Abstract
Exposure to air pollution is associated with adverse effects on health. In particular, a strong epidemiologic association is observed between acute and chronic exposures to particulate matter and the occurrence of cardiovascular events, coronary artery disease, cerebrovascular disease and venous thromboembolism, especially among older people and people with diabetes and previous cardiovascular conditions. Multiple mechanisms have been postulated to cause the increase in atherothrombotic and thromboembolic events, including the activation by particulate matter of inflammatory pathways and hemostasis factors, production of reactive oxygen species through the oxidative stress pathway, alterations in vascular tone, and decreased heart rate variability (a marker of cardiac autonomic dysfunction and a predictor of sudden cardiac death and arrhythmias). Current knowledge on the biologic mechanisms and the clinical effect of short- and long-term exposure to particulate air pollutants is discussed, emphasizing that life expectancy improved significantly in sites where air pollutants were controlled.
Introduction
Urban air pollution is a leading problem for environmental health.1 Even though much of the early research focused on respiratory diseases, more recent epidemiologic studies have shown that air pollution substantially contributes to the onset and aggravation of cardiovascular diseases and that these effects occur at relatively low levels of exposure.2-4 Air pollution definitely increases rates of morbidity and mortality because of atherothrombotic events, causes more frequent hospitalizations, and worsens preexisting cardiac diseases (such as congestive heart failure and arrhythmias).5 In addition, recent preliminary findings suggest an association between air pollution and venous thromboembolism.3 In line with these observations, the World Health Organization (WHO) calculates that urban air pollution is an important cause of global mortality, being responsible for ≥ 700 000 premature deaths.6 For the person, the risk of cardiovascular disease triggered by air pollution is relatively small. However, the public health burden is very important, because of the unavoided exposure of large populations to this risk.7
Both gaseous air pollutants (ie, ozone, sulfur and nitric oxides, carbon monoxide) and particulate matter (PM) cause adverse effects on health. However, the most serious effects are related to PM, because particles contain a broad range of toxic substances and are considered reliable indicators of other pollutants (such as nitric oxides) and hence of the global adverse effect of air pollution.8-10 Ambient PM is distinguished, according to aerodynamic diameter (AD), in coarse (PM10, AD between 2.5 and < 10 μm), fine (PM2.5, AD < 2.5 μm), and ultrafine (AD < 0.1 μm) particles. On inhalation, the coarser particles reach only the nasal cavities and upper airways, but fine particles reach the lower airways and alveoli. Composition of PM varies greatly and depends on geographic, meteorologic, and source-specific variables. In metropolitan areas, primary particles directly emitted in the ambient are a complex and heterogeneous mixture of solid and liquid components, including inorganic substances (sulfates, nitrates, ammonium, chloride, metals), elemental and organic carbon, crustal material, and biologic components (bacteria, spores, pollens).11 In addition, chemical conversion of gaseous species to aerosol particles in the atmosphere leads to the formation of secondary particles. In metropolitan areas most environmental PM is anthropogenic and originates from the combustion of fossil fuels, mainly from automobile traffic and domestic heating.10 There are also soil-related sources of PM that originate from road dust, smokestacks, and windblown soil. The latter source can lead to the phenomenon of long-range transport and spread of airborne-suspended PM.
This review focuses on the relationship between ambient air pollution, thrombogenicity, and cardiovascular diseases through the analysis of the putative biologic mechanisms and the strength of the epidemiologic evidence.
Biologic mechanisms
Even though the mechanisms by which air pollutants influence the risk of cardiovascular events are still under investigation, a number of plausible explanations are available.10 One possible pathway involves the finest particles (< 2.5 nm) that on inhalation penetrate beyond the upper respiratory tract into the parenchymal region of the lung, where they may be translocated into the circulating blood by interstitium, macrophage phagocytosis, as well as epithelial cell endocytosis.12 After PM inhalation, the lung releases locally and into the systemic circulation pro-oxidative (ie, reactive oxygen species) or proinflammatory (ie, cytokines such as IL-6 and TNF-α) mediators or both and vasoactive hormones such as endothelins.13-20 Acute-phase reactions take place and lead to the increase in plasma of such reactants as C-reactive protein and fibrinogen. In the lung the secretion of adhesion molecules by inflamed endothelial cells results in binding and activation of leukocytes and platelets, formation of platelet-leukocyte conjugates, and generation of hemostatically active, tissue factor-bearing microparticles.21-23
A series of studies conducted in animals have shown that acute exposure to proxies of particulate air pollutants leads to the activation of platelets and coagulation enzymes.23 Nemmar et al24 found that in hamsters the intratracheal instillation of ultrafine polystyrene particles enhanced thrombus formation, mainly through a mechanism involving platelet activation. In subsequent experiments that used diesel exhaust as proxy of ambient PM heightened thrombus formation did follow particle penetration into the circulating blood.25 In another study a persistent increase in thrombosis susceptibility to diesel exhaust particles was seen after as long as 24 hours. This prolonged effect was mitigated by pretreatment with sodium cromoglycate (a stabilizer of mast cells and basophil degranulation), suggesting that thrombosis was secondary to histamine release.26 These data provided evidence for a direct link among diesel exhaust, particle-induced release of histamine, and pulmonary inflammation, which in turn caused thrombosis through systemic inflammation and platelet activation. However, pulmonary inflammation and thrombosis appeared to be associated events only at the latest time points, because histamine was increased in plasma only 6 and 24 hours after exposure to diesel exhaust, and diphenhydramine (a histamine H1-receptor antagonist) mitigated thrombosis at 6 and 24 hours but not at 1 hour.27 It was hypothesized that direct effects of PM constituents reaching the circulation may be responsible for the earliest prothrombotic effects. Pulmonary instillation of carbon nanotubes triggered neutrophil lung influx, and circulating platelet-leukocyte conjugates were elevated 6 hours after exposure, whereas procoagulant microvesicular tissue factor activity and the peripheral thrombotic potential were increased 24 hours later.27 Inhibition of P-selectin (which mediates the adhesion of activated platelets to circulating leukocytes) abrogated these responses, showing that rapid activation of circulating platelets by the pulmonary deposition of PM plays a crucial role.28
This series of experimental studies suggests that the delayed release in the lung of cell-derived mediators (eg, histamine), together with the earlier activation of circulating platelets by lung inflammation by P-selectin–dependent mechanisms, may mediate the prothrombotic effects of the proxies of particulate air pollution. It must be emphasized that the particles used in these studies do reproduce the situation of exposure to ambient PM in terms of size but not in composition, because ambient PM contains an array of toxic substances that cause adverse effects well beyond their size. For instance, the content of transition metals such as iron may be particularly toxic through the oxidative stress pathways, because they yield highly reactive oxygen species, including the hydroxyl radical through the Fenton reaction.
In humans, increased exposure to air pollution is associated with the increase in plasma of several hemostasis components. For instance, in 3256 randomly selected people plasma viscosity increased markedly during an air pollution episode that lasted 13 days.29 Healthy volunteers experimentally exposed to concentrated PM had increased plasma levels of fibrinogen, an acute-phase inflammatory protein, an important determinant of increased viscosity and an established risk factor for venous and arterial thromboses.18 Another marker of thrombophilia such as plasma homocysteine is positively associated with exposure to traffic-related pollutants, especially PM and black carbon.30 A study conducted in 1218 healthy persons found that the degree of chronic exposure to air pollution was associated with the shortening of such a global coagulation test as the prothrombin time.31 The same study confirmed that homocysteine increases in proportion to the degree of exposure to gaseous and particulate air pollutants, albeit only in smokers.32 Bonzini et al33 demonstrated heightened thrombin formation in workers from a steel-production plant exposed to high concentrations of inhalable particles. In these workers the link between inflammation and hypercoagulability was emphasized by the concomitant increase in C-reactive protein.33
The fibrinolytic system is an important regulator of thrombus formation, propagation, and vascular occlusion. In a series of double-blind experiments performed by Mills et al,34-36 healthy persons as well as patients with stable coronary artery disease were randomly exposed to filtered air or high concentrations of diesel exhaust (300 μg/mm3) while performing intermittent exercise. The release from endothelial cells of tissue plasminogen activator, a key player in the regulation of endogenous fibrinolysis, was reduced on exposure to diesel exhaust. This antifibrinolytic effect persisted for ≥ 6 hours after exposure and was of similar magnitude to that seen in heavy smokers.
On the whole, these findings in humans indicate that secondary pulmonary inflammation or microparticles penetrating directly into the blood or both cause a hypercoagulable state and hypofibrinolysis, that in turn may trigger thrombotic cardiovascular events because of heightened thrombus formation in blood vessels.37
Another mechanism for the occurrence of cardiovascular disease is perhaps related to the alterations induced by PM to the autonomic control of the heart.38,39 Animals exposed to diesel exhaust had reduced heart rate variability.40,41 These experimental data were substantiated by a number of clinical studies that showed an inversely proportional relationship between PM concentration and heart rate variability.42-48 Decreased heart rate variability indicates the existence of a state of cardiac autonomy dysfunction and is a risk factor for sudden cardiac death because of arrhythmias. The adverse effects of particulate pollutants on the cardiac autonomic balance have also been documented through their effects on vascular tone and reactivity, leading to increased blood pressure.49-52 The underlying mechanisms responsible for the increase of the sympathetic drive remain unclear but may involve activations of pulmonary neural reflex arcs and direct effects of pollutants on cardiac ion channels.53
In addition to these mechanisms, several studies in humans and animals have shown that exposure to PM results in the progression of atherosclerotic lesions through proinflammatory mechanisms.54 For instance, intratracheal administration of ambient PM10 in hyperlipidemic rabbits55 or long-term exposure to PM2.5 of genetically susceptible mice (apolipoprotein E deficient) enhanced growth of atherosclerotic plaque.56,57 Apolipoprotein E-null mice exposed to ultrafine particles exhibited significantly more and larger atherosclerotic lesions than mice exposed to filtered air, through pro-oxidative effects and the inhibition of the anti-inflammatory capacity of plasma high-density lipoprotein.58
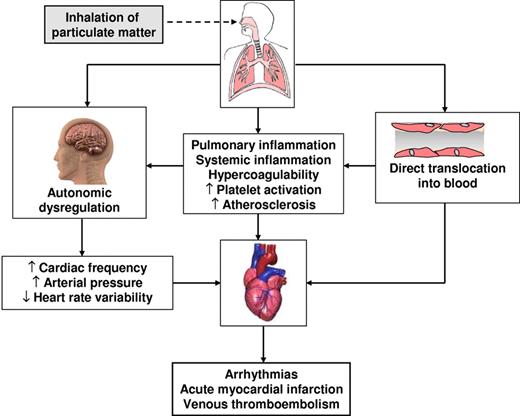
Thus, the aforementioned experimental and clinical studies suggest that heightened thrombus formation over atherosclerotic plaques is probably an important mechanistic player in the occurrence of cardiovascular events, as supported by the close temporal relationship that exists between short-term peaks of pollutants and concomitant increases in atherothrombotic events such as acute myocardial infarction (AMI) and ischemic stroke (see “Short-term effects”). The mechanism and pathways that probably involved in the cardiovascular effects of ambient air pollution are summarized in Figure 1.
Adverse effects of air pollution exposure: possible mechanisms of action of cardiovascular events.
Adverse effects of air pollution exposure: possible mechanisms of action of cardiovascular events.
Population studies
The relationships between ambient air pollution and cardiovascular events are usually represented by dose-response exposure functions that express the relative risk of adverse events for a specified increase in air pollutants. The clinical studies can be broadly divided into short-term and long-term studies. This artificial distinction is justified by the fact that the former investigate the effects of acute transient peaks of exposure to air pollution with emphasis on mortality and hospitalization and includes mostly time series analyses over a few days of increased PM exposure (changes generally ranging from 10 to 30 μg/mm3). The long-term studies, comprising cohort survival analyses over many years of exposure, evaluate the chronic, persistent effect of exposure to air pollutants on the risk of cardiovascular events.59-61 In terms of putative mechanisms, although short-term exposure affects mainly endothelium-mediated processes (impaired vasodilation, greater vasoconstriction, and hemostasis activation in the specific pulmonary bed) because of an increase of the sympathetic drive, more systemic mechanisms (such as oxidative stress, inflammation, and hypercoagulability) are probably predominant pertaining to long-term effects, through an acceleration of atherosclerosis and a heightened thrombotic tendency.62
Short-term effects
Several epidemiologic studies have unequivocally shown a strong association between acute peaks of particulate air pollutants and the occurrence of cardiovascular events.63-79 The National Morbidity, Mortality and Air Pollution Study, which involved 50 million people in the 20 largest cities in the United States, found that total mortality rates were independently associated with particle concentrations and that, on the day before death, each 20 μg/m3 rise in PM10 was associated with a 0.6% increase for cardiopulmonary mortality.64 A stronger association between adverse health outcomes and air pollution was shown by the Air Pollution and Health European Approach study.67 For 43 million people living in 29 European cities, the estimated daily increase in cardiovascular mortality was 1.5% for each 20 μg/m3 rise in PM10. A recent update of the Meta-analysis of Italian Studies on the short-term effects of air pollution,66 which collected data from 9 million people from 15 Italian cities through the period 1996-2002, has shown an increased daily rate of cardiorespiratory mortality associated with increased levels of several air pollutants (NO2, CO, SO2, and PM10).
Other studies have analyzed the adverse cardiovascular effects of air pollution in terms of morbidity (ie, coronary artery disease, arrhythmias, and heart failure) rather than mortality. For instance, in a case-crossover study conducted in 691 German patients who had developed AMI, the time spent in cars, public transport, motorcycles, or bicycles was consistently linked to the time of onset of symptoms, thus indicating that exposure to air pollution because of road traffic is a risk factor for AMI (odds ratio, 2.92).65 The increased risk was somewhat greater in cyclists (odds ratio, 3.94) than in automobile users (odds ratio, 2.60), suggesting an adverse interaction between physical activity and traffic-related air pollution. In a case-crossover study a 25 μg/mm3 PM2.5 rise within the 2 hours preceding the event was associated with a 48% increased risk of AMI.63 The acute effect of fine particulate air pollution on elderly people was analyzed by Dominici et al,64 who studied between 1999 and 2002 a US population of 11.5 million persons aged > 65 years to evaluate whether there was an association between daily concentrations of PM2.5 and changes in hospital admissions for cardiovascular and respiratory diseases. The largest association was for congestive heart failure, with a 1.3% increased risk per 10 μg/m3 rise in same-day PM2.5 concentration. From 1986 to 1999 the relationship between particulate air pollution and hospital admissions for congestive heart failure was investigated in 7 US cities: a 10 μg/m3 rise in PM10 was associated with a 0.7% increase in the rate of admission with this diagnosis on the same day. In the Intermountain Heart Collaborative Study, a rise by 10 μg/m3 of short-term exposure to PM2.5 was associated with a 4.5% increased rate of acute coronary events.70 The association between traffic-borne air pollutants and AMI is also supported by the European Health Effects of Air Pollution among Susceptible Subpopulations study,71 air pollution being associated with an increased risk of cardiac readmission to hospital in patients with preexisting AMI.72 In a study on the relationship between air pollution and emergency room admissions in Boston, a positive association was found among NO2, PM2.5, and the risk of hospitalization for AMI.73
A higher incidence of ischemic and hemorrhagic stroke has also been reported, with higher mortality and more hospital admissions related to short-term increases in airborne PM.74 For instance, in the US Medicare population of elderly patients, there was an increase in hospitalization for stroke on the same day of exposure to higher PM2.5 levels.68 A study in 9 US cities of people aged > 65 years confirmed this association, with a daily rise of 1.03% in hospital admissions for ischemic stroke for every 23 μg/m3 increase in PM10.75 A positive association between stroke mortality and daily concentrations of fine particles was also found in persons aged > 65 years from Helsinki.76 A more recent study conducted in Denmark confirmed also for ultrafine particles the association with hospital admissions for stroke.77
Although all the aforementioned epidemiologic studies provide solid and unequivocal evidence of a positive relationship between short-term peaks of air pollution and atherothrombotic disease, the evidence for an effect on venous thromboembolism is more preliminary and less established.78 Dales et al79 reported that the short-term increase in hospital admissions for venous thrombosis and pulmonary events in Santiago (the capital city of Chile) was proportional to the concentration of particulate and gaseous air pollutants. These findings are not surprising, because there is evidence that venous and arterial thromboses are not distinct entities but share a number of common risk factors.80 Thus, given that acute peaks of exposure to air pollution contribute to pulmonary and systemic inflammation and hypercoagulability, an epidemiologic link between short-term PM peaks and changes in venous thrombosis events is plausible, but additional evidence is warranted.
In all, these findings show that short-term elevations in ambient PM (that are also a proxy for gaseous pollutants) are capable of triggering atherothrombotic events and perhaps also clinical manifestations of venous thromboembolism such as deep vein thrombosis and pulmonary embolism. Detailed results of the largest short-term studies are shown in Table 1. It must be emphasized that the degree of elevation in ambient PM that, according to these studies, causes a substantial increase in mortality and morbidity rates for cardiovascular disease may indeed occur very frequently in metropolitan areas, especially where and when there is a combination of intense automobile traffic, high atmospheric pressure, and air stagnation.
Summary of the largest population studies on short-term cardiovascular effects of ambient air pollution
| First author, year (study acronym) . | Population . | Many findings . | Reference . |
|---|---|---|---|
| Peters, 2001 | Case cross-over study of 772 patients with AMI | A PM2.5 increase of 25 μg/m3 within the 2 hours before the event was associated with a 48% increased risk of AMI | 63 |
| Dominici, 2003 (NMMAPS) | Time-series study included 50 million people from 20 US cities | 0.6% daily increase of cardiorespiratory deaths for each 20-μg/m3PM10 increase | 64 |
| Peters, 2004 (AMIR) | Case cross-over study of 691 patients with AMI | Increased risk of AMI (OR, 2.92) associated with acute exposure to car traffic 1 hour before the event | 65 |
| Biggeri, 2004 (MISA) | Meta-analysis of 9 million people from 15 Italian cities | 0.4% daily increase of cardiovascular deaths for each 10-μg/m3 increase in NO2, of 0.9% for each 1-mg/m3 increase in CO, of 1.1% for each 10-μg/m3 increase in SO2, and of 0.5% for each 10-μg/m3 increase in PM10 | 66 |
| Analitis, 2006 (APHEA-2) | Time-series study of 43 million people from 29 European cities | 1.5% daily increase in cardiovascular deaths for each 20-μg/m3 PM10 increase | 67 |
| Dominici, 2006 | Time-series study of 11.5 million US people aged > 65 years | 1.3% daily increase in the risk of CHF and 1.2% increase in the risk of stroke for each 10-μg/m3 PM2.5 increase. | 68 |
| Wellenius, 2006 | Time-series study of 292 918 admissions for CHF in 7 US cities | 0.7% increase in the rate of admission for heart failure for each 10-μg/m3 PM10 increase | 69 |
| Pope, 2006 (IHCS) | Case cross-over study of 12 865 US patients undergoing coronary arteriography | 4.5% daily increase of acute coronary events for each 10-μg/m3 PM2.5 increase | 70 |
| Lanki, 2006 (HEAPPS) | 26 854 cases of first AMI in 5 European cities | A positive association with AMI of the same day CO (RR, 1.021) per 0.2 mg/m3 and particle number concentration (RR, 1.058) per 10 000 particles/cm2 | 71 |
| von Klot, 2005 (HEAPPS) | 22 066 AMI survivors in 5 European cities | Rate ratio for cardiac readmissions was 1.021 for each 10-μg/m3 in PM10 increase | 72 |
| Zanobetti, 2006 | 15 578 admission for cardiovascular and respiratory diseases in Boston | Significant association between NO (12.7% increase) and PM2.5 (8.6% increase) and the risk of emergency hospitalization for AMI | 73 |
| Wellenius, 2005 | 155 503 ischemic and 19 314 hemorrhagic stroke admissions in people aged ≥ 65 years from 9 US cities | One interquartile range increase in PM10 was associated with a 1.03% daily increase in hospitalization for ischemic stroke | 75 |
| Kettunen, 2007 | 3265 deaths from stroke among people aged ≥ 65 years from Finland | Daily stroke mortality was positively associated with current and previous-day PM2.5 levels | 76 |
| Andersen, 2010 | Case cross-over study of 7485 admissions for hemorrhagic and ischemic stroke in Denmark | Exposure to ultrafine particles led to a 21% increase in hospitalization for ischemic stroke | 77 |
| Dales, 2010 | Times-series study of a population of 5.4 million residents in Santiago (Chile) | The relative risk of hospitalization for venous thrombosis was 1.05 for a 20-μg/m3 PM2.5 increase | 79 |
| First author, year (study acronym) . | Population . | Many findings . | Reference . |
|---|---|---|---|
| Peters, 2001 | Case cross-over study of 772 patients with AMI | A PM2.5 increase of 25 μg/m3 within the 2 hours before the event was associated with a 48% increased risk of AMI | 63 |
| Dominici, 2003 (NMMAPS) | Time-series study included 50 million people from 20 US cities | 0.6% daily increase of cardiorespiratory deaths for each 20-μg/m3PM10 increase | 64 |
| Peters, 2004 (AMIR) | Case cross-over study of 691 patients with AMI | Increased risk of AMI (OR, 2.92) associated with acute exposure to car traffic 1 hour before the event | 65 |
| Biggeri, 2004 (MISA) | Meta-analysis of 9 million people from 15 Italian cities | 0.4% daily increase of cardiovascular deaths for each 10-μg/m3 increase in NO2, of 0.9% for each 1-mg/m3 increase in CO, of 1.1% for each 10-μg/m3 increase in SO2, and of 0.5% for each 10-μg/m3 increase in PM10 | 66 |
| Analitis, 2006 (APHEA-2) | Time-series study of 43 million people from 29 European cities | 1.5% daily increase in cardiovascular deaths for each 20-μg/m3 PM10 increase | 67 |
| Dominici, 2006 | Time-series study of 11.5 million US people aged > 65 years | 1.3% daily increase in the risk of CHF and 1.2% increase in the risk of stroke for each 10-μg/m3 PM2.5 increase. | 68 |
| Wellenius, 2006 | Time-series study of 292 918 admissions for CHF in 7 US cities | 0.7% increase in the rate of admission for heart failure for each 10-μg/m3 PM10 increase | 69 |
| Pope, 2006 (IHCS) | Case cross-over study of 12 865 US patients undergoing coronary arteriography | 4.5% daily increase of acute coronary events for each 10-μg/m3 PM2.5 increase | 70 |
| Lanki, 2006 (HEAPPS) | 26 854 cases of first AMI in 5 European cities | A positive association with AMI of the same day CO (RR, 1.021) per 0.2 mg/m3 and particle number concentration (RR, 1.058) per 10 000 particles/cm2 | 71 |
| von Klot, 2005 (HEAPPS) | 22 066 AMI survivors in 5 European cities | Rate ratio for cardiac readmissions was 1.021 for each 10-μg/m3 in PM10 increase | 72 |
| Zanobetti, 2006 | 15 578 admission for cardiovascular and respiratory diseases in Boston | Significant association between NO (12.7% increase) and PM2.5 (8.6% increase) and the risk of emergency hospitalization for AMI | 73 |
| Wellenius, 2005 | 155 503 ischemic and 19 314 hemorrhagic stroke admissions in people aged ≥ 65 years from 9 US cities | One interquartile range increase in PM10 was associated with a 1.03% daily increase in hospitalization for ischemic stroke | 75 |
| Kettunen, 2007 | 3265 deaths from stroke among people aged ≥ 65 years from Finland | Daily stroke mortality was positively associated with current and previous-day PM2.5 levels | 76 |
| Andersen, 2010 | Case cross-over study of 7485 admissions for hemorrhagic and ischemic stroke in Denmark | Exposure to ultrafine particles led to a 21% increase in hospitalization for ischemic stroke | 77 |
| Dales, 2010 | Times-series study of a population of 5.4 million residents in Santiago (Chile) | The relative risk of hospitalization for venous thrombosis was 1.05 for a 20-μg/m3 PM2.5 increase | 79 |
AMI indicates acute myocardial infarction; PM, particulate matter; NMMAPS, National Morbidity, Mortality and Air Pollution Study; AMIR, Ausburg Myocardial Infarction Registry; OR, odds ratio; MISA, Meta-analysis of Italian Studies on the short-term effects of air pollution; CHF, congestive heart failure; APHEA, the Air Pollution and Health-European Approach; IHCS, Intermountain Health Collaborative Study; HEAPSS, Health Effects of Air Pollution among Susceptible Subpopulations; and RR, relative risk.
Long-term effects
In addition chronic exposure to airborne PM enhances the risk of cardiovascular diseases or mortality.81 In > 8000 adults from 6 US cities (Harvard 6 Cities Study) followed for 14-16 years, overall mortality was 1.26 times higher in the most polluted than in the least polluted cities.82 In an extended follow-up of this study for 8 additional years,83 a significant increase in risk of death was found for every 10 μg/m3 rise in PM2.5, with a relative risk (RR) of 1.28 for cardiovascular death. The American Cancer Society conducted a study between 1982 and 1989 to investigate individual health risks for 552 138 residents of 151 US cities in relation to local ambient air quality, found a RR of 1.17 for all-cause mortality because of increased levels of PM2.5.84 The extended 16-year follow-up of the American Cancer Society study showed that each rise of 10 μg/m3 in the mean PM2.5 daily concentration was associated with a 12% increased risk of death from cardiovascular causes4 and that death from coronary artery disease was the single largest cause of mortality (18%). Mortality from long-term exposure to air pollution was also investigated through the follow-up of 22 905 subjects residing in the Los Angeles urban area during the years 1982-2000.85 Cardiopulmonary mortality and deaths from coronary artery disease increased by 20% and 49%, respectively, per 10 μg/m3 rise in PM2.5 exposure. A study conducted in Sweden on 176 309 male construction workers exposed to PM and compared with 71 778 unexposed workers found that their occupational PM exposure was associated with an increased RR for coronary artery disease (1.13).86 A large Dutch cohort study showed that living near a main road was associated with total mortality (RR, 1.41), an even more significant relationship being found for cardiopulmonary mortality (RR, 1.95).87 Miller et al88 reported that exposure to air pollution over a 6-year period increased the risk of cardiovascular events by 24% and cardiovascular-related death by 76% for every10 μg/m3 PM2.5 rise. Similarly, the same PM2.5 rise was associated with a 35% and 83% increase in risk for stroke and stroke-related mortality, respectively. In the frame of a retrospective cohort study performed in residents of South London who survived a first stroke,89 a positive association was seen between estimated exposure to ambient air pollution and subsequent mortality after stroke.
In addition to the aforementioned strong and consistent evidence that long-term exposure to air pollution is associated with an increase in atherothrombotic clinical events, markers of subclinical atherosclerosis are more frequently found in communities chronically exposed to high levels of ambient air pollution. For instance, Künzli et al90 measured carotid intima medial thickness in 800 residents of Los Angeles. Personal air pollution exposures were estimated with a geostatistical model that mapped their area of residence to PM values recorded by local pollution-monitoring stations. For every 10 μg/m3 rise in PM2.5, intima media thickness increased by 4% after adjustment for potential confounding variables. The same investigators reported an association between air pollution and progression of atherosclerosis (measured as the annual rate of increase in the carotid artery intima media thickness) after the analysis of 5 double-blind randomized trials.91 Similar effects have also been reported for coronary artery calcium content, a biomarker of coronary atherosclerosis. In a prospective cohort study of 4494 persons living close to a main urban road coronary artery calcium scores were increased by 60%.92
The strong evidence supporting the association between atherothrombosis and air pollution is complemented by fewer studies on venous thromboembolism. Baccarelli et al93 examined the relationship between exposure to PM10 and venous thrombosis in a case-control study of 870 patients and 1210 healthy persons and found that for each 10 μg/mm3 rise in PM10 there was a 70% increase of venous thrombosis, independently of other clinical and environmental variables.94 In the same study, exposure to PM was associated with a shortened prothrombin time, extending previous observations that were based on a shorter time window of exposure to pollution.31 The increased risk of venous thrombosis was nearly linearly associated with living in proximity to main traffic roads, extending findings by other investigators who had shown the same association for arterial cardiovascular events.95 However, these results on the risk of venous thromboembolism were not replicated by 2 prospective cohort studies, indicating the need of further studies in this field.96,97
The findings of the largest studies on long-term cardiovascular effects of particulate air pollution are shown in Table 2. Taken together, they show that prolonged exposure to particulate air pollution is associated with adverse cardiovascular events, even when PM10 concentrations become only modestly higher than the thresholds established as target by regulatory agencies (for instance, 20 μg/mm3 by the WHO).98
Summary of the largest population studies on long-term cardiovascular effects of particulate air pollution
| First author, year (Study) . | Population . | Key findings . | Reference . |
|---|---|---|---|
| Laden, 2006 (HSCS) | Prospective cohort of 8111 persons from 6 US cities followed for 28 years | Increase in cardiovascular death (RR, 1.28) for each 10-μg/m3 PM2.5 increase | 83 |
| Pope, 2004 (ACS) | Prospective cohort of 500 000 residents in 150 US cities followed for 16 years | 12% increased risk of cardiovascular deaths and 18% increased risk of coronary artery disease for each 10-μg/m3 increase in long-term PM2.5 exposure | 4 |
| Jerrett, 2005 | Prospective cohort of 22 905 residents in the Los Angeles area | 20% and 49% increase in the risk of cardiopulmonary and coronary artery disease deaths for each 10-μg/m3 increase in long-term PM2.5 exposure | 85 |
| Toren, 2007 | Prospective cohort of 176 309 PM-exposed construction workers and 71 778 PM-unexposed construction workers | Increased risk of coronary artery disease (RR, 1.13) associated with occupational exposure to particulate air pollution | 86 |
| Hoek, 2002 (NLCS) | 4492 Dutch participants | Increased risk of cardiopulmonary mortality (RR, 1.95) associated with living near a main traffic road | 87 |
| Miller, 2007 | Prospective cohort of 65 893 postmenopausal women in 36 US cities followed for 6 years | 24% and 76% increase in the risk of cardiovascular events and cardiovascular mortality for each 10-μg/m3 increase in long-term PM2.5 exposure | 88 |
| Maheswaran, 2010 | Retrospective cohort study of 3320 patients with stroke residing in south London between 1995 and 2005 | Each 10-μg/m3 PM10 increase was associated with a 52% increase in mortality after stroke | 89 |
| Kunzli, 2005 | Measurement of carotid intima media thickness in 798 resident in Los Angeles | Each 10-μg/m3 PM2.5 increase was associated with a 5.9% increase of carotid intima media thickness | 90 |
| Hoffmann, 2007 (Heinz Nixdorf Recall Study) | 4494 German study participants | Compared with participants living > 200 m away from a main road, participants living within 50, 51-100, and 101-200 m had ORs of 1.63, 1.34, and 1.08, respectively, for a high coronary artery calcium content | 92 |
| Baccarelli, 2008 | 870 patients with DVT and 1210 controls in Northern Italy | Each 10-μg/m3 PM10 increase was associated with a 70% increase in the risk of venous thrombosis | 93 |
| Baccarelli, 2009 | 663 patients with DVT and 859 controls in Northern Italy | Risk of venous thrombosis was increased (OR, 1.47) for subjects living near a main traffic road | 94 |
| First author, year (Study) . | Population . | Key findings . | Reference . |
|---|---|---|---|
| Laden, 2006 (HSCS) | Prospective cohort of 8111 persons from 6 US cities followed for 28 years | Increase in cardiovascular death (RR, 1.28) for each 10-μg/m3 PM2.5 increase | 83 |
| Pope, 2004 (ACS) | Prospective cohort of 500 000 residents in 150 US cities followed for 16 years | 12% increased risk of cardiovascular deaths and 18% increased risk of coronary artery disease for each 10-μg/m3 increase in long-term PM2.5 exposure | 4 |
| Jerrett, 2005 | Prospective cohort of 22 905 residents in the Los Angeles area | 20% and 49% increase in the risk of cardiopulmonary and coronary artery disease deaths for each 10-μg/m3 increase in long-term PM2.5 exposure | 85 |
| Toren, 2007 | Prospective cohort of 176 309 PM-exposed construction workers and 71 778 PM-unexposed construction workers | Increased risk of coronary artery disease (RR, 1.13) associated with occupational exposure to particulate air pollution | 86 |
| Hoek, 2002 (NLCS) | 4492 Dutch participants | Increased risk of cardiopulmonary mortality (RR, 1.95) associated with living near a main traffic road | 87 |
| Miller, 2007 | Prospective cohort of 65 893 postmenopausal women in 36 US cities followed for 6 years | 24% and 76% increase in the risk of cardiovascular events and cardiovascular mortality for each 10-μg/m3 increase in long-term PM2.5 exposure | 88 |
| Maheswaran, 2010 | Retrospective cohort study of 3320 patients with stroke residing in south London between 1995 and 2005 | Each 10-μg/m3 PM10 increase was associated with a 52% increase in mortality after stroke | 89 |
| Kunzli, 2005 | Measurement of carotid intima media thickness in 798 resident in Los Angeles | Each 10-μg/m3 PM2.5 increase was associated with a 5.9% increase of carotid intima media thickness | 90 |
| Hoffmann, 2007 (Heinz Nixdorf Recall Study) | 4494 German study participants | Compared with participants living > 200 m away from a main road, participants living within 50, 51-100, and 101-200 m had ORs of 1.63, 1.34, and 1.08, respectively, for a high coronary artery calcium content | 92 |
| Baccarelli, 2008 | 870 patients with DVT and 1210 controls in Northern Italy | Each 10-μg/m3 PM10 increase was associated with a 70% increase in the risk of venous thrombosis | 93 |
| Baccarelli, 2009 | 663 patients with DVT and 859 controls in Northern Italy | Risk of venous thrombosis was increased (OR, 1.47) for subjects living near a main traffic road | 94 |
HSCS indicates Harvard Six Cities Study; RR, relative risk; PM, particulate matter; ACS, American Cancer Society; NLCS, Netherlands Cohort Study; OR, odds ratio; and DVT, deep vein thrombosis.
Conclusions
From an array of experimental, epidemiologic, and clinical studies it emerges that air pollution is an important acquired cardiovascular risk factor that adds to the traditional ones. Alterations induced by exposure to particulate air pollution contribute in the long term to the development and progression of atherosclerosis and, in the frame of short-term exposures, to triggering thrombosis and acute cardiovascular events. The 2 most frequent cardiovascular diseases (ie, coronary artery disease and cerebrovascular disease) are definitely and consistently increased by particulate exposure. Preliminary evidence suggests that PM exposure may be a risk factor also for venous thromboembolism. A recent evaluation of the public health relevance of triggers of AMI found that exposure to automobile traffic had the greatest population effect on this event, being an ongoing factor to which a huge number of persons are exposed.7
Further studies on the mechanisms of cardiovascular effects related to air pollution are necessary, and changes in the hemostatic system warrant special attention. For instance, the role of platelets in thrombus formation has been relatively poorly studied in humans99 at variance with the evidence gained in animal models, that, however, were based on artificial proxies of ambient air pollutants. The many toxic substances contained in the anthropogenic PM (eg, metals) may cause oxidative stress and platelet hyperactivity. Of the other components of hemostasis, coagulation and fibrinolysis are more thoroughly investigated, but more adequate models of PM exposure in humans and animals are needed, because the different particles used in those models fail to mimic the complex composition of PM that are present in the urban ambient because of traffic and domestic heating.
What can be done to control these adverse effects of air pollution? The American Heart Association has recently delivered the following statement39 : “the overall evidence is consistent with a causal relationship between PM2.5 exposure and cardiovascular morbidity and mortality.” A large number of metropolitan areas around the world have PM concentrations well above the WHO target of 20 μg/mm3, especially in Eastern and Southern Europe and Asia. PM exposure is a modifiable risk factor.39 It has been calculated that a reduction by only 10 μg/mm3 in the daily mean concentration of PM10 would decrease by 1.6% the incidence of new cases of myocardial infarction, the decrease being as large as 4.8% if PM10 concentration were decreased by as much as 30 μg/mm.7 Because it has been shown that the decrease of air pollution clearly leads to a parallel increase in life expectancy,83,100 it is a community responsibility to adopt the necessary measures. Efforts directed to manage local air quality should aim to reduce not only PM from anthropogenic but also from soil sources, even if anthropogenic particles appear to have a higher effect on cardiovascular events because of their stronger production of proinflammatory cytokines. Yet, also the single citizen can adopt simple measures to reduce his or her individual risk, such as to avoid walking in the most trafficked roads, performing strenuous outdoor exercise in busy roads, and exposing small children to the high concentration of air pollutants that are present next to the ground.
Acknowledgments
The authors thank Drs Baccarelli and Bertazzi for helpful discussion and advice.
This work was supported in part by grants from the Lombardy Region (P.M.M.).
Authorship
Contribution: M.F. and P.M.M. were the authors of this paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pier Mannuccio Mannucci, Scientific Direction, IRCCS Cà Granda Foundation Maggiore Policlinico Hospital, Via Pace 9, 20122 Milan, Italy; e-mail: pmmannucci@libero.it.