membrane extracts were immunoprecipitated with an anti-VDAC1 (Nter) and complexed with protein G-coated microbeads, then subjected to SDS-PAGE stained with Coomassie blue. The 29-36 kDa bands showed only a very faint signal, consistent with the presence of very low levels of proteins in the RBC membrane. Nevertheless, NanoLC MS/MS analysis of this band clearly identified ANT1,2,3 proteins as well as VDAC3 which coprecipitated with VDAC1. TSPO was only detected with a low level of certainty.
Finally, we used immunostaining for localization of the 3 PBR proteins (Figure 2C). The use of anti-porin 31HL antibody demonstrated the presence of VDAC in the RBC membrane. The same conclusion was obtained for TSPO using anti-TSPO (NCter) antibody and for ANT using anti-ANT (Nter) antibody. These results were confirmed by experiments using anti–VDAC1-Nter (SantaCruz Biotechnology) and anti–TSPO-Cter (Everest Biotech; data not shown) and indicate that the results of Figure 2A and B are not because of contamination with other blood cells.
These data are consistent with previously published reports. Analysis of mRNA expression data from GeneAtlas U133A indicate that 3 isoforms of VDAC, 2 isoforms of ANT and 2 isoforms of TSPO have been found in progenitor cells (http://biogps.gnf.org). VDAC3, TSPO and ANT expression is ubiquitous and essentially nonchanging at the median level from CD34+ cells to CD71+ progenitor cells. A population of receptors with nanomolar affinity for PK 11 195 was found in all blood cells in rank order of density: lymphocytes ≫ platelets > erythrocytes21 and the population of binding sites in erythrocytes was evaluated to 110 ± 22 per cell. TSPO protein23 and VDAC protein have been described in the erythrocyte membrane.24
Because the NPP, allowing both organic and inorganic anions, electroneutral molecules and organic and inorganic monovalent cations to pass, displays many common characteristic features with the PBR/VDAC1 and because the ligands of PBR and are known as potent inhibitors of P falciparum growth in infected RBCs in vitro,25,26 we next considered if the antiplasmodial effects of PBR ligands could correspond to an interaction of the parasite with this native PBR complex through up-regulation of PBR/VDAC activity.
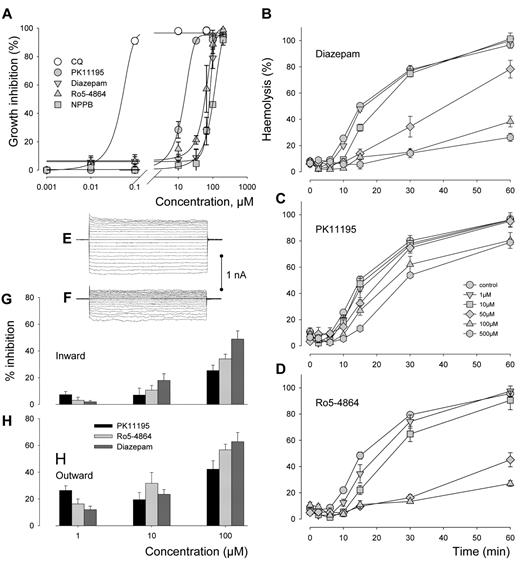
Cultures of the malaria parasite P falciparum were exposed for 72 hours to increasing concentrations of PBR ligands PK11195, Ro5-4864 and diazepam. Growth inhibition curves are shown in Figure 3A. The effects of ligands were compared with those of the reference antimalarial drug chloroquine (CQ) and inhibitor of anionic channels NPPB. The dose dependence curves show that although the inhibitory effects of the ligands remained far below the CQ effect, the IC50 for PK11195 was ∼ 10 times lower than for NPPB while Ro5-4864 and diazepam displayed similar IC50 as NPPB, in the range 100μM. We cannot discard the possibility that at the concentrations used PBR ligands act on nonPBR systems, but if this interaction was via any inhibitory effect of channel activity, we could expect a reduction of cell swelling and hemolysis occurring when infected cells containing the new permeability pathways are exposed to isotonic solutions containing sorbitol. This appearance of increased permeability to otherwise impermeable solutes, was recorded > 2 decades ago.2,27 Figure 3B through D show that the percentage of lysis in cells exposed simultaneously to isotonic solutions containing sorbitol and to various concentrations of the 3 ligands is considerably decreased/delayed. Data analysis carried out according to Krugliak and Ginsburg method20 allowed acurate quantification of the t1/2 of lysis that is inversely proportional to the permeability and of maximal extent of lysis. The derived half-times (t1/2) plotted against the ligand concentration showed that the membrane permeability was significantly decreased (P < .001) when concentration reached 50μM, 1μM, and 10μM for PK11195, Ro5-4864, and Diazepam, respectively. In addition, the maximal extent of lysis declined significantly (P < .001) at 100μM, 50μM, and 100μM for PK11195, Ro5-4864, and Diazepam, respectively.
Antiplasmodial effects of PBR ligands. (A) Cultures of the 3D7 strain synchronized at ring stage (in 96-well plates at 1.5% parasitemia and 2% hematocrit) were exposed to different concentrations of PBR ligands PK11195, Ro5-4864 and diazepam during 72 hours at 37°C. Their effects were compared with effects of antimalarial drug chloroquine (CQ) and anionic channels NPPB. Inhibition of parasite growth was evaluated by comparison of the total parasitemia to the negative control where cultures were treated with the solvent (DMSO) only. The lines connecting the experimental points were drawn according to nonlinear regression analysis of the experimental results converted into percent values. Each count was made in triplicate and each point on the curves corresponds to the mean (± SEM) of 3 separate experiments. (B,C,D) In sorbitol lysis experiments, culture suspensions were prepared as described in “Electrophysiology.” The effects of diazepam (B), PK11195 (C) and Ro5-4864 (D) added at t = 0 minutes of lysis experiments at concentrations below and above their IC50s were tested by comparison to nontreated cells when haemolysis was at maximum at t = 60 minutes. Each count was made in triplicate and each point on the curves correspond to the mean (± SEM) of 3 separate experiments. Note that Ro5-4864 was not tested at 500 μM because of solubility limitation. (E-H) The whole-cell membrane conductance of infected RBCs was calculated by measurement of the amplitude of currents obtained by evoking a series of test potentials (VT) from −100 to +100 mV in 10 mV steps for 500 ms from a holding potential (VH) of 0 mV in the whole-cell configuration of the patch-clamp technique before and 15 minutes after addition of a ligand. The examples of panels E and F were obtained before and 15 minutes after addition of 100μM PK11195 to the bathing solution containing (in mmol/l) 115 NaCl, 5 KCl, 10 MgCl2, 5 CaCl2, 10 Hepes, 10 glucose, 1% human serum, pH 7.4. The pipette solution contained 155 NMDG-Cl, 1 MgCl2, 10 HEPES (pH 7.4). The calcium concentration was adjusted to pCa 3 in the bathing solution and to pCa 7 in the pipette solution. The impacts of the 3 different ligands were assessed by calculating the percentage reduction of inward conductance (G), (cord conductance between −100 mV and 0 mV) and outward conductance (H), (cord conductance between 0 mV and +100 mV). Bars are means ± SEM from 6 experiments.
Antiplasmodial effects of PBR ligands. (A) Cultures of the 3D7 strain synchronized at ring stage (in 96-well plates at 1.5% parasitemia and 2% hematocrit) were exposed to different concentrations of PBR ligands PK11195, Ro5-4864 and diazepam during 72 hours at 37°C. Their effects were compared with effects of antimalarial drug chloroquine (CQ) and anionic channels NPPB. Inhibition of parasite growth was evaluated by comparison of the total parasitemia to the negative control where cultures were treated with the solvent (DMSO) only. The lines connecting the experimental points were drawn according to nonlinear regression analysis of the experimental results converted into percent values. Each count was made in triplicate and each point on the curves corresponds to the mean (± SEM) of 3 separate experiments. (B,C,D) In sorbitol lysis experiments, culture suspensions were prepared as described in “Electrophysiology.” The effects of diazepam (B), PK11195 (C) and Ro5-4864 (D) added at t = 0 minutes of lysis experiments at concentrations below and above their IC50s were tested by comparison to nontreated cells when haemolysis was at maximum at t = 60 minutes. Each count was made in triplicate and each point on the curves correspond to the mean (± SEM) of 3 separate experiments. Note that Ro5-4864 was not tested at 500 μM because of solubility limitation. (E-H) The whole-cell membrane conductance of infected RBCs was calculated by measurement of the amplitude of currents obtained by evoking a series of test potentials (VT) from −100 to +100 mV in 10 mV steps for 500 ms from a holding potential (VH) of 0 mV in the whole-cell configuration of the patch-clamp technique before and 15 minutes after addition of a ligand. The examples of panels E and F were obtained before and 15 minutes after addition of 100μM PK11195 to the bathing solution containing (in mmol/l) 115 NaCl, 5 KCl, 10 MgCl2, 5 CaCl2, 10 Hepes, 10 glucose, 1% human serum, pH 7.4. The pipette solution contained 155 NMDG-Cl, 1 MgCl2, 10 HEPES (pH 7.4). The calcium concentration was adjusted to pCa 3 in the bathing solution and to pCa 7 in the pipette solution. The impacts of the 3 different ligands were assessed by calculating the percentage reduction of inward conductance (G), (cord conductance between −100 mV and 0 mV) and outward conductance (H), (cord conductance between 0 mV and +100 mV). Bars are means ± SEM from 6 experiments.
Electrophysiologic tests confirmed that this loss of permeability occurred via the inhibition of a conductive pathway. In a representative experiment shown in Figure 3E and F, an infected RBC displays a reduction in whole-cell membrane conductance at both positive and negative potentials over the first 15 minutes after addition of PK11195 at the concentration of 100μM. The dose-dependence of inhibition evoked after only 15 minutes by the 3 ligands is presented in Figure 3G and H for inward (G) and outward (H) currents and confirms that the immediate reduction in the membrane permeability of Plasmodium-infected RBCs occurs to a large extent through inhibition of conductive pathways. This effect was more pronounced for diazepam and Ro5-4864 than for PK11195. With regard to the quickness of the observed response, hemolysis and electrophysiology experiments suggest a direct effect on channel activity rather than an effet on parasite fitness.
Discussion
Seeking clues for the molecular identification of the RBC native maxi-anion channel, this study links together for the first time the presence of PBR proteins in the RBC membrane and the NPP up-regulated in infected RBC. The experimental data substantiate the hypothesis that dormant PBR/VDAC become the so-called “new permeability pathways” in infected erythrocytes after up-regulation by P falciparum.
A basic RBC membrane anionic conductance of < 100 pS,28 is in fair agreement with the value calculated from experiments on cell suspensions29 and indicates that most of the time, the channel carrying the anionic conductance is deactivated, but that it could be transiently activated. The molecular nature of the gating mechanism is still unclear, but activation could take place by voltage changes as is the case for VDAC, caused for example by transient activation of the Gardos channel on membrane deformation as recently shown.30 Once activated, VDAC possesses multiple sub-conductance levels similar to those displayed by RBC's maxi-anion channels (in the range 350-450 pS for large openings).31 Highest VDAC conductance states are observed at low potentials with a marked preference for anions (eg thiocyanate, phosphate, and chloride), whereas the selectivity is favorable to small cations at higher positive or negative potentials.31 Mitochondrial VDAC is located at the interface between the mitochondria and the cell cytosol and appear to control the fluxes of ions and metabolites in and out of the mitochondria.32 The same role could be played by VDAC in the PBR complex between the RBC interior and the extracellular milieu in health and in disease.
In health, depending on the conformational state, this channel could be involved in a large range of dynamic changes in red cell homeostasis and membrane permeability.32 We may conclude from our experiments that VDAC forms a PBR complex with TSPO and ANT molecules clearly identified in the RBC membrane despite their low quantity. A population of approximately 100-150 PBR complexes is more likely since it fits with our previous electrophysiologic evaluation of anion channels28 and with the calculated number of receptors with nanomolar affinity for PK 11 195.21 It is likely that a hundred copies of a pathway for small ions (Na+, K+, Cl−)33 as well as large anions (glutamate,34 ATP35 ) large cations (Tris36 ) and divalent cations, such as Ca2+37,38 plays an important role in the physiologic processes of metabolite transport and volume regulation in the erythrocyte.39 It is tempting to explain the observation that the oldest RBCs are light, high in Na and low in K40 by final activation of VDAC in its low-conductance (cationic) mode, thus reversing the densification trend prevailing in younger cells.40
We previously demonstrated that the channels up-regulated by P falciparum are of endogenous origin28 and, since the present work demonstrate that PK11195, Ro5-4864 and diazepam block parasite growth and induce rapid reductions in permeation and conductance, we suggest that the new permeability pathways are largely carried by PBR/VDAC even though we cannot discard the possibility that ANT and TSPO contribute in the global conductance. Early pharmacologic studies using isotopic fluxes and sorbitol lysis experiments came to the conclusion that NPP is a large poorly selective anion channel with a selectivity SCN > I > Br > Cl > acetate > lactate > glutamate corresponding to the Eisenman sequence number1 for anions and also allows sugars, purines, amino-acids and organic and inorganic cations to pass,1 as is the case for VDAC.32 NPP displays a selectivity over 0.6 kDa for organic solutes similar to VDAC (almost 1.0 kDa41 ) they both allow cation movements,41,42 carry ATP35,43 and are modulated by serum components,44,45 oxydo-reduction states46,47 and PKA-dependent phosphorylation.48,49 The pore radius of NPP was estimated at 0.70 nm27 and theVDAC pore radius calculated to be 0.85 nm.50 The fact that NPP properties resemble those of VDAC provides further support to the conclusion that the 2 channels are one and the same protein. Thus, a single species of multipotent channel able to transport different structurally unrelated solutes has the potential to meet the parasite requirements for selective nutrient uptake, removal of waste products and volume regulation by playing sequentially with a wide range of conductances, selectivities and permeabilities controlled by several modulators such as small molecules (calcium,37,38 glutamate,34 reactive oxygen species51 ), kinases or associated proteins.48,49 Although it appears that NPPs are not parasite-engendered channels as previously suggested,3 it is conceivable, in the light of recent findings,52 that some parasite-encoded proteins such as those encoded by the 2 clag3 genes, contributes to up-regulation of this native pathway.53 In our electrophysiologic single-channel recordings, it is evident that the NPP are permanently active at the low conductance levels but may display occasionally (in approximately 5% of recordings) brief episodes of high conductance initially attributed to superimposition of another type of outwardly rectifying anion channel.
The present work demonstrates that peripheral-type benzodiazepin receptors are an important new contributing factor for consideration in understanding red cell physiology and pathophysiology of malaria and other diseases.54 It validates the PBR complex as an antimalarial target and suggests that the pharmacopoeia of benzodiazepine as well as benzodiazepine scaffolds for the production of new inhibitors55 could become a novel strategic approach for future antimalarial chemotherapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs J. F. Hoffman, P. Bennekou, and V. L. Lew for invaluable advice and help during the course of this work. In addition they thank D. Vaulot and D. Marie (Station Biologique de Roscoff) for expert assistance in flux cytometry experiments.
This study was supported by research funding from Agence Nationale de la Recherche (ANR; -08-MIEN-031-02) and the Doris Duke Charitable Foundation (P.G.G.). E.G. is supported by ANR (-08-MIEN-031-02).
Authorship
Contribution: A.C. and G.B. contributed equally to experimental design, execution, and interpretation; J.K., E.G., S.E., Y.M., and P.G.G. contributed to experimental design, execution, and interpretation; S.L.Y.T. conceived the project and analyzed data; and S.L.Y.T. and P.G.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Serge L. Y. Thomas, CNRS-UPMC, UMR 7150, Station Biologique, BP 74, 29682 Roscoff cedex, France; e-mail: thomas@sb-roscoff.fr.
References
Author notes
G.B. and A.C. contributed equally to this article.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal