Abstract
The antiphospholipid syndrome (APS) is an autoimmune disease characterized by thromboembolic events and/or fetal loss in the presence of antiphospholipid antibodies (aPLs). The mechanisms underlying the pathogenicity of aPLs are still poorly understood. Here we show that 3 human monoclonal aPLs as well as IgG fractions from patients with the APS increase mRNA expression of the intracellular toll-like receptor (TLR) 7 in plasmacytoid dendritic cells and TLR8 in monocytes. Simultaneously they induce the translocation of TLR7 or TLR8 from the endoplasmic reticulum to the endosome. These effects depend on the uptake of aPLs into the endosome, subsequent activation of endosomal NADPH oxidase, and generation of superoxide. As a consequence cells are dramatically sensitized to ligands for TLR7 and TLR8. This observation delineates a novel signal transduction pathway in innate immunity originating from the endosome. Because the overexpression of TLR7 can also be detected in plasmacytoid dendritic cells from patients with the APS ex vivo, our results provide an explanation for proinflammatory and procoagulant effects of aPLs. Because inappropriate expression of TLR7 has been implicated in the development of systemic autoimmunity, these findings may also be relevant for the understanding of autoimmunity.
Introduction
The antiphospholipid syndrome (APS) is an autoimmune disease characterized by venous and/or arterial thrombosis or by recurrent fetal loss associated with persistently elevated titers of antiphospholipid antibodies (aPLs).1,2 APLs are a heterogeneous family of autoantibodies that bind negatively charged phospholipids such as cardiolipin, phosphatidylserin, and lysobisphosphatidic acid and phospholipid binding proteins such as β2-glycoprotein I (β2GPI).3 The pathogenetic role of aPL has been well established in various animal models of thrombosis and pregnancy.1,4,5 Furthermore, several potentially procoagulant properties of aPLs have been described in cell culture models, the most prominent being their ability to induce inappropriate expression of tissue factor in monocytes and endothelial cells.6 However, the underlying signal transduction is still not fully understood. A major problem has been the lack of a well-defined cellular epitope of aPL.
Recently TLR2 and 4 have been implicated in the signaling cascade induced by aPLs in fibroblasts and endothelial cells, respectively.7-9 We have shown by using human monoclonal aPL and IgG fractions from patients that aPLs induce TLR8 expression and signaling in monocytes, leading to the secretion of inflammatory cytokines.10 However, conclusive evidence that aPLs bind to any of the TLRs has not been presented. Another model proposes that aPLs specific for β2GPI induce the formation of antibody-linked dimers of β2GPI that can activate the apolipoprotein E-receptor 2 in platelets.11,12 This concept implies that aPLs do not bind to any cellular receptors but rather contribute to the formation of an illegitimate ligand. It remains an open question, whether these apparently very different signaling pathways have a common denominator or may be explained by the broad heterogeneity of aPL specificities ranging from anionic phospholipids to proteins such as β2GPI or even by the different cell types analyzed.
The aim of the present study was to further delineate the mechanisms underlying the effects of aPL on TLR8 in monocytes. The 3 monoclonal aPLs used here have been shown previously to induce procoagulant activity in human monocytes.13,14 HL5B can also inhibit yolk sac growth in vitro.15 It should be noted that HL5B and HL7G show extensive somatic mutations, whereas the third aPL, RR7F, exhibits a germline sequence. In addition, we extended our work to a second endosomal TLR, namely TLR7 in human plasmacytoid dendritic cells (pDCs), which is closely related to TLR8 and has been implicated in the development of autoimmunity.16,17
Methods
Reagents
Reagents were purchased as follows: IgG control antibody (ACRIS); limulus amoebocyte assay (Cambrex Bioscience); R848 (InvivoGen); immunoregulatory DNA sequences IRS869, IRS66118 modified by phosphothioate linkages (IBA); Ro106-9920 (Torcis); RNase A (QIAGEN); niflumic acid (NFA), N-acetylcysteine (NAC), nystatin, methyl-β-cyclodextrin (MβCD), exogenous polyethylene-glycolated superoxide dismutase (PEG-SOD), tert-butyl-hydroperoxide (all from Sigma-Aldrich); FITC labeling kit (Thermo Fisher Scientific); BDCA-4 isolation kit and anti–BDCA-2 antibody (Miltenyi Biotec); Lyso-Tracker Red DND-99 and albumin coupled dihydro-2′,4,5,6,7,7′-hexafluorofluorescein (ie, H2HHF-BSA, Oxy Burst; Invitrogen). Antibodies and fluorochrome-labled reagents included streptavidin-APC, streptavidin–Alexa Fluor 546, anti–TNFα-PerCP, anti-FcγI-III–appropriate isotype controls (BD Pharmingen), anti–IFNα-FITC (ImmunoKontact; Abingdon Oxon), monoclonal anti-TLR7 antibody for Western blot (Thermo Fisher Scientific), biotin-labeled monoclonal anti-TLR8 (ACRIS), biotin-labeled anti–rabbit IgG antibody (KPL), anti–β-actin antibody, anti-calnexin, anti-EEA1, rabbit anti–mouse Alexa Fluor 488, and polyclonal anti-TLR7 antibody for FACS staining (all Abcam).
Human samples
Human monoclonal aPLs have been described in detail previously.10,13,14,19 In brief, HL5B and HL7G were cloned from a patient with primary APS. They have numerous somatic mutations indicating antigen driven maturation. RR7F was cloned from a patient with APS and systemic lupus erythematosus (SLE). The sequence of RR7F is almost identical to a germline sequence. IgG fractions and PBMCs of patients and controls were obtained as previously described.10 All antibody preparations had < 0.1 U endotoxin/mL as determined by limulus amoebocyte assay. All patients provided informed consent according to the ethical guidelines following the Declaration of Helsinki. The study was approved by the local ethical committee of the University Medical Center Mainz.
Mice
Age- and sex-matched (6-10 weeks) C57BL/6 and Tlr7−/− mice on the same background were used for RNA internalization experiments. Mice were killed by cervical dislocation, and spleens were excised. All animal procedures were performed in accordance with the legal and institutional guidelines.20
Cell isolation and culture
The isolation of pDCs from buffy coats by the use of the BDCA-4 kit and the subsequent cell culture and confirmation of cell purity > 98% was performed as described.10,16 In brief, pDCs were cultured for 16 hours at 37°C in serum-free medium (Macrophage-SFM; GIBCO/Invitrogen) before we added the different stimuli. MonoMac1 cells were maintained in RPMI-1640 medium supplemented with 10% FCS, l-glutamine, and sodium pyruvate. At 16 hours before the stimulation assays MonoMac 1 cells were transferred to serum-free RPMI-1640 medium. Murine splenic CD11c+ cells were isolated by MACS in a manner similar to pDC isolation described previously in this section. Cells were cultured for 16 hours at 37°C in RPMI-1640 medium supplemented with 10% FCS, l-glutamine, and sodium pyruvate.
Stimulation and inhibition of cells
The stimulatory reagents were added as indicated on 0.5 × 106 cells/mL cultured in serum-free Macrophage-SFM medium (GIBCO/Invitrogen). All inhibitors were added to the cells 30 minutes before the stimuli. The NFκB inhibitor Ro106-9920 and TLR inhibitors (IRS661 and IRS869) were complexed to DOTAP as previously described.10 All other inhibitors were dissolved and applied in culture medium only.
RNA isolation and quantitative RT-PCR
RNA isolation and quantitative RT-PCR was performed as described by Döring et al.10 Primer sequences for TLR7 and IFNα were as follows:
TLR7 forward: CTGTGTGGTTTGTCTGGTGG; reverse: AGATCACACTTTGGCCCTTG; and IFNα forward: GCAAGCCCAGAAGTATCTGC; reverse: ACTGGTTGCCATCAAACTCC.
SDS-PAGE and Western blot analysis of TLR7 expression
Western blot analysis was performed as described.10 The antibodies were used according to the manufacturer's instructions. A loading control with β-actin was performed on the same membranes after stripping twice for 10 minutes in 1.5% glycine wt/vol, 0.1% SDS wt/vol, and 1% Tween20 vol/vol; pH 2.2.
Flow cytometry
Surface and intracellular staining of cells was performed by the use of standard protocols. The antibodies were used according to the manufacturer's instructions. Flow cytometric analyses were performed with a FACS Canto cytometer (Becton Dickinson). The analysis was performed with the use of FlowJo 7.2 software (TreeStar Inc).
RNA40 uptake assay
Cells were pretreated with either HL5B or IgG for 10 minutes. After 3 washing steps, fluorescently labeled RNA40 was added. Uptake of RNA40 was detected by flow cytometry after different time intervals. Extensive washing steps prevented binding of RNA40 to the cell surface.
Live cell imaging by confocal laser scanning microscopy
HL5B and IgG control antibody were labeled with FITC by the use of a standard FITC Antibody Labeling Kit from Thermo Fisher Scientific according to the manufacturer's instructions. Internalization was shown with a Zeiss LSM 710 NLO confocal laser scanning microscope and a 1.4 Oil Dic M27 63 × plan apochromat objective (Zeiss). Cells were cultured in RPMI without phenol red, stimulated, and imaged directly in chambers maintained at 37°C (Nunc).
Detection of cellular reactive oxygen species formation
The method for measurement of cellular reactive oxygen species (ROS) was previously described21 and only slightly modified. In brief, for intracellular ROS detection, cells (1 × 106/mL) were incubated in PBS (1mM Ca2+/Mg2+) with 100μM L-012 for 20 minutes at 37°C in the presence of solvent control, IgG antibody control, or HL5B antibody at different concentrations. For identification of potential ROS sources, inhibitors for NADPH oxidase (apocynin) and xanthine oxidase (allopurinol) were used along with a superoxide scavenging enzyme (PEG-SOD). For extracellular ROS detection, cells (1 × 106/mL) were incubated in PBS with 100μM luminol/0.1μM HRP. Chemiluminescence was detected in a Centro LB960 chemiluminescence plate reader from Berthold at 20 minutes.
The detection of endosomal generation of ROS was performed by use of the fluorogenic reagent OxyBURST Green H2HFF-BSA.22 Cells were grown on chamber slides in PBS containing 1mM Ca2+, 1.5mM Mg2+, and 5.5mM glucose for 2 hours then incubated in 10 μg/mL H2HFF-BSA for 2 minutes with or without PEG-SOD, NFA, or NAC at 37°C, and then stimulated with HL5B. Cell fluorescence was analyzed by confocal microscopy or by flow cytometry.
Verification of superoxide formation
The formation of 2-hydroxyethidium (2HE) from dihydroethidium as indicator of superoxide production was analyzed by HPLC as previously described.23,24 In brief, cells were incubated with 50μM dihydroethidium (DHE) for 20 minutes at 37°C in PBS buffer. The cells were centrifuged for 5 minutes at 8000g, and the supernatant was discarded. The pellet was resuspended in 50% acetonitrile/50% PBS for lysis. A total of 50 μL of the supernatant was subjected to HPLC analysis. The system consisted of a control unit, 2 pumps, mixer, detectors, column oven, degasser, and an autosampler (AS-2057 plus) from Jasco and a C18-Nucleosil 100-3 (125 × 4) column from Macherey & Nagel. A high-pressure gradient was used with acetonitrile (with 10% water) and 25mM citrate buffer, pH 2.2, as mobile phases with the following percentages of the organic solvent: 0 minutes, 36%; 7 minutes, 40%; 8-12 minutes, 95%; and 13 minutes, 36%. The flow was 1 mL/min, and DHE was detected by its absorption at 355 nm, whereas 2HE and ethidium were detected by fluorescence (Ex. 480 nm/Em. 580 nm). The signal was normalized on the number of cells.
Immunofluorescence
To show translocation of TLR8 on stimulation, cells were fixed and stained with TLR8 and anticalnexin as marker for the endoplasmic reticulum (ER) or anti-EEA1 as marker for early endosomes as described by Latz et al.25 Fluorescence was visualized with a Zeiss LSM 710 NLO confocal laser scanning microscope
ELISA for cytokines
TNFα was measured by use of the DuoSet ELISA Development System from R&D Systems. IFNα secretion was determined by use of the IFNα Platinum ELISA from Bender MedSystems.
Statistics
Statistical analyses were performed by independent 2-tailed Student t test.
Results
Induction of TLR7, IFNα, and TNFα by aPL and the role of NFκB
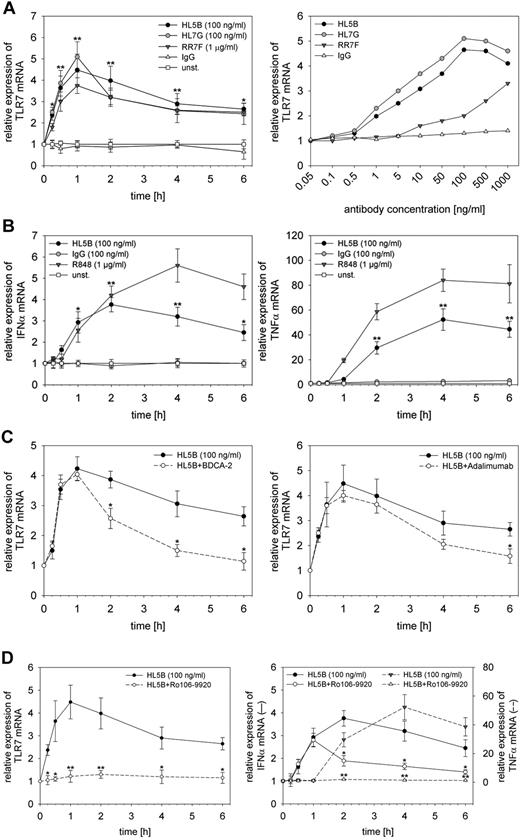
Incubation of human pDCs with the monoclonal human aPL HL5B, HL7G, or RR7F led to a dose-dependent and fast induction of TLR7 mRNA (Figure 1A). As early as 15 minutes after the addition of the antibody, the increase in TLR7 mRNA was detectable, reached a maximum after 1 hour, and could be observed for several hours. The maximal response to HL5B and HL7G was observed with 100-500 ng/mL, which has been used in all additional experiments. The dose-response to the germline antibody RR7F was shifted to the right but otherwise identical. TLR7 mRNA overexpression was accompanied by an increase of TLR7 protein (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In all further experiments, we concentrated on HL5B because the effects of the 3 aPLs were nearly identical.
Activation of peripheral blood pDCs with aPLs. pDCs were incubated with HL5B, HL7G, RR7F, or irrelevant IgG. (A) Time course (left) and dose response curve after 1 hour (right) are shown. Relative expression of TLR7 mRNA was determined by quantitative RT-PCR normalized to β-actin. Values represent mean ± SD of at least 5 independent experiments. *P < .02 and **P < .001 for aPLs versus IgG. (B) Relative expression of IFNα (left) and TNFα (right) mRNA normalized to β-actin. Values represent mean ± SD of at least 5 independent experiments. *P < .01, **P < .001 for HL5B versus IgG. (C) Effect of inhibition of IFNα by BDCA-2 (1 μg/mL; left) or inhibition of TNFα by adalimumab (0.1 μg/mL; right) on TLR7 expression. *P < .03 for HL5B versus HL5B plus inhibitor. (D) Effect of inhibition of NFκB by Ro106-9920 (5μM). Expression of TLR7 (left) and IFNα (right, solid line) or TNFα (right, dotted line). *P < .03, **P < .001 for HL5B versus HL5B plus inhibitor.
Activation of peripheral blood pDCs with aPLs. pDCs were incubated with HL5B, HL7G, RR7F, or irrelevant IgG. (A) Time course (left) and dose response curve after 1 hour (right) are shown. Relative expression of TLR7 mRNA was determined by quantitative RT-PCR normalized to β-actin. Values represent mean ± SD of at least 5 independent experiments. *P < .02 and **P < .001 for aPLs versus IgG. (B) Relative expression of IFNα (left) and TNFα (right) mRNA normalized to β-actin. Values represent mean ± SD of at least 5 independent experiments. *P < .01, **P < .001 for HL5B versus IgG. (C) Effect of inhibition of IFNα by BDCA-2 (1 μg/mL; left) or inhibition of TNFα by adalimumab (0.1 μg/mL; right) on TLR7 expression. *P < .03 for HL5B versus HL5B plus inhibitor. (D) Effect of inhibition of NFκB by Ro106-9920 (5μM). Expression of TLR7 (left) and IFNα (right, solid line) or TNFα (right, dotted line). *P < .03, **P < .001 for HL5B versus HL5B plus inhibitor.
Incubation with HL5B rapidly induced both IFNα and TNFα mRNA (Figure 1B) and protein (supplemental Figure 1B). We therefore asked whether TLR7 mRNA induction is mediated by IFNα or TNFα. Both cytokines can induce TLR7 mRNA when added to pDCs (not shown). When IFNα gene expression was blocked by coincubation with anti-BDCA-2,26 TLR7 induction within the first hour was unchanged but decreased faster than in the absence of anti-BDCA-2 (Figure 1C, supplemental Figure 1C). A similar phenomenon is observed when TNFα was captured in the cell-culture medium by the specific antibody adalimumab (Figure 1C). These experiments demonstrate that early induction of TLR7 mRNA by HL5B is neither mediated by IFNα nor TNFα, whereas the sustained increase in TLR7 expression is at least in part mediated by these cytokines.
In a next step we found that inhibition of NFκB signaling by Ro106-9920 completely abolished the effect of HL5B on TLR7 mRNA induction, indicating that NFκB is required for this process. However, the increase in IFNα expression could not be fully suppressed by Ro106-9920, whereas induction of TNFα expression was again fully dependent on NFκB signaling (Figure 1D, supplemental Figure 1D). In experiments with 2 other inhibitors of NFκB (Bay11-7085 and celastrol), similar results were obtained (data not shown).
Effect of TLR7 inhibitors and ligands on HL5B-induced expression of TNFα and IFNα
To further analyze the mechanisms underlying the induction of TLR7, TNFα, and IFNα by HL5B, we investigated the effects of TLR inhibitors and ligands. Specific inhibition of TLR7 signaling in pDC by IRS661 completely prevented the induction of IFNα and TNFα mRNA and protein expression by HL5B, whereas the TLR9 inhibitor IRS869 had no effect (Figure 2A, supplemental Figure 2A). Neither inhibitor affected the induction of TLR7 mRNA by HL5B (not shown). Vice versa, coincubation with HL5B and R848, a synthetic ligand of TLR7/8, potentiated the individual effects of HL5B and R848 on IFNα and TNFα mRNA expression and release (Figure 2B, supplemental Figure 2B). This finding indicates that the induction of TLR7 mRNA by HL5B is independent of TLR signaling, whereas the induction of IFNα and TNFα mRNA expression fully depends on TLR7 signaling.
Effect of TLR inhibitors and ligands and addition of RNase. (A, B) pDCs were stimulated with HL5B either alone or together with IRS661, IRS869 (each 0.5μM), or R848 (1 μg/mL) as indicated. Relative expression of IFNα and TNFα mRNA normalized to β-actin. All values represent mean ± SD of 5 independent experiments. *P < .05; **P < .005 for HL5B versus HL5B plus inhibitor or activator, respectively. (C) Human pDCs were stimulated with HL5B (100 ng/mL) or a combination of HL5B and R848 (1 μg/mL) either alone or in the presence of RNase (10 μg/mL). RNAse was added 30 minutes before the other stimuli. Quantitative RT-PCR was performed after different periods of time, for up to 6 hours. Relative expression of TLR7 (left) or IFNα (right) was normalized to β-actin. Values represent mean ± SD of at least 5 independent experiments. *P < .01, **P < .005 for HL5B versus HL5B plus RNase.
Effect of TLR inhibitors and ligands and addition of RNase. (A, B) pDCs were stimulated with HL5B either alone or together with IRS661, IRS869 (each 0.5μM), or R848 (1 μg/mL) as indicated. Relative expression of IFNα and TNFα mRNA normalized to β-actin. All values represent mean ± SD of 5 independent experiments. *P < .05; **P < .005 for HL5B versus HL5B plus inhibitor or activator, respectively. (C) Human pDCs were stimulated with HL5B (100 ng/mL) or a combination of HL5B and R848 (1 μg/mL) either alone or in the presence of RNase (10 μg/mL). RNAse was added 30 minutes before the other stimuli. Quantitative RT-PCR was performed after different periods of time, for up to 6 hours. Relative expression of TLR7 (left) or IFNα (right) was normalized to β-actin. Values represent mean ± SD of at least 5 independent experiments. *P < .01, **P < .005 for HL5B versus HL5B plus RNase.
RNase abolishes the effect of HL5B on TNFα and IFNα expression and release
Under the assumption that it is unlikely that HL5B directly activates TLR7 and that cell culture media might always contain low-molecular-weight RNA, ie, ligands for TLR7/8, we performed experiments in the presence of RNase in the culture medium. RNase was added 30 minutes before addition of the aPL. Although RNase had no effect on the induction of TLR7 expression by HL5B, it completely abolished the increase in IFNα and TNFα mRNA and protein expression (Figure 2C, supplemental Figure 2C). If RNase was added at the same time as the antibody, this effect was significantly blunted, indicating that it depends on degradation of RNA present in the culture medium rather than on the mere presence of RNase (not shown). As expected, RNase had no effect on the potentiation of the effects of the synthetic ligand R848, which is not degradable by this enzyme. Similar results were obtained for TLR8 in monocytes (not shown). These data suggest that the induction of IFNα and TNFα mRNA observed after incubation with HL5B depends on RNA present in the cell culture medium. Because the induction of TLR7 mRNA was not affected by RNase, our data indicate that HL5B sensitizes the cells to the effects of natural and synthetic ligands of TLR7/8.
RNA uptake induced by HL5B
To further characterize the mechanisms leading to RNA uptake, we first analyzed whether HL5B can bind to RNA. HL5B did not bind to any nuclear antigens associated with lupus in the conventional diagnostic assays, including assays for antinuclear antibodies, dsDNA, native DNA, Sm, and other extractable nuclear antigens. Addition of RNA to the ELISA assay did not compete binding of HL5B to cardiolipin (not shown). We therefore hypothesized that HL5B may prime cells for uptake of RNA. To test this hypothesis, we incubated human pDCs with HL5B or control IgG in fresh medium for 10 minutes, removed the medium, and washed the cells twice. Thereafter, either fresh medium or the conditioned medium in which the cells had been cultured the before addition of HL5B or control IgG was added to the cells.
Although the addition of fresh medium had no effect on TNFα production of cells incubated with HL5B, the addition of conditioned medium containing RNA induced TNFα significantly (Figure 3A, supplemental Figure 3). Control IgG had no effect in this system. This finding shows clearly that the physical presence of HL5B for RNA uptake is not needed. However, HL5B can apparently prime the cells to internalize RNA. To further substantiate this, we performed the same experiment with fluorescently labeled RNA40. By flow cytometry, we could show that priming with HL5B leads to significant uptake of RNA40 (Figure 3B). When we performed this experiment with CD11c+ cells from TLR7 knockout mice, no uptake of RNA40 could be detected (Figure 3C-D) indicating that uptake in our cell system is dependent on the presence of TLR7.
Analysis of cellular uptake of RNA mediated by HL5B. (A) Human pDCs were stimulated with HL5B or control IgG (100 ng/mL each) in fresh medium for 10 minutes. Thereafter, the medium was removed and the cells were washed twice before adding, either fresh medium or the conditioned medium in which the cells had been cultured before. Relative expression of TNFα mRNA was normalized to β-actin. (B-D) Human pDCs (B) or CD11c+ cells from C57BL/6 (C) or Tlr −/− mice (D) were pretreated for 10 minutes with either HL5B or IgG. Uptake of fluorescently labeled RNA40 was measured by flow cytometry.
Analysis of cellular uptake of RNA mediated by HL5B. (A) Human pDCs were stimulated with HL5B or control IgG (100 ng/mL each) in fresh medium for 10 minutes. Thereafter, the medium was removed and the cells were washed twice before adding, either fresh medium or the conditioned medium in which the cells had been cultured before. Relative expression of TNFα mRNA was normalized to β-actin. (B-D) Human pDCs (B) or CD11c+ cells from C57BL/6 (C) or Tlr −/− mice (D) were pretreated for 10 minutes with either HL5B or IgG. Uptake of fluorescently labeled RNA40 was measured by flow cytometry.
aPL binding to mononuclear cells
Previous attempts to identify the cellular epitope of HL5B by biochemical techniques have been unsuccessful. Therefore, we analyzed cellular binding of FITC-labeled HL5B. For reasons of availability MonoMac1 cells, which respond to HL5B as human blood monocytes (not shown), were used in these experiments. Confocal laser scanning microscopy revealed that MonoMac1 cells internalize HL5B within minutes. The intracellular distribution overlaps with the acidic compartments of the cell, ie, endosomes and lysosomes (Figure 4). Similar data were obtained with human pDC (not shown). This localization is identical to that described for a monoclonal antibody against LBPA.27 Because HL5B also binds to LBPA in an ELISA (supplemental Figure 4A) we assume that HL5B, like other aPLs, is internalized and binds to LBPA in endosomes.28 Interestingly, up-regulation of TLR8 can be induced in MonoMac1 with the commercially available mouse monoclonal antibody against LBPA (6C4; supplemental Figure 4B).27
Uptake of HL5B into cells as analyzed by confocal microscopy. MonoMac1 cells were pretreated for 15 minutes with Lyso-Tracker (50nM) and then incubated with IgG-FITC or HL5B-FITC conjugates (500 ng/mL each). After 7 minutes cells were imaged by confocal fluorescence microscopy under exactly identical instrument settings for all stimuli. To block cellular uptake of HL5B the aPL was coadministered with HLP (250 ng/mL), anti-FcγRII (0.5 μg/mL), or MβCD (1mM) as indicated.
Uptake of HL5B into cells as analyzed by confocal microscopy. MonoMac1 cells were pretreated for 15 minutes with Lyso-Tracker (50nM) and then incubated with IgG-FITC or HL5B-FITC conjugates (500 ng/mL each). After 7 minutes cells were imaged by confocal fluorescence microscopy under exactly identical instrument settings for all stimuli. To block cellular uptake of HL5B the aPL was coadministered with HLP (250 ng/mL), anti-FcγRII (0.5 μg/mL), or MβCD (1mM) as indicated.
Coincubation with HLP, a peptide specific for the antigen binding site of HL5B,29 abrogated intracellular accumulation of HL5B (Figure 4), indicating that it depends on the antigen binding domain of the aPL. In addition, antibodies against the Fc-γ-receptor (FcγR)-II (Figure 4) but not against FcγRI or FcγRIII (not shown), completely block uptake of HL5B. Similarly, substances interfering with raft structure, such as MβCD (Figure 4) or nystatin, inhibit uptake of HL5B. Thus, accumulation of HL5B in the endosomal compartment depends on its antigen binding site and interaction with FcγRII and rafts. Functional analysis showed that all agents inhibiting endosomal accumulation of HL5B also inhibited induction of TLR8 expression (supplemental Figure 4C). The results with different inhibitors suggest that, indeed, uptake into the endosome rather than binding to an unidentified cell surface structure is critical for the induction of cellular effects by HL5B.
Induction of superoxide generation in endosomes by aPLs
Endosomal superoxide production has recently been described as signaling mechanism.22,30 We therefore analyzed whether HL5B could induce the production of superoxide or other ROS in endosomes. When MonoMac1 cells were loaded with the ROS-sensitive dye L-012 and stimulated with HL5B, a dose-dependent intracellular ROS generation was observed, whereas the control IgG had no effect (Figure 5A). Neither antibody had an effect on extracellular ROS as determined by incubation with luminol/HRP (Figure 5B). When the inhibitors of ROS generation apocynin and allopurinol were added to the incubation, only the NADPH oxidase inhibitor apocynin but not the xanthineoxidase inhibitor allopurinol reduced HL5B-induced ROS generation (Figure 5C). These data indicate that superoxide generation by NADPH oxidase was the basis for the intracellular ROS signal. This finding is further supported by the complete suppression of the HL5B effect by exogenous PEG-SOD in the cell culture medium, which is known to enter the endosomal route (Figure 5C).
Induction of ROS by HL5B. HL5B or IgG were added to MonoMac1 cells in the concentrations indicated. (A) Induction of intracellular ROS production as determined by L-012 chemiluminescence. (B) Induction of extracellular ROS production as determined by luminol/HRP chemiluminescence. (C) Effects of apocynin (100μM), PEG-SOD (100 U/mL), and allopurinol (100μM) on intracellular ROS production. (D) Quantification by HPLC of 2HE and ethidium generated in response to incubation with HL5B and IgG. (E) Flow cytometric analysis of ROS production induced by HL5B and the effect of inhibitors. Cells were loaded for 2 minutes with H2HFF-BSA at 37°C followed by incubation with HL5B (100 ng/mL) alone or with NFA (0.1mM), PEG-SOD (500 U/mL) or NAC (10mM) as indicated. Tert-butyl-hydroperoxide (25μM) serves as a positive control. Means ± SD of MFI values of 5 independent experiments are presented. *P < .005, **P < .001 for HL5B versus HL5B plus inhibitor. (F) Relative expression of TLR8 normalized to β-actin. Values represent mean ± SD of at least 5 independent experiments. *P < .005, **P < .001 for HL5B versus HL5B plus inhibitor. (G) ROS generation analyzed by confocal microscopy. MonoMac1 cells were loaded for 2 minutes with H2HFF-BSA and stimulated for 15 minutes with HL5B or IgG (500 ng/mL each) and imaged by confocal fluorescence microscopy with identical instrument settings. The acidic organelles were visualized by the use of Lyso-Tracker (50nM). Fluorescent H2HFF-BSA indicative of ROS production was only observed after incubation with HL5B. The majority colocalized with LysoTracker (arrows). ROS production could be almost completely blocked by incubation with PEG-SOD (500 U/mL).
Induction of ROS by HL5B. HL5B or IgG were added to MonoMac1 cells in the concentrations indicated. (A) Induction of intracellular ROS production as determined by L-012 chemiluminescence. (B) Induction of extracellular ROS production as determined by luminol/HRP chemiluminescence. (C) Effects of apocynin (100μM), PEG-SOD (100 U/mL), and allopurinol (100μM) on intracellular ROS production. (D) Quantification by HPLC of 2HE and ethidium generated in response to incubation with HL5B and IgG. (E) Flow cytometric analysis of ROS production induced by HL5B and the effect of inhibitors. Cells were loaded for 2 minutes with H2HFF-BSA at 37°C followed by incubation with HL5B (100 ng/mL) alone or with NFA (0.1mM), PEG-SOD (500 U/mL) or NAC (10mM) as indicated. Tert-butyl-hydroperoxide (25μM) serves as a positive control. Means ± SD of MFI values of 5 independent experiments are presented. *P < .005, **P < .001 for HL5B versus HL5B plus inhibitor. (F) Relative expression of TLR8 normalized to β-actin. Values represent mean ± SD of at least 5 independent experiments. *P < .005, **P < .001 for HL5B versus HL5B plus inhibitor. (G) ROS generation analyzed by confocal microscopy. MonoMac1 cells were loaded for 2 minutes with H2HFF-BSA and stimulated for 15 minutes with HL5B or IgG (500 ng/mL each) and imaged by confocal fluorescence microscopy with identical instrument settings. The acidic organelles were visualized by the use of Lyso-Tracker (50nM). Fluorescent H2HFF-BSA indicative of ROS production was only observed after incubation with HL5B. The majority colocalized with LysoTracker (arrows). ROS production could be almost completely blocked by incubation with PEG-SOD (500 U/mL).
To further characterize the intracellular ROS signal induced by HL5B, cells were incubated with DHE and the production of ethidium, and 2HE was quantified by HPLC (supplemental Figure 5). In the presence of HL5B in the cell-culture medium, the intracellular concentration of 2HE is significantly greater than in the presence of control IgG (Figure 5D). Because 2HE is only generated in the presence of superoxide, this proves that the initial ROS generated in response to HL5B is superoxide.31
Endosomal superoxide production has been shown to depend on the activity of an endosomal anion channel, chloride channel 3.22,30 We therefore analyzed whether anion channel inhibitors, eg, NFA, inhibit the ROS signal. This was performed by flow cytometry with the redox-sensitive fluorescent dye H2HFF-BSA. As expected, no ROS production could be induced by HL5B in the presence of NFA or other inhibitors (Figure 5E). In further experiments we could show that incubation with NFA or ROS scavengers such as PEG-SOD or NAC abolished the induction of TLR8 mRNA by HL5B (Figure 5F). Similarly, the induction of TNFα production was completely inhibited in MonoMac1 cells (supplemental Figure 6). Similar results could be observed with TLR8 in human monocytes and TLR7 in pDC (not shown).
Finally, we wanted to unequivocally localize superoxide production to the endosome. To this end, cells were loaded with H2HFF-BSA and the induction of ROS generation was followed by confocal microscopy. These experiments showed that ROS generation was indeed localized in an acidic compartment of the cell, most likely the endosome (Figure 5G).
Effect of aPLs on translocation of TLR7/8 from the ER to the endosome
As shown by the RNase experiments, HL5B does not induce TLR signaling itself but sensitizes the cells to TLR7/8 ligands. These data might be explained if aPLs induce translocation of TLR7 and TLR8 from the ER to the endosome. This would sensitize cells to natural or synthetic ligands of these TLRs. This hypothesis would also explain our observation that inhibition of NFκB can fully block the production of TNFα but only partially block the early production of IFNα in response to HL5B (Figure 1D). TLR7 induces IFNα production by an NFκB-independent pathway from the early endosome.32 If we assume that HL5B translocates TLR7/8 to the endosome where they are activated by RNA present in the cell culture medium, NFκB-independent generation of IFNα would be expected. This should be suppressed by RNase treatment, which is indeed the case (Figure 2C).
To visualize the localization of TLR8, MonoMac1 cells were incubated with a fluorescently labeled antibody against TLR8 and a marker for early endosomes (EEA1) or for the ER (calnexin), respectively. In resting cells or cells incubated with control IgG, there was significant overlap between TLR8 and calnexin, whereas there was no colocalization of TLR8 and EEA1 (Figure 6A). When cells were incubated with HL5B, there was no longer colocalization of TLR8 and calnexin. Instead, colocalization of TLR8 and EEA1 could be detected (Figure 6B). This experiment provides evidence that HL5B induces translocation of TLR8 from the ER to the endosome.
aPLs induce translocation of TLR8. Confocal images of MonoMac1 cells stained with anti-TLR8 (red) and anti-calnexin or anti-EEA1 (green). Nuclei were visualized with DAPI (blue). Before fixation cells were either stimulated with IgG or HL5B (100 ng/mL) for 30 minutes. In cells incubated with IgG (A) TLR8 colocalized with calnexin but not with EEA1. In addition, some TLR8 did not colocalize with either marker. In cells incubated with HL5B (B) colocalization of TLR8 with calnexin was not detectable, whereas there was significant overlap with EEA1. Again, some TLR8 did not colocalize with either marker. To allow better appreciation of the cellular features, a montage of representative cells from the same experiment on the same slide was prepared.
aPLs induce translocation of TLR8. Confocal images of MonoMac1 cells stained with anti-TLR8 (red) and anti-calnexin or anti-EEA1 (green). Nuclei were visualized with DAPI (blue). Before fixation cells were either stimulated with IgG or HL5B (100 ng/mL) for 30 minutes. In cells incubated with IgG (A) TLR8 colocalized with calnexin but not with EEA1. In addition, some TLR8 did not colocalize with either marker. In cells incubated with HL5B (B) colocalization of TLR8 with calnexin was not detectable, whereas there was significant overlap with EEA1. Again, some TLR8 did not colocalize with either marker. To allow better appreciation of the cellular features, a montage of representative cells from the same experiment on the same slide was prepared.
IgG fractions from APS patients induce TLR7
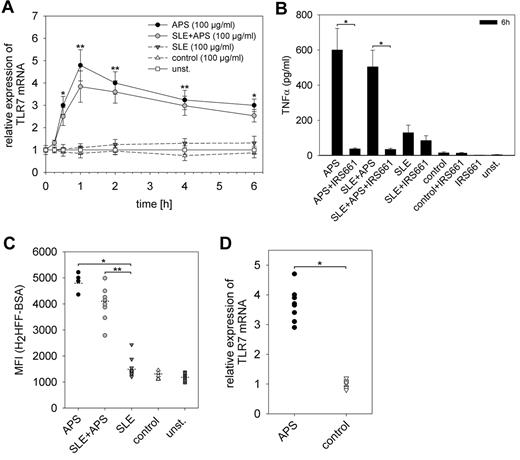
To clarify whether the stimulatory activity of the 3 monoclonal aPLs is representative for the heterogeneous group of aPL, we performed similar stimulation assays with IgG fractions from APS patients. These included 4 patients with the primary APS (m/f = 1/3; mean age 51 years, range 39-72 years) and 10 patients with secondary APS (m/f = 2/8; mean age 42 years, range 21-54 years) according to the Sapporo criteria.1 The latter all had SLE as the underlying autoimmune disease. As comparison a group of 10 SLE patients without discernible aPL or lupus anticoagulant were analyzed (m/f = 2/8; mean age 44.5 years, range 28-64 years). In addition, 10 healthy control probands were analyzed (m/f = 2/8; mean age 37 years, range 24-65 years). IgG fractions of APS patients induced an increased expression of TLR7 in human pDC irrespective of the presence of SLE (Figure 7A). This is accompanied by enhanced TNFα release, which is blocked by the specific TLR7 inhibitor IRS661 (Figure 7B). In contrast, stimulation with IgG fractions of patients with SLE without discernable aPLs did not induce TLR7 mRNA (not shown) and only a weak increase in TNFα production, which could not be fully reversed by IRS661. IgG from healthy control subjects did not show any significant stimulatory effect (Figure 7A-B). As expected, incubation with APS-IgG induced intracellular ROS production, whereas SLE-IgG had again only mild activity (Figure 7C).
IgG fractions and PBMCs of APS patients. Human pDCs were stimulated with IgG fractions of 4 patients with primary APS, 10 patients with secondary APS and SLE, and 10 healthy control donors. (A) Real-time RT-PCR after stimulation for 15 minutes to 6 hours. TLR7 mRNA expression was normalized to β-actin. Values represent mean ± SD of 5 independent experiments with all 24 IgG fractions *P < .05; **P < .005 for APS or SLE + APS versus SLE. (B) TNFα secretion was measured by the use of ELISA. Inhibition of TLR7 signaling was performed using IRS661 as described previously. *P < .005. (C) Production of ROS was determined by the use of flow cytometry as described in Figure 7E. *P < .005, **P < .02. (D) TLR7 mRNA levels in PBMCs from 8 different APS patients and 8 healthy controls were measured as duplicates by real-time RT-PCR. TLR7 mRNA was normalized to the expression of β-actin. *P < .005.
IgG fractions and PBMCs of APS patients. Human pDCs were stimulated with IgG fractions of 4 patients with primary APS, 10 patients with secondary APS and SLE, and 10 healthy control donors. (A) Real-time RT-PCR after stimulation for 15 minutes to 6 hours. TLR7 mRNA expression was normalized to β-actin. Values represent mean ± SD of 5 independent experiments with all 24 IgG fractions *P < .05; **P < .005 for APS or SLE + APS versus SLE. (B) TNFα secretion was measured by the use of ELISA. Inhibition of TLR7 signaling was performed using IRS661 as described previously. *P < .005. (C) Production of ROS was determined by the use of flow cytometry as described in Figure 7E. *P < .005, **P < .02. (D) TLR7 mRNA levels in PBMCs from 8 different APS patients and 8 healthy controls were measured as duplicates by real-time RT-PCR. TLR7 mRNA was normalized to the expression of β-actin. *P < .005.
TLR7 mRNA expression in PBMCs of APS patients
If aPLs induce TLR7 expression, one might expect an increased expression of TLR7 mRNA in the PBMCs of APS patients. TLR7 mRNA expression ratio was measured in PBMCs in a subgroup of the patients with APS and healthy blood donors. There was a significant increase of TLR7 mRNA expression level in PBMCs of APS patients in comparison with healthy control patients (Figure 7D). This finding provides evidence that the observed effects of aPL in vitro are relevant for the in vivo situation of APS patients and may participate in the pathogenesis of the APS.
Discussion
We describe here in the context of the APS a novel signal transduction pathway that originates from activation of endosomal NADPH oxidase and leads to a dramatic sensitization of pDC and monocytes toward ligands for TLR7 and TLR8. Besides its obvious relevance for the understanding of the APS, this novel pathway has also significant implications for innate immunity and autoimmunity in general. It is involved in the control of intracellular distribution and gene expression of TLR7 in pDC and TLR8 in monocytes. Human aPLs are able to activate this pathway. The exaggerated response to ligands for TLR7 and TLR8 could explain the proinflammatory and procoagulant effects observed with aPL. For example, TNFα, which is induced by aPL in our cell culture experiments, is long known to induce tissue factor expression in monocytes or endothelial cells.33
We would like to stress the fact that the monoclonal aPL HL5B used in our investigation does not bind β2GPI, nor is it dependent on this protein cofactor. The highly related monoclonal aPL HL7G, which differs only in few amino acids, acquired the capability to bind β2GPI but retained its cardiolipin binding.13,14 A third monoclonal aPL, RR7F, binds also exclusively to anionic phospholipids without the need for a protein cofactor. It is very similar to a known germline sequence and has the same effects in our system, indicating that antigen maturation may not be a precondition for activation of TLR7/8. This also underscores the fact that antibodies binding solely to anionic phospholipids definitively can have proinflammatory properties.
Another important aspect relating to the binding specificity of the monoclonal aPL is the fact that they do not bind to RNA or RNA-protein complexes. The uptake of RNA induced by the aPL is not dependent on their physical presence. Our data provide evidence that aPL prime cells to internalize RNA and that this depends on the presence of TLR7 because pDCs isolated from Tlr7−/− mice do not internalize RNA after incubation with HL5B. We propose that similar to TLR923 TLR7 also appears in the cell membrane during translocation to the endosome and can thereby internalize its ligand.
Our data confirm previous data that aPLs can accumulate in the endosomal compartment,27,28 supporting the relevance of lipid antigens in autoimmunity. We can now show that aPLs induce endosomal NADPH oxidase to generate superoxide. Currently, the NADPH oxidases NOX1 and NOX2 have been found in endosomes.34 Interestingly, besides its function in bacterial killing, NOX2 has been shown to be involved in the regulation of antigen processing in dendritic cells by controlling phagosomal pH.35 Thus, fine regulation of the activity of these enzymes may be of profound relevance for innate and adaptive immunity. Dendritic CD11c+ cells isolated from spleens of mice with a targeted deletion of gp91phox the catalytic subunit of NOX2 are unable to respond to stimulation with aPL (N.P., K.J.L., unpublished observations, April 2011). The exact mechanism of how aPLs activate endosomal NADPH oxidase is not known but is likely to be related to interaction of the antibodies with endosomal lysobisphosphatidic acid. Activation of NADPH oxidase leads to activation of NFκB and a subsequent increase in the expression of the genes for TLR7 and TLR8.
Although the activation of NFκB by superoxide is not surprising, the involvement of ROS in the translocation of TLRs from the ER to the endosome was unexpected. The latter process is obviously independent of NFκB activation. Translocation of TLR7/8 to the endosome dramatically sensitizes cells to natural ligands of these TLRs, ie, single-stranded RNA. Because at the same time gene expression of TLR7/8 is increased, the overall effect is a substantial and inappropriate sensitization of the cells. This is likely to lead to an inflammatory response to stimuli that are usually subthreshold or to an exaggerated response to an appropriate stimulus as may be present during viral or bacterial infection. In this context, it is of interest that infections are known to precipitate the so called “catastrophic APS” an acute APS-like syndrome with severe, widespread microthrombosis.36
The broader in vivo relevance of our findings has been underlined by the observation that IgG fractions from patients with the APS can activate this novel pathway. More importantly, we could show that PBMCs from patients with the APS have an increased expression of TLR7. This is analogous to our previous observation of an increased expression of TLR8 in PBMC of APS patients.10 This is strong evidence that the up-regulation of the TLR7/8 system is not a mere cell culture phenomenon but also present in vivo.
Translocation of endosomal TLRs 3, 7, 8, and 9 from the ER to the endolysosome has been described recently. Among others, the process is dependent on the membrane protein UNC93B1 and the ER-resident chaperone gp96.37,38 However, little is known about the regulatory control of translocation of TLRs besides the fact that TLR ligands, eg, ssRNA or DNA, can induce receptor translocation. Here, we show that aPLs that are not TLR ligands are able to induce translocation of TLR7/8 to the endosome and that this is dependent on the activation of endosomal NADPH oxidase and superoxide production. Inhibitors of endosomal superoxide production fully block all effects of the aPLs, indicating that all other effects observed, for example, the NFκB-dependent induction of TLR7/8 expression, are downstream from the activation of NADPH oxidase in the endosome. It should be mentioned here that our data also support the notion that superoxide is instrumental for TLR translocation induced by the TLR7/8 ligand R848 because we can inhibit TNFα and IFNα production by blocking endosomal superoxide production with the anion channel blocker NFA (not shown).
Another intriguing result of our study is the massive sensitization of the TLR7/8 axis caused by aPLs. Many data in the literature support the notion that TLR7 and TLR9 responses to self-nucleotides predispose to autoimmune disease.17,39 Mice overexpressing TLR7 because of a duplication of the gene are prone to the development of SLE,40,41 which has led to the hypothesis that sequestration of intracellular TLRs to the ER protects the immune system from activation by self-nucleotides.32,38,42 Our results show that aPLs are able to break this mechanism of self-tolerance and that APS patients show increased expression of TLR7 in vivo. This finding implies that aPLs could be much more relevant in the initiation and/or propagation of SLE in humans than previously thought. The frequent finding of aPLs in SLE patients, which are usually considered secondary to SLE, would have to be interpreted in a different light
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a grant from the Stiftung Pathobiochemie und Molekulare Diagnostik of the German United Society of Clinical Chemistry and Laboratory Medicine.
Authorship
Contribution: N.P. performed and planned most of the experiments, with technical help from I.P., M.L., and A. Degreif; N.C. performed a series of seminal experiments in the early phase of the project; D.S. provided the methodology for confocal microscopy; A. Daiber planned and performed the photometry and HPLC experiments for ROS detection; P.S., M.R., H.S., and S.B. provided many helpful suggestions and methods for the project; P.v.L. and K.J.L planned and supervised all experimentation; and N.P. and K.J.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl J. Lackner, Institute of Clinical Chemistry and Laboratory Medicine, University Medical Centre Mainz, Langenbeckstr 1, D-55131 Mainz, Germany; e-mail: karl.lackner@unimedizin-mainz.de.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal