In this issue of Blood, Schaniel and colleagues demonstrate that combined disruption of canonical and noncanonical Wnt signaling in the hematopoietic stem cell (HSC) niche results in a loss of quiescent HSCs and exhaustion of their self-renewal capacity in young adult mice under conditions of systemic stress.1
Hematopoietic stem cells give rise to our entire blood cell repertoire throughout adult life, and hence the balance of self-renewal and differentiation of HSCs needs to be tightly controlled. Intrinsic signals and signals from the HSC niche microenvironment, including osteoblasts, are key to maintaining this balance.2 The Wnt signaling pathway has been repeatedly implicated in HSC fate decisions; however, its role remains controversial.
The Wnt system involves a large network of proteins that mediate signaling through canonical or noncanonical pathways. Wif1 is an antagonist of both pathways, acting to sequester secreted Wnt proteins in the extracellular space, preventing their binding to Frizzled receptors.3 Here, Schaniel and colleagues identified that Wnt signaling molecules were enriched in the HSC-supporting stromal cell line, AFT024, compared with nonsupporting stromal cell lines. They therefore overexpressed Wif1 in AFT024 cells, which resulted in a significant impairment in their potential to support cobblestone area forming cells (CAFCs), which are a surrogate measure of HSCs.
To further investigate the role of Wif1 in the HSC niche, Schaniel et al generated transgenic (Tg) mice in which Wif1 was overexpressed specifically in a relatively mature population of osteoblasts. Wif1 Tg mice showed no change in bone length, diaphysis width, or cortical bone thickness. It was unclear, however, if trabecular bone parameters or the numbers or activities of cells involved in regulating bone formation (in particular osteoblasts and osteoclasts) were altered in Wif1 Tg mice compared with wild-type (WT) mice.
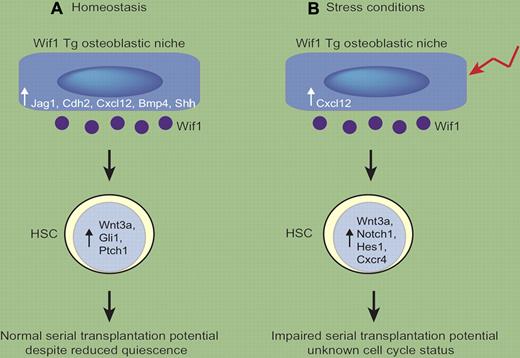
Wif1 Tg mice had normal content of mature hematopoietic cells in their bone marrow. In contrast, significant increases in immunophenotypical populations of primitive HSCs and progenitor cells were evident in the bone marrow and spleen of 8- to 12-week-old Wif1 Tg mice. Interestingly, however, the differences were only obvious when particular cell immunophenotypes were used (LSK, CD48, CD34, and Flk2) and lost when CD150 was used for additional analysis. Analysis of cell-cycle status of the LSKCD48 cells revealed that there were significantly fewer of these cells in the quiescent G0 phase, accompanied by increased numbers of cycling G1 cells in Wif1 Tg mice compared with WT mice. Surprisingly, however, HSCs obtained from Wif1 Tg mice had similar serial transplantation potential compared with those obtained from WT mice (see figure panel A).
Osteoblastic overexpression of Wif1 alters HSC potential in a context-dependent manner. (A) In Wif1 Tg mice in homeostatic conditions, osteoblasts have increased expression of HSC regulatory molecules such as Jag 1, Cdh2, Cxcl12, Bmp4, and Shh. HSCs have elevated expression of Wnt3a, Gli1, and Ptch1. Increased numbers of cycling HSCs are observed but the HSCs have normal serial transplantation potential. (B) In stress situations (such as 5-FU), osteoblastic expression of HSC-regulatory molecules is reduced in Wif1 Tg mice compared with that expressed in homeostasis (with the exception of Cxcl12, which is maintained). Wif1 Tg HSCs maintain high expression of Wnt3a, but down-regulate Gli1 and Ptch1 and up-regulate expression of Notch1, Hes1, and Cxcr4. The cell-cycle status of the HSCs was not determined, but HSCs lose their serial transplantation ability.
Osteoblastic overexpression of Wif1 alters HSC potential in a context-dependent manner. (A) In Wif1 Tg mice in homeostatic conditions, osteoblasts have increased expression of HSC regulatory molecules such as Jag 1, Cdh2, Cxcl12, Bmp4, and Shh. HSCs have elevated expression of Wnt3a, Gli1, and Ptch1. Increased numbers of cycling HSCs are observed but the HSCs have normal serial transplantation potential. (B) In stress situations (such as 5-FU), osteoblastic expression of HSC-regulatory molecules is reduced in Wif1 Tg mice compared with that expressed in homeostasis (with the exception of Cxcl12, which is maintained). Wif1 Tg HSCs maintain high expression of Wnt3a, but down-regulate Gli1 and Ptch1 and up-regulate expression of Notch1, Hes1, and Cxcr4. The cell-cycle status of the HSCs was not determined, but HSCs lose their serial transplantation ability.
The most dramatic effects on HSCs were observed in the context of stress situations, when Wif1 Tg mice were subjected to myeloablative therapies. Control mice, but not Wif1 Tg mice, survived repeated rounds of treatment with the chemotherapeutic agent 5-fluorouracil (5-FU), which kills dividing cells. Furthermore, WT HSCs harvested 4 months after transplantation into lethally irradiated Wif1 Tg mice had reduced capacity to repopulate secondary WT recipients compared with HSCs obtained from WT primary recipients (see figure panel B). Whether a longer exposure of HSCs to a Wif1 Tg environment under homeostatic conditions impacts on HSC repopulating potential remains to be determined. Alternatively, the Wif1 Tg microenvironment may have an altered response to stress situations such as irradiation and 5-FU, which in turn may have caused the HSC defects observed. Both of these possibilities are worthy of further exploration.
Interestingly, inhibition of the Wnt pathway in osteoblasts significantly altered expression of other signaling pathways in osteoblasts and HSCs. Furthermore, this response was significantly impaired after 5-FU treatment. Molecules known to play roles in the microenvironmental control of HSC quiescence, retention, and homing activities (Bmp4, Cdh2, Jag 1, Cxcl12, and Shh) were up-regulated in osteoblasts isolated from Wif1 Tg mice, leading Schaniel et al to hypothesize the osteoblastic HSC niche was trying to compensate for the Wif1-mediated loss of HSC quiescence. Furthermore, HSC populations obtained from Wif1 Tg mice had elevated transcript expression of the Shh pathway transcripts Gli1 and Ptch1. Strikingly, Wif1 overexpression in the osteoblasts activated autocrine Wnt signaling in HSCs, likely because of elevated expression of Wnt3a in these cells.
Interestingly, the up-regulated expression of HSC niche-associated transcripts (Jag1, Cdh2, Bmp4, and especially Shh) was markedly reduced in osteoblasts obtained from Wif1 Tg mice after 5-FU treatment, further indicating that Wif1 Tg osteoblasts may have an altered response to stress signals. Interestingly, the expression of Wnt3a in HSCs isolated from 5-FU-treated Wif1 Tg mice did not change compared with HSCs obtained from nontreated Wif1 Tg mice. In contrast, 5-FU treatment resulted in down-regulation of Gli1 and Ptch1, although expression of these remained higher than those obtained from 5-FU-treated WT mice. Furthermore, expression of Cxcr4, Notch1, and its target Hes1 were significantly up-regulated in HSCs isolated from 5-FU-treated Wif1 Tg mice.
The deregulated expression of Shh pathway targets in Wif1 Tg HSCs is interesting. A series of studies has indicated that Shh signaling increases proliferation of HSCs, which may account for the HSC phenotype observed in homeostatic Wif1 Tg mice. In this regard it is of interest to determine whether HSCs from 5-FU–treated Wif1 Tg mice had similar cell-cycle kinetics to those in homeostasis. Further studies will be required to delineate the involvement of Shh (and other deregulated pathways) in the osteoblast (and in turn) HSC phenotypes in these mice, especially in stress situations.
This report by Schaniel and colleagues adds to the complexity of the roles of the Wnt signaling pathway in regulating hematopoiesis. Overexpression of the canonical Wnt-signaling antagonist Dkk1 in osteoblasts diminished quiescent HSCs and inhibited their self-renewal capacity.4 Unlike the HSCs from Wif1 Tg mice, the long-term repopulation and serial transplantation capacity of Dkk1 Tg HSCs was not maintained. The HSCs from Dkk1 Tg mice also showed reduced Wnt signaling, in direct contrast to HSCs obtained from Wif1 Tg mice, which had elevated Wnt signaling. However, Dkk1 Tg mice also had a distinct bone phenotype, which may have contributed to the HSC defects observed in these mice. Sfrp1 is another member of the Wnt pathway that acts to sequester Wnts, and can activate Frizzled receptors in a noncanonical manner.3,5 Interestingly, mice deficient in Sfrp1 had increased numbers of quiescent HSCs, and this was mediated by the microenvironment.6
Schaniel et al have provided us with new insight into Wnt signaling in the osteoblastic HSC niche and its effects on HSCs. In light of previous publications on Wnt signaling and hematopoiesis, it is interesting to note that molecules within the same signaling pathway can have varying effects on the HSC microenvironment and in turn HSCs, and highlights the necessity to dissect the role of all components within a signaling pathway.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal