Abstract
Current management of hemophilia B entails multiple weekly infusions of factor IX (FIX) to prevent bleeding episodes. In an attempt to make a longer acting recombinant FIX (rFIX), we have explored a new releasable protraction concept using the native N-glycans in the activation peptide as sites for attachment of polyethylene glycol (PEG). Release of the activation peptide by physiologic activators converted glycoPEGylated rFIX (N9-GP) to native rFIXa and proceeded with normal kinetics for FXIa, while the Km for activation by FVIIa–tissue factor (TF) was increased by 2-fold. Consistent with minimal perturbation of rFIX by the attached PEG, N9-GP retained 73%-100% specific activity in plasma and whole-blood–based assays and showed efficacy comparable with rFIX in stopping acute bleeds in hemophilia B mice. In animal models N9-GP exhibited up to 2-fold increased in vivo recovery and a markedly prolonged half-life in mini-pig (76 hours) and hemophilia B dog (113 hours) compared with rFIX (16 hours). The extended circulation time of N9-GP was reflected in prolonged correction of coagulation parameters in hemophilia B dog and duration of effect in hemophilia B mice. Collectively, these results suggest that N9-GP has the potential to offer efficacious prophylactic and acute treatment of hemophilia B patients at a reduced dosing frequency.
Introduction
Factor IX (FIX) is a vitamin K–dependent glycoprotein and an essential protease of the hemostatic system. The domain organization of FIX is shared with factors VII, X, and protein C and comprises an N-terminal domain rich in γ-carboxyglutamic acid (Gla), 2 epidermal growth factor-like repeats and a C-terminal trypsin-like protease domain.1 Together they form a 55-kDa single-chain protease precursor circulating in plasma at a concentration of approximately 90nM (5 μg/mL), defined as 1 IU/mL. FIX is converted to the 2-chain activated form by the tissue factor (TF)–factor VIIa (FVIIa) complex or factor XIa (FXIa). Activation occurs by limited proteolysis at Arg145 and Arg180 in the protease domain and liberates a 35-amino acid activation peptide that carries the only 2 N-linked glycans in the protein.2,3 Subsequent assembly of FIXa with the cofactor VIIIa on the activated platelet surface greatly enhances the proteolytic activity of FIXa toward its substrate factor X (FX) and is essential for propagation of the coagulation response.4 The importance of this activity is reflected by the occurrence of the bleeding disorder hemophilia B (HB) in individuals carrying mutations in the FIX gene. The prevalence of HB is approximately 1 in 25 000 males, and it has been estimated that approximately 84 000 people are affected worldwide.5
The mainstay in HB treatment is substitution therapy by infusion of plasma-derived or recombinant FIX (rFIX). The therapeutic goal is to prevent bleeding episodes and to provide safe and efficacious treatment of bleedings when they occur. Because of the relatively short half-life of FIX (18-24 hours6-8 ), the recommended prophylaxis regimen consists of 2 to 3 weekly infusions of 40-100 IU/kg9 FIX to maintain trough levels above 1% and thus shifting patients from a severe to a milder phenotype. When adhered to, prophylaxis in patients without severe joint disorder is efficacious with a frequency of only 0-2 breakthrough bleeds per year in the majority of patients.8,10 However, the need for multiple weekly infusions present challenges in terms of repeated venous access which may affect adherence to treatment and necessitate placement of indwelling catheters with the inherent risk of infection.11
Various approaches have been pursued to extend the half-life of coagulation factors including genetic fusions to albumin and the Fc fragment of IgG as well as chemical modification by hydrophilic polymers such as polyethylene glycol (PEG).12-15 The prolonged circulation time endowed by the latter approach is believed to arise from a reduction in the efficiency of various elimination processes such as renal excretion, receptor mediated uptake, and proteolytic degradation.16 With several PEGylated protein-based therapeutics on the market since 1990, the efficacy and safety of this technology has undergone extensive clinical validation.17 For any protraction strategy applied on FIX, the gain in circulation time should be balanced by the need to preserve biologic activity and normal regulation of the activated form. Being remote from known interaction sites in FIX and present only in the circulating zymogen form, the N-glycans of the activation peptide offer potential acceptor sites for PEGylation that may fulfil these requirements. In the present study we demonstrate the feasibility of targeted glycoPEGylation and show that this approach confers a long plasma half-life to FIX in animal models while preserving its biologic activity.
Methods
Production and biochemical characterization of N9-GP
Production and biochemical characterization of glycoPEGylated rFIX (N9-GP) is described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Reference FIX
BeneFIX (Pfizer) was used as a source of recombinant FIX (rFIX) for reference purposes. The product was reconstituted according to the manufacturer's instructions and stored in aliquots at −80°C.
In vitro functional characterization
Kinetics of FIX activation by FXIa or FVIIa-TF.
Activation was initiated by addition of 10pM FXIa (Haematologic Technologies) or 1nM rFVIIa (Novo Nordisk) and 0.1nM lipidated TF (Innovin; Siemens) to varying concentrations of FIX in 25mM HEPES, 137mM NaCl, 3.5mM KCl, 5mM CaCl2, 0.1% BSA, pH 7.4 at 25°C. Reactions were quenched after 30 minutes by addition of 30mM EDTA and generated FIXa measured at 405 nm in the presence of 31% ethylene glycol and 0.5mM Spectrozyme IXa substrate (American Diagnostica).
Kinetics of FX activation.
FX activation experiments were performed as described by Christiansen et al18 (see supplemental Methods for a full description).
Binding of FIX to endothelial cells.
Primary HUVECs (Cambrex BioScience) were maintained in EBM-2 medium as described by the manufacturer. Binding experiments were performed in 48-well plates at 95% confluence essentially as described by Cheung et al.19 Briefly, cells were exposed to 125I-rFIX (0.5-1nM) and unlabeled competitor in 0.15 mL of buffer (10mM HEPES, 150mM NaCl, 4mM KCl, 5mM CaCl2, 11mM glucose, 1 mg/mL BSA, pH 7.4) and kept on ice for 3 hours followed by 3 washes with ice-cold buffer. Finally, cells were lyzed and radioactivity measured in a Packard Cobra II γ-counter. Results were analyzed in GraphPad Prism (GraphPad Software Inc).
FIX clot analysis.
Coagulant activity was estimated using a 1-stage FIX clotting assay (instrument and reagents were from Instrumentation Laboratories). Test sample was mixed with equal volumes of FIX immunodepleted plasma, activated partial thromboplastin time (aPTT) reagent (SynthAFax), and 25mM CaCl2. The time to clot formation was measured on an ACL9000 instrument using HemosIL reference plasma calibrated against the 3rd WHO international FIX concentrate standard (96/854; National Institute for Biological Standards and Control). Control experiments using N9 and free PEG confirmed that PEG did not interfere with the assay as previously noted for some aPTT reagents.20
Thromboelastography in hemophilia B whole blood.
Blood was obtained from 5 moderate and 3 severe HB patients in a study approved by The Danish National Committee on Biomedical Research Ethics (no. [KF] 01 299489, add. 18 186). For comparative purposes blood was also obtained from 11 healthy volunteers. All participants received oral and written information and signed informed consent before blood sampling. Healthy donors had not taken acetyl salicylic acid for 10 days or other anti-inflammatory drugs for 72 hours. All patients had not received factor concentrate for minimum 72 hours. Blood was sampled in 3.2% citrate and rested 90 minutes before analysis. Equimolar amounts of N9-GP or rFIX (0.08-76nM) were added and clotting initiated by kaolin (Haemoscope Corporation). A normal reference range was generated based on blood from the healthy volunteers. Clot (R) time, maximum thrombus generation (MTG) and maximum amplitude (MA) parameters were used for statistical analysis. Data were fitted to a concentration-response-curve in a nonlinear model. Parameter estimates for efficacy and EC50 were subsequently analyzed on log-scale in a normal linear mixed effects model allowing for random subject-to-subject and random day-to-day variation.
In vivo pharmacokinetics and pharmacodynamics
Animals.
HB mice (B6.129P2-F9tm1Dws) were originally obtained from Darrel W. Stafford (University of North Carolina).21 Mice were of both sexes (50:50) and between 12 and 16 weeks old. Male Göttingen mini-pigs were obtained from Ellegaard Göttingen Minipigs A/S. Studies were approved by and performed according to guidelines from the Danish Animal Experiments Council, the Danish Ministry of Justice. Studies in an immunologically tolerized HB dog were conducted at the Chapel Hill colony under the supervision of T.C.N.22 and approved by the Institutional Animal Care and Use Committee at the University of North Carolina. The methods used for tolerization and characterization of the level of transgenic human FIX in the dog are described in supplemental Methods.
Calculation of doses.
Doses were calculated based on protein content using the same molecular weight (55 kDa) for rFIX, N9, and N9-GP. Hence, at a given milligram per kilogram dose, equimolar amounts of the molecules were administered.
Quantitative analysis.
FIX activity and Ag levels were quantified in citrated plasma samples by FIX clot analysis as described above and by sandwich ELISA using an anti-FIX Gla-domain Ab for capture and a HRP-conjugated sheep anti-FIX detection Ab (Fitzgerald Industries International).
Pharmacokinetic analysis.
Unless otherwise stated, pharmacokinetic analysis was carried out by noncompartmental methods (NCA) using WinNonlin software (Pharsight Corporation).
Pharmacokinetics in hemophilia B mice and mini-pigs.
N9-GP or rFIX was given intravenously to HB mice as a 1.5 mg/kg bolus injection in the tail vein. Blood samples were collected according to a staggered design consisting of 3 mice per time point and 3 samples per mouse. Six mini-pigs (9.7 ± 1.2 kg) divided in 2 groups received an intravenous bolus injection of 0.2 mg/kg N9-GP or rFIX. Blood samples were collected for PK analysis up to 14 days after administration. Data were fitted to a 2-compartment model and estimated parameters used to simulate elimination profiles in a multiple dose setting using NONMEM software (GloboMax/ICON).
Acute effect in tail bleeding model.
Bleeding was induced in HB mice by amputating 4 mm of the tail tip as described previously.23 Briefly, 10 minutes before amputation, the tail was placed in a test tube containing 14 mL of saline (37°C) and 5 minutes later vehicle (buffer), rFIX, or N9-GP were administered randomized and blinded at 10 mL/kg through the carotid artery. The total bleeding time and blood loss (expressed as nanomoles of hemoglobin) were recorded for 30 minutes.23
Duration of effect in tail bleeding model.
HB mice were administered 0.75 mg/kg rFIX or N9-GP in the tail vein. At timed intervals, bleeding time and blood loss were determined as described for measurement of acute effect.
Duration of effect in ferric chloride-induced carotid artery injury model.
Each group of HB mice received 0.75 mg/kg rFIX or N9-GP randomized and blinded through the tail vein, except for mice tested after 5 minutes which were dosed through the jugular vein. At timed intervals after dosing, injury was induced by exposure of the carotid artery to 10% (w/v) ferric chloride for 3 minutes and time to arrest of blood flow measured as described by Møller and Tranholm.24
Pharmacodynamics in hemophilia B dog.
A female HB dog (18 kg) immunologically tolerant to human FIX was used in the study.25 rFIX and N9-GP were infused at 0.4 mg/kg. Blood samples for pharmacokinetic (PK) analysis, thromboelastography (TEG; no stabilizer), and whole-blood clotting time (WBCT; no stabilizer) were drawn at regular intervals up to 45 days after administration.
Results
Production and characterization of N9
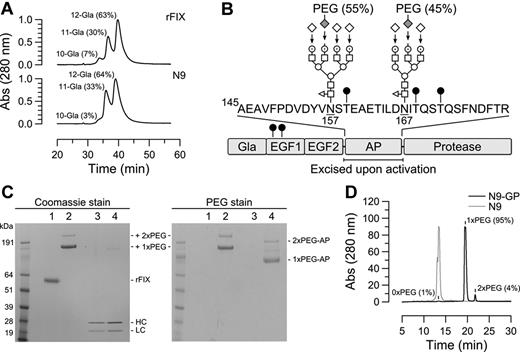
rFIX (N9) was expressed in CHO cells which are known to support proper maturation and posttranslational modification of FIX.26-28 Correct identity of the purified product was confirmed by N-terminal sequencing and peptide mapping (results not shown), and the molecular weight determined to be 54 512 Da by MALDI-MS. Of particular importance to the activity of FIX is the γ-carboxylation of several glutamic acid residues in the Gla domain. Twelve acceptor sites are present but occupancy of 10 are sufficient for FIX to attain full activity.29 Gla profiling by anion exchange HPLC demonstrated a predominance of 11 (33%) and 12 (64%) Gla forms with an average content of 11.6 Gla per molecule, which is similar to the composition of rFIX but less than the 12 Gla residues present in plasma-derived FX (pdFX; Figure 1A).28 Further analysis of posttranslational modifications identified as expected partial β-hydroxylation of Asp64 and O-glycans attached to Ser53 and Ser61 in the EGF1 module.30 In the activation peptide, partial occupancy of the O-linked glycan acceptor sites Thr159, Thr169, and Thr172 was observed as described for rFIX30 and pdFIX,31 whereas N-glycans were uniformly present at Asn157 and Asn167. As illustrated in Figure 1B these were predominantly of the core fucosylated tri- and tetra-antennary complex type. Consistent with the high structural similarity of N9, rFIX and pdFIX, the specific aPTT clot activities of the proteins were not significantly different (260 ± 28, 250 ± 15, and 260 ± 21 IU/mg, respectively), and similar pharmacokinetics of N9 and rFIX were observed in HB mice (results not shown).
Modifications in native and glycoPEGylated N9. (A) Separation of Gla species in rFIX and N9 by anion exchange HPLC using a linear ammonium acetate gradient. The fractional content of indicated Gla species is given in parentheses and was calculated by relating the area of the corresponding peak to the total peak area. (B) Domain structure of N9 and location of identified N- and O-linked (●) carbohydrates. The 2 N-linked glycans in the activation peptide (AP) were predominantly core-fucosylated tri- and tetra-antennary complex structures composed of fucose (◁), N-acetylglucosamine (□), mannose (○), galactose (⊙), and sialic acid (♢). Enzymatic transfer of 40k-PEG-sialic acid to desialylated N9, and sialylation of remaining exposed galactoses are indicated by arrows. The relative distribution of PEG between the 2 N-glycans is given in parentheses. (C) N9-GP (lane 2) separated into 2 bands by SDS-PAGE corresponding to mono- and di-PEGylated (1x and 2xPEG, respectively) forms. Incubation with 2.5nM FXIa for 6 hours at pH 7.4 and 25°C converted 500nM N9-GP (lane 4 left panel) into heavy and light chains with the same mobility as activated N9 (lane 3 left panel) concomitant with the release of mono and di-PEGylated activation peptides (1x and 2xPEG-AP, respectively) evident by barium iodide (PEG) staining (lane 4 right panel). (D) The distribution of PEGylated species was determined by rpHPLC using a linear acetonitrile gradient which resolved N9-GP into unmodified (1%), mono-PEGylated (95%), and di-PEGylated (4%) forms. In comparison, N9 eluted as a single peak.
Modifications in native and glycoPEGylated N9. (A) Separation of Gla species in rFIX and N9 by anion exchange HPLC using a linear ammonium acetate gradient. The fractional content of indicated Gla species is given in parentheses and was calculated by relating the area of the corresponding peak to the total peak area. (B) Domain structure of N9 and location of identified N- and O-linked (●) carbohydrates. The 2 N-linked glycans in the activation peptide (AP) were predominantly core-fucosylated tri- and tetra-antennary complex structures composed of fucose (◁), N-acetylglucosamine (□), mannose (○), galactose (⊙), and sialic acid (♢). Enzymatic transfer of 40k-PEG-sialic acid to desialylated N9, and sialylation of remaining exposed galactoses are indicated by arrows. The relative distribution of PEG between the 2 N-glycans is given in parentheses. (C) N9-GP (lane 2) separated into 2 bands by SDS-PAGE corresponding to mono- and di-PEGylated (1x and 2xPEG, respectively) forms. Incubation with 2.5nM FXIa for 6 hours at pH 7.4 and 25°C converted 500nM N9-GP (lane 4 left panel) into heavy and light chains with the same mobility as activated N9 (lane 3 left panel) concomitant with the release of mono and di-PEGylated activation peptides (1x and 2xPEG-AP, respectively) evident by barium iodide (PEG) staining (lane 4 right panel). (D) The distribution of PEGylated species was determined by rpHPLC using a linear acetonitrile gradient which resolved N9-GP into unmodified (1%), mono-PEGylated (95%), and di-PEGylated (4%) forms. In comparison, N9 eluted as a single peak.
GlycoPEGylation of N9
Selective glycoPEGylation used the substrate promiscuity of ST3GalIII sialyl transferase which allowed for the transfer of CMP-activated sialic acid-6′-40k PEG to terminal galactoses of the N-glycans in the activation peptide of desialylated N9. Remaining exposed galactosyl residues were subsequently sialylated by addition of excess unmodified CMP-sialic acid. By optimizing reaction conditions the conjugation stoichiometry could be controlled to yield a product (N9-GP) containing mainly mono-PEGylated N9 (95%) and a minor proportion of the di-PEG form (5%) as determined by SDS-PAGE and reversed-phase (rp) HPLC (Figure 1C-D). By the latter method the proportion of unPEGylated N9 was estimated to be 1%. The content of activated FIX was measured by the amidolytic method32 and found to be 0.03% compared with a level of 0.13%-0.18% in rFIX.
Localization of PEG to the activation peptide was confirmed by incubation of N9-GP with FXIa which resulted in quantitative conversion of the single-chain molecule to heavy and light chains with the same electrophoretic mobility as activated N9. Staining for PEG revealed 2 bands with the same relative intensities as unactivated N9-GP but lower apparent molecular weights, which were identified by N-terminal sequencing as the liberated activation peptide (Figure 1C). Further mapping used a markedly prolonged elution time of PEG by rpHPLC which allowed for the isolation of PEGylated peptides after digestion of N9-GP with trypsin and endoproteinase Glu-C to separate the N-glycans on different peptides. N-terminal sequencing of the isolated PEGylated fragments showed the PEG to be almost equally distributed among peptides containing either the Asn157 (55%) or Asn167 (45%) N-glycan acceptor site (see Figure 1B). No other PEGylated peptides were identified.
In vitro functional properties of N9-GP
To address the effects of glycoPEGylation on the functional properties of N9, we examined individually the reactions in the coagulation process relevant to the activation or subsequent proteolytic action of N9-GP. The kinetics of N9/N9-GP activation catalyzed by FXIa or the FVIIa-TF complex was measured in amidolytic assays. As shown in Figure 2A, the activation of N9 and N9-GP by FXIa proceeded with essentially identical kinetics (Table 1). In contrast, the catalytic efficiency (kcat/Km) of N9-GP activation by the lipidated TF-FVIIa complex was reduced to 47% of N9 (Figure 2B), which was mainly because of an increased Km value from 74 ± 3 to 122 ± 3nM (Table 1).
Impact of glycoPEGylation on the activation and functional properties of N9. The kinetics of N9 or N9-GP activation was determined in the presence of (A) 10pM FXIa or (B) 100pM lipidated TF saturated with 1nM FVIIa. After incubation for 30 minutes at pH 7.4, 5mM Ca2+, and 25°C, further activation was quenched by addition of excess EDTA and rates of FIXa generation determined from the amidolytic activity. Data (mean ± SD, n = 3) were fitted to the Michaelis-Menten equation. (C) Binding of FVIIIa to activated N9 and N9-GP was analyzed from titrations of 0.1nM FIXa with 0-3nM FVIIIa in the presence of 25μM 25:75 phosphatidylserine: phosphatidylcholine (PS:PC) vesicles and 100nM FX at pH 7.4, 5mM Ca2+, and 37°C. After 30 seconds of incubation, reactions were quenched with EDTA and initial rates of FXa generation determined from the amidolytic activity. Fit of data (mean ± SD, n = 3) to a 1:1 binding isotherm yielded apparent dissociation constants (K½,FVIIIa) of 1.14 ± 0.03 and 1.21 ± 0.06nM for activated N9 and N9-GP, respectively. (D) The kinetics of FX (0-100nM) activation by activated N9 or N9-GP (20pM) were determined in the presence of saturating FVIIIa (2.7nM) and 25μM 25:75 PS:PC vesicles. Initial rates of FX activation (mean ± SD, n = 3) were fitted to the Michaelis-Menten equation. Kinetic parameters are listed in Table 1.
Impact of glycoPEGylation on the activation and functional properties of N9. The kinetics of N9 or N9-GP activation was determined in the presence of (A) 10pM FXIa or (B) 100pM lipidated TF saturated with 1nM FVIIa. After incubation for 30 minutes at pH 7.4, 5mM Ca2+, and 25°C, further activation was quenched by addition of excess EDTA and rates of FIXa generation determined from the amidolytic activity. Data (mean ± SD, n = 3) were fitted to the Michaelis-Menten equation. (C) Binding of FVIIIa to activated N9 and N9-GP was analyzed from titrations of 0.1nM FIXa with 0-3nM FVIIIa in the presence of 25μM 25:75 phosphatidylserine: phosphatidylcholine (PS:PC) vesicles and 100nM FX at pH 7.4, 5mM Ca2+, and 37°C. After 30 seconds of incubation, reactions were quenched with EDTA and initial rates of FXa generation determined from the amidolytic activity. Fit of data (mean ± SD, n = 3) to a 1:1 binding isotherm yielded apparent dissociation constants (K½,FVIIIa) of 1.14 ± 0.03 and 1.21 ± 0.06nM for activated N9 and N9-GP, respectively. (D) The kinetics of FX (0-100nM) activation by activated N9 or N9-GP (20pM) were determined in the presence of saturating FVIIIa (2.7nM) and 25μM 25:75 PS:PC vesicles. Initial rates of FX activation (mean ± SD, n = 3) were fitted to the Michaelis-Menten equation. Kinetic parameters are listed in Table 1.
Enzyme kinetic parameters
| Enzyme/cofactor . | Substrate . | Km, nM . | kcat, s−1 . | kcat/Km, M−1s−1 . |
|---|---|---|---|---|
| FXIa* | ||||
| N9 | 45 ± 2 | 0.81 ± 0.01 | (18 ± 1) × 106 | |
| N9-GP | 46 ± 6 | 0.76 ± 0.03 | (17 ± 2) × 106 | |
| FVIIa† | ||||
| TF/PS:PC | N9 | 74 ± 3 | 0.50 ± 0.01 | (6.7 ± 0.2) × 106 |
| TF/PS:PC | N9-GP | 122 ± 10 | 0.38 ± 0.02 | (3.1 ± 0.1) × 106 |
| N9a‡ | ||||
| FVIIIa/PS:PS | FX | 11.4 ± 0.3 | 8.7 ± 0.8 | (7.6 ± 0.5) × 108 |
| [N9-GP]a‡ | ||||
| FVIIIa/PS:PC | FX | 12.9 ± 0.9 | 9.2 ± 0.1 | (7.1 ± 0.4) × 108 |
| Enzyme/cofactor . | Substrate . | Km, nM . | kcat, s−1 . | kcat/Km, M−1s−1 . |
|---|---|---|---|---|
| FXIa* | ||||
| N9 | 45 ± 2 | 0.81 ± 0.01 | (18 ± 1) × 106 | |
| N9-GP | 46 ± 6 | 0.76 ± 0.03 | (17 ± 2) × 106 | |
| FVIIa† | ||||
| TF/PS:PC | N9 | 74 ± 3 | 0.50 ± 0.01 | (6.7 ± 0.2) × 106 |
| TF/PS:PC | N9-GP | 122 ± 10 | 0.38 ± 0.02 | (3.1 ± 0.1) × 106 |
| N9a‡ | ||||
| FVIIIa/PS:PS | FX | 11.4 ± 0.3 | 8.7 ± 0.8 | (7.6 ± 0.5) × 108 |
| [N9-GP]a‡ | ||||
| FVIIIa/PS:PC | FX | 12.9 ± 0.9 | 9.2 ± 0.1 | (7.1 ± 0.4) × 108 |
Michaelis-Menten parameters were measured in the presence of relevant cofactors as indicated. N9a and [N9-GP]a denote activated N9 and N9-GP, respectively.
FX indicates factor X; FV, factor V; TF, tissue factor; PS, phosphatidylserine; and PC, phosphatidylcholine.
See Figure 2A.
See Figure 2B.
See Figure 2D.
To compare the functional properties of activated N9 and N9-GP, tenase complexes were assembled on a phospholipid surface by combining the respective FXIa-activated forms and thrombin-activated FVIII. Conversion of FX to FXa by the complexes was measured from the generated amidolytic activity. By varying the concentration of FVIIIa in the assay, the kinetically derived apparent dissociation constants (K½,FVIIIa) for the interaction with FVIIIa-phospholipid were estimated and found to be similar for activated N9 (1.14 ± 0.03nM) and N9-GP (1.21 ± 0.06nM; Figure 2C). Likewise, both catalyzed FX activation with indistinguishable kinetics in the presence of phospholipid and saturating FVIIIa (Figure 2D, Table 1).
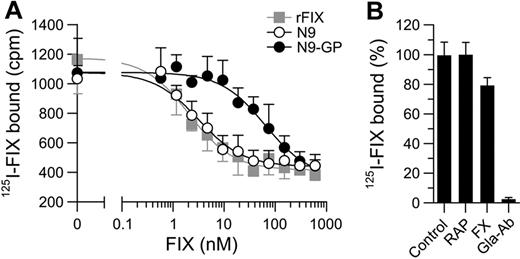
Among the coagulation proteases FIX is unique in its ability to bind collagen IV associated with the vascular endothelium.33 Binding is calcium-dependent and mediated by residues in the Gla domain.34 The effect of glycoPEGylation on the binding to endothelial cells (HUVECs) was investigated in vitro. In accordance with previous reports, rFIX and N9 were both capable of competitively displacing 125I-labeled rFIX with low-nanomolar Ki values of 1.6 and 2.4nM, respectively (Figure 3A). Specificity was confirmed by the ability of an anti-FIX Gla-domain Ab to abrogate binding, whereas high concentrations of FX or the LRP scavenger receptor antagonist RAP (receptor-associated protein) had no effect (Figure 3B). N9-GP also competitively displaced 125I-rFIX. However, 20-fold higher concentrations were required corresponding to a Ki of 48nM (Figure 3A).
Effect of glycoPEGylation on the binding of N9 to endothelial cells. (A) Competitive displacement of 1nM 125I-rFIX with increasing concentrations of unlabeled rFIX, N9, or N9-GP (0.6-600nM). Monolayers of HUVEC cells were incubated with compound for 3 hours at 4°C before counting of bound radioactivity. Binding of rFIX was analyzed according to a homologous 1-site binding model and the estimated apparent Kd of 1.6nM used to derive Ki values for N9 (2.4nM) and N9-GP (48nM). (B) HUVECs were incubated with 0.5nM 125I-rFIX with or without molar excess of anti-FIX Gla domain Ab (Gla-Ab; 33nM), FX (400nM), or RAP (500nM) for 3 hours at 4°C. All data are shown as mean ± SD (n = 4).
Effect of glycoPEGylation on the binding of N9 to endothelial cells. (A) Competitive displacement of 1nM 125I-rFIX with increasing concentrations of unlabeled rFIX, N9, or N9-GP (0.6-600nM). Monolayers of HUVEC cells were incubated with compound for 3 hours at 4°C before counting of bound radioactivity. Binding of rFIX was analyzed according to a homologous 1-site binding model and the estimated apparent Kd of 1.6nM used to derive Ki values for N9 (2.4nM) and N9-GP (48nM). (B) HUVECs were incubated with 0.5nM 125I-rFIX with or without molar excess of anti-FIX Gla domain Ab (Gla-Ab; 33nM), FX (400nM), or RAP (500nM) for 3 hours at 4°C. All data are shown as mean ± SD (n = 4).
Activity in plasma and whole-blood–based assays
Having established the basic molecular properties of N9-GP, we next assessed its ability to support coagulation in plasma and whole-blood–based assays. In the 1-stage aPTT assay, the specific activity of N9-GP was 190 ± 10 IU/mg, that is, slightly lower (73%) than that measured for N9 (260 ± 28 IU/mg). To further assess the activity of N9-GP, clot formation was examined in whole blood obtained from 8 HB patients with the moderate (5) and severe (3) phenotype. In this and the subsequent studies, rFIX was included for comparison. Clot formation was initiated by addition of kaolin and monitored by TEG.35 Kaolin is considered an intrinsic pathway activator. However, a contribution from the extrinsic FVIIa-TF-driven pathway was evident because addition of an inhibitory TF Ab to FIX-depleted blood resulted in a 30% increase of the R time. Representative TEG profiles from a severe and a moderate patient are shown in Figure 4A and B before and after addition of 28nM N9-GP or rFIX (about 30% of the normal level) to approximate the plasma level in HB patients receiving a standard prophylactic dose.7 Baseline TEG profiles were found to differ somewhat between patients; however, all were outside the normal range and thus displayed markedly delayed coagulation. Spiking of blood with increasing levels (0.08-76nM) of N9-GP or rFIX led to a concentration-dependent decrease of the R-time and increase in MTG which entered the normal ranges at the highest concentrations (Figure 4C-D). At this concentration, no significant differences in clotting parameters between rFIX and N9-GP were observed indicating similar efficacies. Likewise, the ratio of EC50 values of N9-GP and rFIX estimated based on R time, MTG, and MA values did not differ significantly from unity indicating comparable potencies (supplemental Table 1).
Comparison of N9-GP and rFIX in human hemophilia B whole blood. TEG traces of whole blood from a severe (A) and a moderate (B) HB patient before (stippled line) and after spiking with 28nM N9-GP (black line) or rFIX (gray line). Clot formation was induced by addition of kaolin. R-time and MTG values are shown as a function of added N9-GP or rFIX in blood from the same (C) severe or (D) moderate patient. The normal range ± 2 SD are indicated by horizontal lines.
Comparison of N9-GP and rFIX in human hemophilia B whole blood. TEG traces of whole blood from a severe (A) and a moderate (B) HB patient before (stippled line) and after spiking with 28nM N9-GP (black line) or rFIX (gray line). Clot formation was induced by addition of kaolin. R-time and MTG values are shown as a function of added N9-GP or rFIX in blood from the same (C) severe or (D) moderate patient. The normal range ± 2 SD are indicated by horizontal lines.
Acute and prolonged hemostatic effect in hemophilia B mice
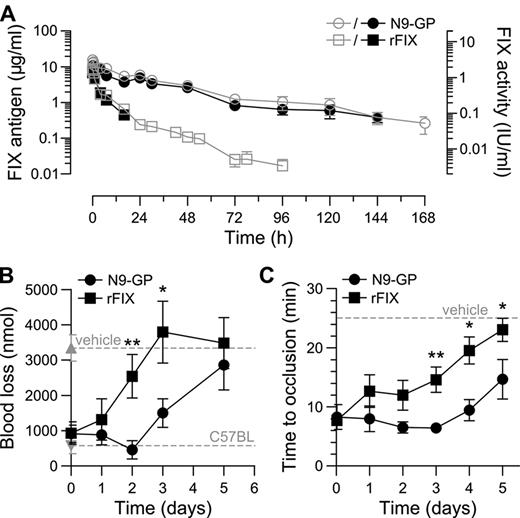
The hemostatic potentials of N9-GP and rFIX were further compared in vivo in HB mice where bleeding was induced by tail-tip transection 5 minutes after dosing. In vehicle-treated mice bleeding time and blood loss were significantly increased compared with normal C57BL mice. Both compounds demonstrated a clear dose-response with no effect at the lowest dose of 0.01 mg/kg (corresponding to 1.9 IU/kg N9-GP based on the aPTT clot activity) and normalization of both parameters at 0.4-0.75 mg/kg (76-143 IU/kg N9-GP; Figure 5A-B). Consistent with the results obtained in whole blood, the dose-response relationship for the 2 compounds did not differ significantly. The plasma level of FIX was determined at the end of each 30-minute observation period and found to be proportional to the dose given but, interestingly, on average 2.1 times higher for N9-GP than rFIX at equimolar doses (Figure 5C). The higher in vivo recovery of N9-GP was confirmed in HB mice administered N9-GP or rFIX but not subjected to injury (Table 2). Measurement of plasma levels in these mice up to 1 week after infusion furthermore demonstrated a prolonged pharmacokinetic profile of N9-GP characterized by a half-life (T½) of 41 hours compared with 17 hours for rFIX based on ELISA (Figure 6A, Table 2). Superimposable profiles were obtained by measurement of clot activities indicating that N9-GP retained functionality in circulation (Figure 6A).
Acute hemostatic effect in tail bleeding model in hemophilia B mice. N9-GP or rFIX was given IV at 0-0.75 mg/kg to HB mice 5 minutes before tail-tip transection (4 mm). (A) Bleeding time and (B) blood loss were recorded for the after 30 minutes. Data are shown as mean ± SEM (n = 6-14/group). The bleeding pattern in normal (C57BL) and vehicle-treated HB mice are included for comparison. (C) FIX levels were measured by ELISA at the end of each observation period. Linear regression analysis yielded slopes of 3.8 ± 0.3 and 1.8 ± 0.3 (μg/mL) (mg/kg)−1 for N9-GP and rFIX, respectively.
Acute hemostatic effect in tail bleeding model in hemophilia B mice. N9-GP or rFIX was given IV at 0-0.75 mg/kg to HB mice 5 minutes before tail-tip transection (4 mm). (A) Bleeding time and (B) blood loss were recorded for the after 30 minutes. Data are shown as mean ± SEM (n = 6-14/group). The bleeding pattern in normal (C57BL) and vehicle-treated HB mice are included for comparison. (C) FIX levels were measured by ELISA at the end of each observation period. Linear regression analysis yielded slopes of 3.8 ± 0.3 and 1.8 ± 0.3 (μg/mL) (mg/kg)−1 for N9-GP and rFIX, respectively.
Summary of pharmacokinetic parameters
| Species/compound . | Dose, mg/kg (IU/kg) . | Cmax, ng/mL (IU/mL) . | Half-life, h . | Clearance, mL/h/kg . |
|---|---|---|---|---|
| HB mouse | ||||
| rFIX | 1.5 (375) | 8777 (1.70) | 17 (6*) | 36 (50*) |
| N9-GP | 1.5 (285) | 15 500 (1.78) | 41 (67) | 3.6 (4.8) |
| HB dog | ||||
| rFIX | 0.4 (100) | 3680 (0.98) | 25 (16) | 7 (9.8) |
| N9-GP | 0.4 (76) | 5130 (1.20) | 156 (113) | 0.6 (0.6) |
| Mini-pig | ||||
| rFIX | 0.2 (50) | 1850 (nd) | 16 (nd) | 12 (nd) |
| N9-GP | 0.2 (38) | 1993 (nd) | 76 (nd) | 1.7 (nd) |
| Species/compound . | Dose, mg/kg (IU/kg) . | Cmax, ng/mL (IU/mL) . | Half-life, h . | Clearance, mL/h/kg . |
|---|---|---|---|---|
| HB mouse | ||||
| rFIX | 1.5 (375) | 8777 (1.70) | 17 (6*) | 36 (50*) |
| N9-GP | 1.5 (285) | 15 500 (1.78) | 41 (67) | 3.6 (4.8) |
| HB dog | ||||
| rFIX | 0.4 (100) | 3680 (0.98) | 25 (16) | 7 (9.8) |
| N9-GP | 0.4 (76) | 5130 (1.20) | 156 (113) | 0.6 (0.6) |
| Mini-pig | ||||
| rFIX | 0.2 (50) | 1850 (nd) | 16 (nd) | 12 (nd) |
| N9-GP | 0.2 (38) | 1993 (nd) | 76 (nd) | 1.7 (nd) |
All compounds were administered intravenously. Noncompartmental parameters were estimated from plasma profiles obtained by Ag or aPTT clot activity (in parentheses) measurements.
rFIX indicates recombinant factor IX; and aPTT, activated partial thromboplastin time.
Based on few data points (0-16 hours) due to fast plasma elimination below detection limit of the assay.
The longer circulation time of N9-GP compared with rFIX was reflected in a prolonged duration of effect in the tail-bleeding model. After infusion of 0.75 mg/kg N9-GP (143 IU/kg) or rFIX (188 IU/kg) bleeding was normalized acutely. In addition, N9-GP maintained blood loss in the normal nonhemophilic range until day 2 followed by a gradual return to the hemophilic baseline at day 5. In mice receiving rFIX, blood loss was already significantly elevated at day 2 and back at baseline at day 3 (Figure 6B).
Pharmacokinetics and duration of effect in hemophilia B mice. (A) N9-GP (circles) or rFIX (squares) was administered at 1.5 mg/kg. Blood samples were analyzed for FIX clot activity (closed symbols) and Ag (open symbols) and are shown as mean ± SD (n = 3). (B) Duration of effect of 0.75 mg/kg N9-GP (143 IU/kg) and rFIX (188 IU/kg) in the tail bleeding model. Blood loss was recorded for 30 minutes at the indicated time points starting at 5 minutes postadministration. Blood loss in normal (C57BL) mice and vehicle-treated HB mice are included for comparison (----). Results are shown as mean ± SEM (n = 8-16/group); *P < .05 and **P < .01 are relative to rFIX using 1-way ANOVA and Bonferroni posttest. (C) Duration of effect in the ferric chloride–induced carotid artery injury model. The occlusion time was determined at indicated intervals after infusion of 0.75 mg/kg N9-GP or rFIX with the first time point after 5 minutes. Occlusion was defined as complete lack of blood flow for > 5 minutes. If occlusion did not occur within the 25-minute observation period, as was the case in vehicle-treated mice, the occlusion time was reported as 25 minutes (----). Results are shown as mean ± SEM (n = 6-11/group); *P < .05 and **P < .01 are relative to rFIX using the Kruskal-Wallis test and the Dunn posttest.
Pharmacokinetics and duration of effect in hemophilia B mice. (A) N9-GP (circles) or rFIX (squares) was administered at 1.5 mg/kg. Blood samples were analyzed for FIX clot activity (closed symbols) and Ag (open symbols) and are shown as mean ± SD (n = 3). (B) Duration of effect of 0.75 mg/kg N9-GP (143 IU/kg) and rFIX (188 IU/kg) in the tail bleeding model. Blood loss was recorded for 30 minutes at the indicated time points starting at 5 minutes postadministration. Blood loss in normal (C57BL) mice and vehicle-treated HB mice are included for comparison (----). Results are shown as mean ± SEM (n = 8-16/group); *P < .05 and **P < .01 are relative to rFIX using 1-way ANOVA and Bonferroni posttest. (C) Duration of effect in the ferric chloride–induced carotid artery injury model. The occlusion time was determined at indicated intervals after infusion of 0.75 mg/kg N9-GP or rFIX with the first time point after 5 minutes. Occlusion was defined as complete lack of blood flow for > 5 minutes. If occlusion did not occur within the 25-minute observation period, as was the case in vehicle-treated mice, the occlusion time was reported as 25 minutes (----). Results are shown as mean ± SEM (n = 6-11/group); *P < .05 and **P < .01 are relative to rFIX using the Kruskal-Wallis test and the Dunn posttest.
The hemostatic protection provided by N9-GP and rFIX was also compared in the ferric chloride–induced carotid artery injury model in HB mice.24 Injury was induced up to 5 days after administration of 0.75 mg/kg and the time to occlusion recorded. Except for the first time point at 5 minutes, the average occlusion time in mice receiving N9-GP was consistently shorter than in mice treated with rFIX (Figure 6C). This difference was also reflected in the fraction of animals which developed stable occluding thrombi within 25 minutes after injury. One to 4 days after dosing occlusion was observed in 90%-100% of N9-GP treated mice, whereas only 70% of mice receiving rFIX occluded at day 1 and 44% at day 4. Five days after dosing 56% of N9-GP mice still occluded compared with 13% of mice treated with rFIX.
Pharmacokinetics and pharmacodynamics in hemophilia B dog
The pharmacodynamics of N9-GP was investigated in a single HB dog tolerized to human FIX by neonatal retrovial gene transfer25 which allowed for inclusion of rFIX in a crossover design. N9-GP and rFIX were both given at 0.4 mg/kg corresponding to 76 and 100 IU/kg, respectively. As shown in Figure 7A, plasma clearance of N9-GP was greatly reduced and the plasma concentration did not reach the 1% level (50 ng/mL) until 40 days after administration compared with 8 days for rFIX. This differences was reflected in a functional half-life of N9-GP (113 hours) that was 7 times longer than rFIX (16 hours) and a clearance rate that was reduced 16-fold from 9.8 to 0.6 mL/kg/h (Table 2). Profiles determined by ELISA and clotting activity were super-imposable until day 16 at which the detection limit of the functional assay was reached providing further evidence of preserved functionality of N9-GP during the extended residence in circulation.
Pharmacokinetics and pharmacodynamics in hemophilia B dog and mini-pig. A tolerized HB dog received successive infusions of 0.4 mg/kg rFIX (100 IU/kg) and N9-GP (76 IU/kg) separated by a washout period. Blood samples were collected at indicated time points to measure (A) FIX clot activity (closed symbols) and Ag levels (open symbols) and to evaluate the ex vivo hemostatic potential by (B) WBCT and (C-D) TEG. The standard WBCT range in nonhemophilic dogs is indicated. For the TEG experiments, coagulation was triggered with kaolin. (C) Representative TEG traces recorded pre-dose (gray), at 5 minutes and 3, 19, 26, 31, 40, and 43 days (from left to right in black) after administration of N9-GP. (D) MTG values at indicated time points after administration. (E) Plasma concentrations of rFIX and N9-GP in mini-pig (3 per group) shown as individual Ag measurements after administration of 0.2 mg/kg (50 and 38 IU/kg, respectively). Solid lines represent best fits to a 2-compartment model. (F) Fitted parameters were used to simulate pharmacokinetic profiles in a multiple dose setting. Simulations were performed with N9-GP (black line) dosed every 7 (solid) or 14 (stippled) days and rFIX (gray line) dosed every 2.5 days, all at 0.2 mg/kg. Horizontal stippled lines represent 100% (top) and 1% (bottom) levels, respectively.
Pharmacokinetics and pharmacodynamics in hemophilia B dog and mini-pig. A tolerized HB dog received successive infusions of 0.4 mg/kg rFIX (100 IU/kg) and N9-GP (76 IU/kg) separated by a washout period. Blood samples were collected at indicated time points to measure (A) FIX clot activity (closed symbols) and Ag levels (open symbols) and to evaluate the ex vivo hemostatic potential by (B) WBCT and (C-D) TEG. The standard WBCT range in nonhemophilic dogs is indicated. For the TEG experiments, coagulation was triggered with kaolin. (C) Representative TEG traces recorded pre-dose (gray), at 5 minutes and 3, 19, 26, 31, 40, and 43 days (from left to right in black) after administration of N9-GP. (D) MTG values at indicated time points after administration. (E) Plasma concentrations of rFIX and N9-GP in mini-pig (3 per group) shown as individual Ag measurements after administration of 0.2 mg/kg (50 and 38 IU/kg, respectively). Solid lines represent best fits to a 2-compartment model. (F) Fitted parameters were used to simulate pharmacokinetic profiles in a multiple dose setting. Simulations were performed with N9-GP (black line) dosed every 7 (solid) or 14 (stippled) days and rFIX (gray line) dosed every 2.5 days, all at 0.2 mg/kg. Horizontal stippled lines represent 100% (top) and 1% (bottom) levels, respectively.
WBCT is a marker for hemostatic efficacy in HB dogs and was used to evaluate the pharmacodynamics of N9-GP.22 Because of low endogenous levels of FIX in the tolerized dog as a result of neonatal gene transfer (< 0.1% of the normal level),25 the WBCT pre-dose value (22 minutes) was shorter than for naive HB dogs (> 60 minutes) but sufficiently above the normal range (10-15 minutes) to allow for detection of pharmacologic effect. Normalization of the WBCT was observed 5 minutes after administration of rFIX and lasted for 4 days followed by a gradual return to pre-dose levels at day 12 (Figure 7B). A similar immediate correction of the clotting time was observed with N9-GP. However, the WBCT remained in the normal range for 12-19 days and then slowly increased toward the pre-dose level at day 45 in accordance with its reduced elimination kinetics (Figure 7B). A similar pattern was observed when blood samples were analyzed by TEG which is more sensitive to trace amounts of FIX. Here, clot formation was corrected until approximately day 26 followed by a return of the R time (data not shown) and MTG to baseline values (Figure 7C-D).
Multiple dose simulations
To further verify the pharmacokinetics of N9-GP in a larger animal model and to generate a dataset sufficient for reliable pharmacokinetic modeling, clearance studies were performed in mini-pigs. As shown in Figure 7E, the variation between animals (n = 3) was small and the elimination profiles for N9-GP and rFIX declined in a biphasic manner that was well-described by a 2-compartment model. The estimated terminal half-life of N9-GP was 76 hours and thus significantly longer than rFIX (16 hours). Based on the fitted parameters, plasma profiles were simulated in a multiple dose setting assuming a fixed dose level of 0.2 mg/kg given at regular intervals. This amount corresponds to 38 and 50 IU/kg N9-GP and rFIX, respectively, and represents a typical prophylactic dose.7 For rFIX, a trough of 1% of the normal level was observed 2 days after dosing, which mirrors the typical 2-3 times weekly dosing regimen in HB patients. In comparison, the plasma level of N9-GP was 4% after 1 week and 1% 2 weeks after administration (Figure 7F).
Discussion
PEGylation is a well-established strategy for extending the plasma half-life of proteins. The application of classic nonspecific means of attachment are, however, often associated with an unwanted loss of activity.36 To circumvent this problem, we applied enzymatic glyco-conjugation to direct PEGylation to the native N-glycans of FIX which are attached to the activation peptide and remote from known interaction sites of functional importance. The attached PEG moiety is thus present only on the circulating zymgen form and removed on its activation. Confinement of PEG to the activation peptide was confirmed by peptide mapping and corroborated by functional characterization demonstrating unaltered enzymatic properties of the activated forms of N9-GP and N9 (Figure 2C-D) including normal reactivity toward the principal plasma inhibitor antithrombin III37 (L.C.P., unpublished results, February 10, 2011). These results indicate that, once activated, N9-GP retains full activity and is subjected to normal physiologic regulation.
The ability of FIX to exert its function is dependent on its conversion to the activated form. FXIa and FVIIa-TF both recognize FIX by extended interactions38,39 characterized by Km values in the low nanomolar range (Table 1), which could compensate for steric hindrance by the PEG. Indeed, activation of N9 by FXIa was found to be insensitive to glycoPEGylation, while the membrane-dependent activation by the FVIIa-TF was reduced by approximately 50% primarily as a result of an increased Km (Figure 2A-B). Interestingly, no significant differences in the efficacy and potency of N9-GP and rFIX were observed in whole blood from HB patients (Figure 4) or in the tail-bleeding model in HB mice (Figure 5) although both models exhibit TF dependency.40 Thus, despite the slightly slower rate of activation, N9-GP appears to retain near normal specific activity and the ability to stop bleeds with the same efficiency as rFIX suggests that it may be efficacious for acute treatment of bleeding episodes.
The mechanisms by which FIX is cleared from the circulation have been addressed in a few studies. High-affinity binding sites associated with the vascular endothelium have been implicated in the recovery and initial disappearance of FIX.41 In addition, uptake by the vascular endothelium or other cell types expressing the neonatal Fc receptor (FcRn) has been demonstrated by genetic fusion of FIX to the Fc fragment of IgG. Using this concept, Peters et al14 obtained a 3- to 4-fold extension of FIX half-life in mice that was dependent on endogenous FcRn expression, and similar half lives of approximately 47 hours were determined in monkey and HB dog. The pharmacokinetic studies conducted in the present work are in accord with published results on rFIX14,42 and furthermore demonstrate a significant beneficial effect of glycoPEGylation on the circulation time of FIX. The half-life of N9-GP was found to increase according to body weight from 41 hours in mice to 76 and 113 hours in mini-pig and dog, respectively (Table 2). The differential impact of PEG and Fc-fusion on FIX half-life in larger animal models suggests that multiple pathways contribute to the clearance of FIX and that PEG may act by distinct or additional retention mechanisms than previously used to prolong FIX survival in the circulation.12,14 Importantly, the longer circulation time of N9-GP compared with rFIX was evident as an extended hemostatic protection in HB mice and normalization of coagulation parameters in HB dog (Figures 6, 7B-D). Accordingly, several lines of evidence suggest that N9-GP may support a considerably longer dosing interval than feasible with existing therapy, a conclusion which is further supported by multiple dose simulations using the plasma profiles obtained in mini-pig as a model for human pharmacokinetics. At a standard prophylaxis dose, the predicted 1% trough level of N9-GP was not reached until 2 weeks after administration compared with 2 days for rFIX (Figure 7F).
The lower in vivo recovery of rFIX compared with plasma-derived products is well documented in humans6 and animal models42 and is compensated for in current dosage recommendations. The difference has been ascribed to variations in posttranslational modifications although no definitive results have been presented.30,43 Interestingly, an up to 2-fold higher in vivo recovery of N9-GP compared with rFIX was found across species (Table 2). Considering the structural similarity of N9 and rFIX, this difference is most likely related to effects of carbohydrate modification, that is, PEGylation or the subsequent capping with sialic acid. Incomplete sialylation of the N-linked carbohydrates may target FIX for hepatic degradation and has been proposed as one cause of the lower recovery.44,45 The fact that N9 is capped with sialic acid as the last step in the glycoPEGylation process could protect N9-GP from this pathway and consequently increase recovery. Another contributing factor may relate to the presence of high-affinity binding sites for FIX on collagen IV associated with the vascular endothelium.33 Cheung et al34 localized the binding site on FIX to the Gla domain and a FIX variant (K5A) devoid of collagen IV binding was shown by Gui et al41 to exhibit increased recovery and accelerated plasma clearance in mice. Endothelial cell binding by N9-GP was addressed in vitro and found to be reduced by approximately 20-fold (Ki 48nM) compared with N9 and rFIX which bound with similar high affinities (Figure 3). As the plasma levels achieved after infusion are in the range of the estimated dissociation constant for N9-GP, it is conceivable that the reduced affinity would allow for a greater fraction of N9-GP to remain in circulation in the initial phase after administration. Pre-occupancy of vascular binding sites by endogenous FIX may reduce the contribution of this pathway to initial FIX disappearance37 and is consistent with the only marginally higher in vivo recovery of N9-GP compared with rFIX in nonhemophilic mini-pigs in contrast to HB mice and dogs (Table 2). A recent study suggested that lack of collagen binding was associated with a partial hemostatic defect in models involving exposure of vascular collagen including ferric chloride and tail transection models.46 It is presently not clear whether the preserved efficacy of N9-GP in the same models is explained by adequate residual affinity of N9-GP for collagen or by the higher in vivo recovery which may compensate for a reduced accumulation at vascular sites.
In summary, a novel releasable protraction concept has been developed that uses the ability to selectively target naturally occurring carbohydrates in the activation peptide of FIX without the need for amino acid modifications. This approach offers the additional advantages of retained biologic activity and normal physiologic regulation of the activated form, while providing greatly prolonged pharmacokinetics and pharmacodynamics in several animal models. Clinical studies will show whether these properties translate into improved on demand treatment and effective bleeding prophylaxis in HB patients with a once-weekly or less frequent dosing schedule.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their colleagues at Novo Nordisk for their contribution to this work which includes cell line screening and development, purification, and glycoPEGylation process development, assay support, and in vivo studies.
Authorship
Contribution: H.Ø. and M.T. designed and performed research, analyzed data, and wrote the manuscript; J.R.B., L.H., L.C.P., A.A.P., T.E., F.M., M.B.H., P.K.H., T.N.K., J.M.P., M.D.A., H. Ahmadian, K.W.B., and M.L.S.C. designed and performed research, and analyzed data; M.E., B.B.S., H. Agersø, and S.E.B. designed research and analyzed data; K.K. provided clinical input and wrote the manuscript; and T.C.N. designed and performed research.
Conflict-of-interest disclosure: H.Ø., J.R.B, L.H., L.C.P., A.A.P., T.E., F.M., M.B.H., P.K.H., T.N.K., J.M.P., M.E., B.B.S., M.D.A., H. Agersø, H. Ahmadian, K.W.B., M.L.S.C., K.K., S.E.B., and M.T. are employees of Novo Nordisk. T.C.N. declares no competing financial interests.
Correspondence: Henrik Østergaard, Haemostasis Biology, Novo Nordisk A/S, DK-2760 Maaloev, Denmark; e-mail: hqsg@novonordisk.com.