Abstract
Phylogenetically conserved serine protease cascades play an important role in invertebrate and vertebrate immunity. The mammalian coagulation system can be traced back some 400 million years and shares homology with ancestral serine proteinase cascades that are involved in, for example, Toll receptor signaling in insects and release of antimicrobial peptides during hemolymph clotting. In the present study, we show that the induction of coagulation by bacteria leads to immobilization and killing of Streptococcus pyogenes bacteria inside the clot. The entrapment is mediated via cross-linking of bacteria to fibrin fibers by the action of coagulation factor XIII (fXIII), an evolutionarily conserved transglutaminase. In a streptococcal skin infection model, fXIII−/− mice developed severe signs of pathologic inflammation at the local site of infection, and fXIII treatment of wild-type animals dampened bacterial dissemination during early infection. Bacterial killing and cross-linking to fibrin networks was also detected in tissue biopsies from patients with streptococcal necrotizing fasciitis, supporting the concept that coagulation is part of the early innate immune system.
Introduction
Serine protease cascades play an important role in many pathophysiologic processes, including hemostasis, immune response, and wound healing.1 Their activation normally occurs by limited proteolysis, and coagulation and complement are probably the best-characterized serine proteinase cascades in humans. Phylogenetic studies have shown that the 2 systems developed > 400 million years ago,2,3 and it has been proposed that they evolved from a common ancestral origin in eukaryotes.4 Coagulation and complement cascades share a remarkable degree of convergent evolution with other serine protease cascades such as those regulating dorsal-ventral polarity in Drosophila (leading to an activation of Spätzle, the ligand of the Toll receptor) and the hemolymph clotting system in the horseshoe crab.4,5 These findings suggest that the basic motifs of some proteolytic cascades existed long before the divergence of protostomes and deuterostomes.6 Both activation of Spätzle and the horseshoe crab hemolymph clotting system are key components in ancestral immunity, which relies largely on the innate immune system. Whereas the complement system has been considered to be part of the innate immune system for > 30 years, it has only recently been determined that coagulation also partakes in inflammation and the early immune defense.7,8 In the latter studies, a major focus has been on the ability of the clotting cascade to trigger pro- and anti-inflammatory reactions, such as the release of cytokines and the activation of protease-activated receptors. However, it is unknown to what extent coagulation can actively contribute to the elimination of an invading microorganism.
The present study was undertaken to investigate whether activation of the coagulation system in response to bacterial infection contributes to the innate immune system and to elimination of the invading pathogen. Special focus was placed on the role of coagulation factor XIII (fXIII), for which the insect homolog (transglutaminase) was recently found to play a protective role in the immune response against bacterial pathogens in a Drosophila infection model.9 Streptococcus pyogenes was used in the present study, because this bacterium is considered to be one of the most important human bacterial pathogens, being responsible for at least 18 million cases of severe infection worldwide (1.78 million new cases each year) and > 500 000 deaths yearly, as estimated by the World Health Organization.10 Infections with S pyogenes are normally superficial and self-limiting, but can develop into serious and life-threatening conditions such as necrotizing fasciitis and streptococcal toxic shock syndrome, which are associated with high morbidity and mortality.11 The fact that S pyogenes can cause local and systemic infections in the same infection model made it an ideal pathogen to be studied in the present investigation.
Methods
Bacterial strains and culture conditions
Human plasma
Plasma obtained from healthy donors was purchased from Lund University Hospital (Lund, Sweden) and plasma kallikrein–, (PK-), thrombin-, fXII-, and fXIII-deficient plasmas were purchased from George King Bio-Medical.
Measurement of coagulation parameters
Activation of the intrinsic and extrinsic pathway of coagulation was determined by measuring activated partial thromboplastin time (aPTT) and prothrombin time (PT) in human or murine plasma using a coagulometer (Amelung), as described previously.12
Substrate assays
PK activity on the bacterial surface after exposure to normal, PK-, or fXIII-deficient plasma was measured using the chromogenic substrate S-2302 (Chromogenix), as described previously.12 To measure thrombin activity, normal, thrombin-, fXII-, or fXIII-deficient plasma was incubated with 2 × 1010 CFU of S pyogenes in 50mM Tris supplemented with 50μM ZnCl2, 2mM CaCl2, and 1μM phospholipids (Rossix) for 30 minutes at 37°C. The tetrapeptide Gly-Pro-Arg-Pro (Bachem) was added to prevent clotting (1.5 mg/mL final concentration). Samples were washed with Tris and resuspended in Tris/ZnCl2 and 2mM of the chromogenic substrate S-2238. After incubation at 37°C and centrifugation, the absorbance of the supernatants was determined at 405 nm. For fXIII-activity measurements, bacteria were added to normal, thrombin-, fXII-, or fXIII-deficient plasma (diluted 1:100 in sodium citrate), supplemented with zinc, calcium, and phospholipids. After incubation for 15 minutes at 37°C in the presence of a gold-labeled N-ϵ-γ-glutamyl-lysine antibody (153-81D4; GeneTex), samples were subjected to negative-staining electron microscopy.
Bacterial growth in human plasma
Normal and fXIII-deficient plasma was diluted 1:100 in 12.9mM sodium citrate, mixed with 2.5 × 105 CFU of S pyogenes, and 0.5 U of thrombin from human plasma (Sigma) was added before incubation at 37°C. At the indicated time points, 50 μL of the mixture was plated onto blood agar in 10-fold serial dilutions and the number of bacteria was determined by counting colonies after 18 hours of incubation at 37°C; alternatively, the mixture was analyzed by negative-staining electron microscopy.
Generation of plasma clots
Clots from human or murine plasma (normal and fXIII deficient) for electron microscopic analysis were prepared as described previously.9
Cross-linking and immobilization of bacteria within the clot
Human fibrinogen (ICN Biomedicals) was incubated with M1 protein in the absence or presence of thrombin-activated human fXIII (Enzyme Research Laboratories) for 30 minutes at 37°C. For visualization by electron microscopy, the gold-labeled N-ϵ-γ-glutamyl-lysine antibody was added to the reaction mixture.
To analyze bacterial immobilization, clots were generated from normal and fXIII-deficient plasma, washed with PBS, and covered with THB medium. At the indicated time points, 50 μL of the supernatant was plated onto blood agar.
Electron microscopy
Samples for scanning electron microscopy were processed as described previously.14 Transmission electron microscopy and immunostaining using the gold-labeled N-ϵ-γ-glutamyl-lysine antibody were performed as described previously.15 For negative-staining electron microscopy, samples were adsorbed to 400-mesh carbon-coated copper grids for 1 minute, washed with 2 drops of water, and stained with 2 drops of 0.75% uranyl formate. The grids were rendered hydrophilic by glow discharge at low pressure in air. Samples were observed in a 1200 EX transmission electron microscope (JEOL) operated at a 60-kV accelerating voltage.
Animal infection model
CBA/CaOlaHsd wild-type and fXIII−/− mice were from Harlan and CSL Behring, respectively. All animal experiments were approved by the regional ethical committee for animal experimentation (the Malmö/Lund djurforsöksetiska nämnd, Lund District Court, Lund, Sweden; permit M220/08). Mice were subcutaneously infected with 2.5 × 108 CFU of S pyogenes KTL3 as described previously.16 After 24 hours of infection, mice were killed. Skin samples were collected from the local focus of infection and fixed in 3.7% formaldehyde. For plasma analysis, citrated blood was taken from the heart at the time of killing, centrifuged at 2655g for 10 minutes, and frozen at −80°C until use. To determine bacterial loads, blood and homogenates from liver and spleen were plated as described in “Bacterial growth in human plasma.” In some experiments, mice were treated with 200 U/kg body weight of a human fXIII concentrate (FibrogamminP; CSL Behring) subcutaneously at the site of infection 3 hours after bacterial inoculation.
Examination of murine skin samples
Fixed tissue samples were dehydrated in ethanol, embedded in paraffin, and then cut into 3-μm-thick sections. After deparaffination, samples were prepared for scanning electron microscopy or stained with H&E (Histolab) or with Giemsa stain (Merck) for histologic analysis using an Eclipse 80i microscope (Nikon).
Examination of human tissue biopsies
Snap-frozen tissue biopsies collected from the epicenter of infection in 2 patients with necrotizing fasciitis caused by S pyogenes of the M1T1 serotype were stained and compared with biopsies taken from healthy volunteers. The human subjects review committees of the University of Toronto (Toronto, ON) and Karolinska University Hospital (Stockholm, Sweden) approved the studies, and informed consent was obtained from the patients and the volunteers. The biopsies were prepared and immunofluorescent stainings of serial sections were conducted as described previously.17,18 The following antibodies were used in dilutions ranging from 1:250 to 1:10 000: anti N-ϵ-γ-glutamyl-lysine, antifactor XIIIa (Acris), a polyclonal rabbit antiserum specific for the Lancefield group A carbohydrate (Difco), and polyclonal rabbit antiserum against M1 protein. The immunohistochemical stainings were conducted in an RXM microscope with a 25×/0.55 numeric aperture oil objective lens and immunofluorescent stainings in a confocal scanner TCS SP II coupled to a DMR microscope (all from Leica).
Statistical analysis
Data were analyzed using Prism 5 software (GraphPad). The significance between the values of an experimental group was determined by use of a variance analysis (t test).
Results
Contact activation at the surface of S pyogenes leads to an induction of fXIII
Previous studies have shown that the presence of S pyogenes in plasma leads to an assembly and activation of the contact system at the bacterial surface.14 However, those experiments were performed in the absence of calcium and phospholipids, 2 indispensable clotting cofactors required for the activation of coagulation factors upstream of the contact system.19 We therefore wondered whether calcium and phospholipid reconstitution triggers an induction of the remaining clotting cascade at the streptococcal surface. To confirm the previously reported findings, we first measured the PK activity on AP1 bacteria after incubation with normal human plasma supplemented with zinc. As depicted in supplemental Figure 1A (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), substrate hydrolysis was monitored when bacteria were incubated with normal and fXIII-deficient plasma, but not when plasma lacked PK. We then set up experiments to monitor whether bacteria-induced contact activation leads to an induction of the entire coagulation cascade by measuring thrombin activity, the activator of fXIII. Normal plasma was reconstituted with zinc, calcium, and phospholipids. Samples were also supplemented with a tetrapeptide (Gly-Pro-Arg-Pro) to avoid polymerization of thrombin-generated fibrin monomers and subsequent clot formation (for detailed information, see “Methods”). This reaction mixture was added to normal plasma, incubated with AP1 bacteria, and the increase in thrombin activity at the bacterial surface was monitored (supplemental Figure 1B). Similar results were also obtained with fXIII-deficient, but not with fXII-deficient or thrombin-deficient, plasma (supplemental Figure 1B), implying that activation of the contact system at the bacterial surface is required to trigger activation of the remaining clotting factors.
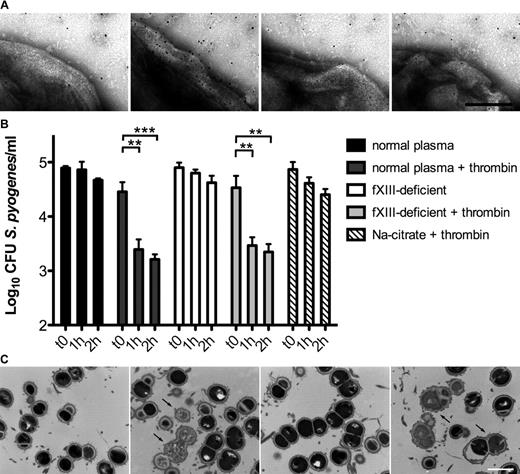
fXIII is one of thrombin's substrates and we therefore tested whether thrombin activation induced by bacteria triggers a conversion of fXIII into its active form. We used immunoelectron microcopy with an antibody directed against N-ϵ-γ-glutamyl-lysine, which specifically recognizes amino acids that are covalently cross-linked by the action of fXIII.20 Because Gly-Pro-Arg-Pro exerted a mild bacteriostatic effect in our experiments, we decided not to use this peptide as an anticoagulant. Instead, plasma was diluted (1:100) to generate a fibrin concentration too low to cause its polymerization when activated by thrombin. Bacteria were incubated with diluted normal, thrombin-, fXII-, and fXIII-deficient plasma in the presence of the gold-labeled antibody, zinc, calcium, and phospholipids. Samples were subsequently analyzed by negative-staining electron microscopy. Figure 1A shows antibody binding to the surface of S pyogenes bacteria treated with normal diluted plasma, whereas only background signals were detected when bacteria were incubated with fXII- or fXIII-deficient plasma (Figure 1A). Similar results were obtained with thrombin-deficient plasma (data not shown). These results suggest that contact activation at the bacterial surface can evoke an induction of the entire coagulation cascade, eventually enabling fXIII to act on S pyogenes surface proteins.
The contact system is activated on the bacterial surface after exposure to plasma, leading to antimicrobial activity. (A) S pyogenes AP1 bacteria were incubated in sodium citrate alone or in normal, fXII-, or fXIII-deficient plasma (all diluted 1:100 in sodium citrate) in the presence of ZnCl2, CaCl2, phospholipids, and the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine for 15 minutes, and then analyzed by negative-staining electron microscopy. The scale bar indicates 100 nm. (B) S pyogenes AP1 bacteria were incubated in nonactivated or thrombin-activated normal and fXIII-deficient plasma (1:100 diluted). At the indicated time points, bacterial numbers were determined by plating of serial dilutions onto blood agar. Bacteria incubated in sodium citrate in the presence of thrombin served as controls. The data represent the means ± SD of 3 independent experiments. **P < .01; ***P < .001. (C) AP1 bacteria were incubated with normal or fXIII-deficient plasma and clotting was initiated by the addition of thrombin. Thin-sectioned clots before (0h) and after 1 hour (1h) of incubation at 37°C are shown. Similar amounts of dead bacteria (arrows) were detected in both samples after incubation. The scale bar indicates 1 μm.
The contact system is activated on the bacterial surface after exposure to plasma, leading to antimicrobial activity. (A) S pyogenes AP1 bacteria were incubated in sodium citrate alone or in normal, fXII-, or fXIII-deficient plasma (all diluted 1:100 in sodium citrate) in the presence of ZnCl2, CaCl2, phospholipids, and the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine for 15 minutes, and then analyzed by negative-staining electron microscopy. The scale bar indicates 100 nm. (B) S pyogenes AP1 bacteria were incubated in nonactivated or thrombin-activated normal and fXIII-deficient plasma (1:100 diluted). At the indicated time points, bacterial numbers were determined by plating of serial dilutions onto blood agar. Bacteria incubated in sodium citrate in the presence of thrombin served as controls. The data represent the means ± SD of 3 independent experiments. **P < .01; ***P < .001. (C) AP1 bacteria were incubated with normal or fXIII-deficient plasma and clotting was initiated by the addition of thrombin. Thin-sectioned clots before (0h) and after 1 hour (1h) of incubation at 37°C are shown. Similar amounts of dead bacteria (arrows) were detected in both samples after incubation. The scale bar indicates 1 μm.
Streptococci are killed in thrombin-activated plasma
It has been shown that contact activation on the surface of S pyogenes leads to the generation of antimicrobial peptides.21 Therefore, we investigated the fate of cross-linked bacteria in activated, but nonclotted, normal and fXIII-deficient plasma. Our results show that bacterial growth was significantly impaired in thrombin-activated normal and fXIII-deficient plasma (diluted 1:100). This effect was dependent on both time and plasma activation (Figure 1B). To determine whether these results were due to an induction of antimicrobial activity, plasma-treated bacteria were subjected to negative-staining electron microscopy. Supplemental Figure 1C shows intact bacteria that were incubated with nonactivated normal plasma. Similar findings were observed when bacteria were incubated with nonactivated fXIII-deficient plasma (supplemental Figure 1C). In the presence of thrombin, however, incubation with normal or fXIII-deficient plasma caused multiple disruptions of the bacterial cell wall and triggered an efflux of cytosolic content (supplemental Figure 1C), a sign of bacterial killing.17 Incubation of S pyogenes with thrombin in the absence of plasma neither impaired bacterial growth (Figure 1B) nor caused cytosolic leakage (data not shown).
To determine whether bacterial killing also occurs within a formed clot, AP1 bacteria and undiluted plasma were mixed and thrombin was added. The clots formed were incubated for 1 hour, thin-sectioned, and analyzed by transmission electron microscopy. Figure 1C shows that a significant number of bacteria in clots generated from normal and fXIII-deficient plasma were devoid of cytosolic content, suggesting a substantial disruption of the cell membrane and bacterial killing. In contrast, only a few dead bacteria were seen when clots were thin-sectioned directly after the addition of thrombin. Statistical analysis revealed an efficient killing of bacteria within the clot regardless of whether the clot was formed from normal or fXIII-deficient plasma (6% at time 0 hours and 35% after 1 hour for normal plasma and 5% at time 0 hours and 36% after 1 hour for fXIII-deficient plasma). These data demonstrate that activation of the coagulation cascade on the surface of S pyogenes leads to an fXIII-independent induction of antimicrobial activity.
Bacterial entrapment within a plasma clot is fXIII dependent
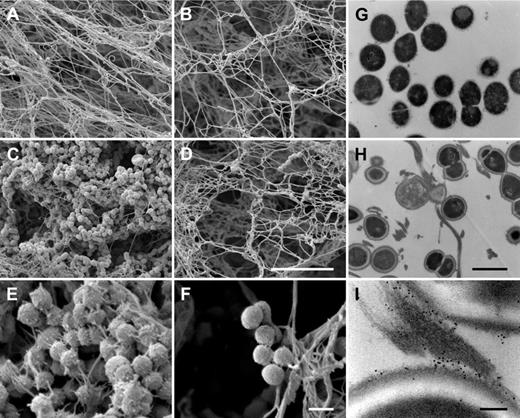
It was recently shown that human fXIII cross-links and immobilizes bacteria of the species Staphylococcus aureus and Escherichia coli inside a plasma clot.9 To determine whether this also applies to S pyogenes, bacteria of strain AP1 were incubated with normal and fXIII-deficient plasma. After activation with thrombin, clots were analyzed by scanning electron microscopy. Figure 2A-B shows clots formed from normal and fXIII-deficient plasma in the absence of bacteria. The micrographs reveal that the clots have a similar morphology, although clots generated from fXIII-deficient plasma appear less dense. However, dramatic changes were observed when clots were formed in the presence of S pyogenes AP1 bacteria. Whereas massive loads of bacteria were entrapped in clots derived from normal plasma (Figure 2C), only a few bacteria were found attached to clots when fXIII-deficient plasma was used (Figure 2D). In addition, fibrin network formation was reduced when bacteria were incubated with normal plasma, which was not seen in fXIII-deficient plasma (Figure 2C-D). At higher resolution, it is noticeable that fibrin fibers and bacteria are in close proximity in the clots generated from normal plasma, and it even appears that the fibers originate from the bacterial surface (Figure 2E). In contrast, bacteria are loosely assembled in clots from fXIII-deficient plasma and no direct interaction with fibrin fibers is visible (Figure 2F). To confirm these findings, clots from normal plasma were thin-sectioned and subjected to transmission electron microscopy, which allows analysis at higher resolution. Figure 2G-I depicts thin-sectioned S pyogenes AP1 bacteria before (Figure 2G) and directly after incubation with normal plasma and subsequent thrombin activation (Figure 2H). Within the clot, bacteria are strung along fibrin fibers and it appears that they have multiple interaction sites. Additional immunostaining with the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine was used to study the mode of interaction between bacteria and fibrin fibers. As expected, numerous cross-linking events within the fibrin fibers were detected. The electron microscopic analysis also revealed that fibrin fibers are avidly cross-linked to the bacterial surface (Figure 2I). Cross-linking activity was not recorded when bacteria were incubated with fXIII-deficient plasma (data not shown).
Entrapment and immobilization of S pyogenes inside the clot. Scanning electron micrographs showing the structure of clots generated from normal plasma (A,C,E) or fXIII-deficient plasma (B,D,F) in the absence (A-B) or presence (C-F) of bacteria. The scale bars indicate 10 μm in panels A through D and 1 μm in panels E and F. The transmission electron micrographs depict S pyogenes alone (G), after exposure to thrombin-activated plasma (H), and after exposure to plasma followed by immunostaining with a gold-labeled N-ϵ-γ-glutamyl-lysine antibody recognizing the fXIII cross-linking site (I). Scale bars indicate 1 μm in panels G and H and 100 nm in panel I.
Entrapment and immobilization of S pyogenes inside the clot. Scanning electron micrographs showing the structure of clots generated from normal plasma (A,C,E) or fXIII-deficient plasma (B,D,F) in the absence (A-B) or presence (C-F) of bacteria. The scale bars indicate 10 μm in panels A through D and 1 μm in panels E and F. The transmission electron micrographs depict S pyogenes alone (G), after exposure to thrombin-activated plasma (H), and after exposure to plasma followed by immunostaining with a gold-labeled N-ϵ-γ-glutamyl-lysine antibody recognizing the fXIII cross-linking site (I). Scale bars indicate 1 μm in panels G and H and 100 nm in panel I.
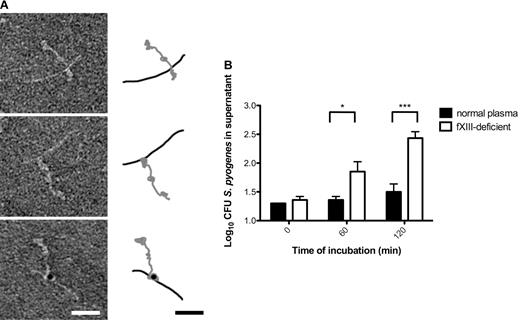
Most streptococcal serotypes have a high affinity for fibrinogen, and the M1 protein has been reported to be the most important fibrinogen receptor of the S pyogenes AP1 strain.22 The respective binding sites were mapped to the aminoterminal region of M1 protein and fragment D, which is part of the terminal globular domain of fibrinogen.22 Negative-staining electron microscopy was used to study the interaction between M1 protein and fibrinogen at the molecular level. The results demonstrate that one terminal region of the streptococcal surface protein is in complex with a globular domain of fibrinogen (Figure 3A top panel), which is in good agreement with the mapping study. The nature of this complex was not altered when activated fXIII was coincubated with the 2 proteins (Figure 3A middle panel). Indeed, additional immunodetection with the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine revealed that the interaction site was covalently cross-linked by fXIII (Figure 3A bottom panel). M proteins are the most abundant surface proteins of streptococci and it is therefore plausible that the M1 protein of S pyogenes AP1 bacteria is one of the major interaction partners that is covalently attached to fibrin fibers by the action of fXIII. However, it cannot be excluded that other streptococcal surface proteins are also targeted by fXIII.
fXIII cross-links the streptococcal M1 protein with fibrinogen, leading to immobilization of bacteria within the clot. (A) The electron micrographs show negatively stained human fibrinogen (characterized by 3 domains) in complex with M1-protein (elongated) before (top) and after fXIII cross-linking (middle). Cross-linking was detected by immunostaining the fibrinogen M1 protein complex with the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine (bottom). A schematic drawing of the fibrinogen (gray) and M1 protein (black) is included to highlight the interaction between fibrinogen and M1 protein. The scale bars indicate 25 nm. (B) Bacteria were incubated with normal or fXIII-deficient plasma and clotting was initiated by the addition of thrombin. Clots were washed briefly, covered with THB medium, and further incubated at 37°C. At the indicated time points, bacterial numbers were determined by plating of serial dilutions of the supernatant onto blood agar. The data represent the means ± SD of 3 independent experiments. *P < .05; ***P < .001.
fXIII cross-links the streptococcal M1 protein with fibrinogen, leading to immobilization of bacteria within the clot. (A) The electron micrographs show negatively stained human fibrinogen (characterized by 3 domains) in complex with M1-protein (elongated) before (top) and after fXIII cross-linking (middle). Cross-linking was detected by immunostaining the fibrinogen M1 protein complex with the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine (bottom). A schematic drawing of the fibrinogen (gray) and M1 protein (black) is included to highlight the interaction between fibrinogen and M1 protein. The scale bars indicate 25 nm. (B) Bacteria were incubated with normal or fXIII-deficient plasma and clotting was initiated by the addition of thrombin. Clots were washed briefly, covered with THB medium, and further incubated at 37°C. At the indicated time points, bacterial numbers were determined by plating of serial dilutions of the supernatant onto blood agar. The data represent the means ± SD of 3 independent experiments. *P < .05; ***P < .001.
Whether cross-linking of bacteria by fXIII has a pathophysiologic function inside the clot was investigated by measuring the escape of S pyogenes AP1 bacteria from clots generated from normal and fXIII-deficient plasma. Streptococci were mixed with undiluted normal or fXIII-deficient plasma and clotting was induced by the addition of thrombin. Clots were then washed with PBS and covered with growth medium. At different time points, samples were collected from the supernatant and the bacterial load was determined. As seen in Figure 3B, fXIII-induced cross-linking significantly reduced the release of bacteria from the clot, suggesting that they are immobilized and killed within the clot. These results show that S pyogenes bacteria are covalently woven into a fibrin network by the action of fXIII and therefore their dissemination from the clot is prevented.
S pyogenes–infected fXIII−/− mice show more signs of inflammation than wild-type animals at the local focus of infection
In vitro data suggest that coagulation is part of the early innate immune response, which in a concerted action triggers the immobilization and killing of S pyogenes inside a clot. We therefore hypothesized that the prevention of bacterial dissemination and their clearance may dampen the inflammatory response at the site of infection. To investigate this, we took advantage of a skin infection model that was established with KTL3, another S pyogenes strain of the M1 serotype.16 Challenge with S pyogenes KTL3 normally causes local infections that eventually disseminate from the infection focus and lead to systemic infection.16 Using scanning electron microscopic analysis, we found that incubation of S pyogenes KTL3 with thrombin-activated human normal or fXIII-deficient plasma in vitro generates clots with a morphology similar to those generated with S pyogenes AP1 bacteria (data not shown). Similar results were also obtained when murine plasma (normal and fXIII-deficient) was incubated with S pyogenes KTL3 bacteria (supplemental Figure 2).
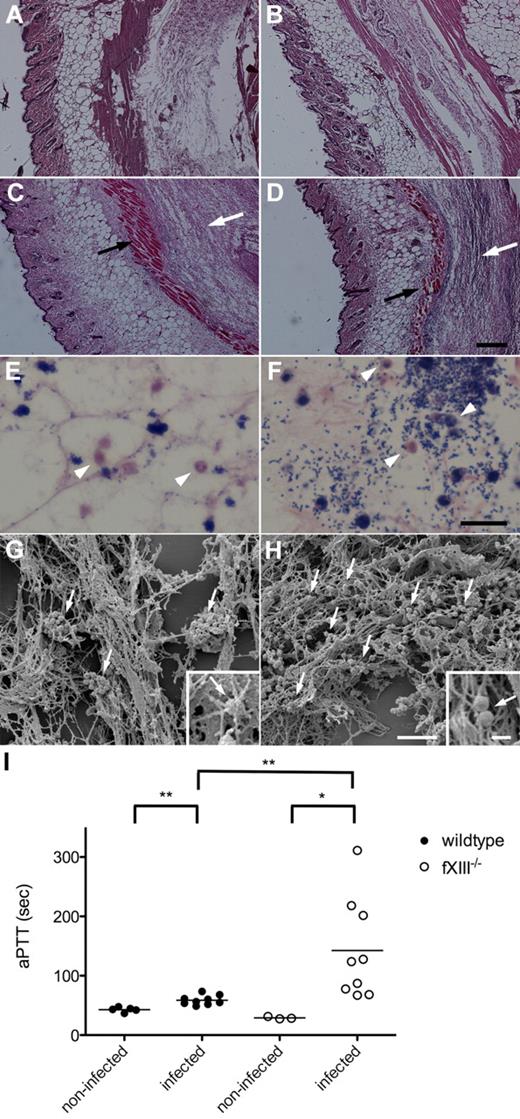
To study the inflammatory response to local infection with S pyogenes, wild-type and fXIII−/− mice were subcutaneously infected with S pyogenes KTL3. Twenty-four hours after infection, mice were killed and the skin from the local focus of infection was surgically removed and stained for histopathologic analysis. Microscopic examination of H&E–stained skin biopsies from noninfected wild-type and fXIII−/− mice revealed no signs of inflammation (Figure 4A-B), whereas cell invasion and tissue damage were seen in biopsies from infected wild-type animals (Figure 4C). The lesions were far more severe in biopsies from infected fXIII−/− mice (Figure 4D). Biopsies were also stained with Giemsa to detect infiltrating cells. Analysis of tissue samples from wild-type animals showed that bacteria were found in clustered patches and some neutrophils had been recruited (Figure 4E). In biopsies from fXIII−/− mice, bacteria were scattered throughout the whole infection site and an increased number of neutrophils was detected (Figure 4F).
Subcutaneous infection of wild-type and fXIII−/− mice with S pyogenes. H&E–stained representative tissue sections from noninfected (A-B) and infected (24 hours after infection; C-D) wild-type (A,C) and fXIII−/− (B,D) mice are shown. Infected animals show signs of inflammation (white arrows) and tissue damage (black arrows). The scale bar indicates 500 μm. Giemsa-stained biopsies from the inflamed area of wild-type (E) and fXIII−/− mice (F) are shown. Arrowheads point to infiltrating inflammatory cells (macrophages/neutrophils). The scale bar indicates 25 μm. Scanning electron micrographs depict biopsies from wild-type (G) and fXIII−/− (H) mice. Arrows indicate bacteria entrapped and clustered within the fibrin meshwork in wild-type mice (G) and scattered throughout the infection area in fXIII−/− animals (H). Scale bars indicate 10 μm in the figure and 1 μm in the insets. (I) aPTT measured in plasma from noninfected and infected wild-type and fXIII−/− mice (24 hours after infection). Data are presented as mean values of plasma samples obtained from 3 or 5 noninfected and 9 infected animals obtained from 3 independent experiments. *P < .05; **P < .01.
Subcutaneous infection of wild-type and fXIII−/− mice with S pyogenes. H&E–stained representative tissue sections from noninfected (A-B) and infected (24 hours after infection; C-D) wild-type (A,C) and fXIII−/− (B,D) mice are shown. Infected animals show signs of inflammation (white arrows) and tissue damage (black arrows). The scale bar indicates 500 μm. Giemsa-stained biopsies from the inflamed area of wild-type (E) and fXIII−/− mice (F) are shown. Arrowheads point to infiltrating inflammatory cells (macrophages/neutrophils). The scale bar indicates 25 μm. Scanning electron micrographs depict biopsies from wild-type (G) and fXIII−/− (H) mice. Arrows indicate bacteria entrapped and clustered within the fibrin meshwork in wild-type mice (G) and scattered throughout the infection area in fXIII−/− animals (H). Scale bars indicate 10 μm in the figure and 1 μm in the insets. (I) aPTT measured in plasma from noninfected and infected wild-type and fXIII−/− mice (24 hours after infection). Data are presented as mean values of plasma samples obtained from 3 or 5 noninfected and 9 infected animals obtained from 3 independent experiments. *P < .05; **P < .01.
Further electron microscopy examination of the tissue biopsies revealed severe bleeding across the infected site in both wild-type and fXIII−/− mice (data not shown). Bacteria were found entrapped and clustered within the fibrin meshwork of infected wild-type mice (Figure 4E), whereas bacteria were distributed throughout the whole infection site in skin biopsies from infected fXIII−/− mice (Figure 4F). Additional statistical analysis revealed approximately 8 bacterial clusters per 100 μm2 in the fibrin network of wild-type animals (Figure 4G), whereas streptococci were mostly seen as single bacteria or small chains at a density of 41 bacteria chains/100 μm2 in fXIII−/− animals (Figure 4H). At higher magnification it appears that bacteria are an integral part of the fibrin network from infected wild-type mice (Figure 4G inset). This was not observed in biopsies from fXIII−/− mice, in which streptococci were found to be associated with, but not a constituent part of, the network (Figure 4H inset). To assess the contribution of immobilization of bacteria to their dissemination, clotting times of the intrinsic pathway of coagulation (aPTTs) were measured. Increased aPTTs are a sign of a systemic response to the infection.12 Plasma samples were recovered 24 hours after infection and clotting times of the intrinsic pathway of coagulation were determined. Figure 4I shows that the aPTTs of plasma samples from infected wild-type mice were moderately but significantly increased, whereas clotting times were extremely high in plasma samples from fXIII−/− mice. The PT remained unaltered after 24 hours of infection in both groups of mice (data not shown). These results demonstrate that the lack of fXIII leads to an increased inflammatory response at the infectious site combined with an induction of systemic reactions.
fXIII cross-linking in patients with necrotizing fasciitis caused by S pyogenes
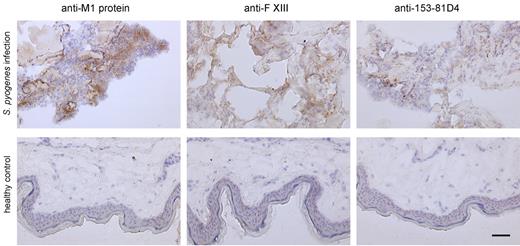
To determine whether the results obtained from the animal studies also apply to the clinical situation, biopsies from patients with necrotizing fasciitis caused by S pyogenes were analyzed by immunohistology and electron microscopy. Figure 5 depicts massive tissue necrosis at the site of infection, and subsequent immunodetection in serial tissue sections showed positive staining for the M1 protein and fXIII at these sites. This suggests an influx of plasma to the infected focus, and indeed cross-linking activity at the same location was recorded (Figure 5 top lane). As controls, biopsies from healthy persons were used, in which no signal was seen when subjected to the same experimental protocol (Figure 5 bottom lane). Tissue sections were further analyzed by confocal immunofluorescence microscopy using antibodies against M1 protein and N-ϵ-γ-glutamyl-lysine. Figure 6A shows positive staining for M1 protein and widespread positive staining for N-ϵ-γ-glutamyl-lysine. In addition, we found a striking colocalization of the 2 antibodies (Figure 6A), suggesting bacterial cross-linking at the infected site. When the biopsies were analyzed by scanning electron microscopy, massive bleeding at the infected site was recorded (data not shown), and bacteria were found to be clustered and entrapped inside the fibrin network (Figure 6B). Specimens were also thin-sectioned and studied by immunotransmission electron microscopy using the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine. Figure 6C shows immunostaining at the bacterial surface in regions in contact with fibrin fibers. The micrographs also reveal that a significant portion (31%) of the entrapped bacteria were not viable, as shown in Figure 6D. These findings are in agreement with the in vitro and in vivo experiments, and illustrate that immobilization of bacteria and generation of antimicrobial activity is seen within the fibrin network in patients with severe and invasive infections with S pyogenes.
Immunohistochemical analysis of human biopsies. Tissue biopsies were obtained from patients with necrotizing fasciitis caused by S pyogenes (top) and from healthy volunteers (bottom). The biopsies were sectioned and immunohistochemically stained for streptococcal M1 protein, fXIII, and N-ϵ-γ-glutamyl-lysine. Stainings without primary antibodies were negative (data not shown). The scale bars indicate 50 μm.
Immunohistochemical analysis of human biopsies. Tissue biopsies were obtained from patients with necrotizing fasciitis caused by S pyogenes (top) and from healthy volunteers (bottom). The biopsies were sectioned and immunohistochemically stained for streptococcal M1 protein, fXIII, and N-ϵ-γ-glutamyl-lysine. Stainings without primary antibodies were negative (data not shown). The scale bars indicate 50 μm.
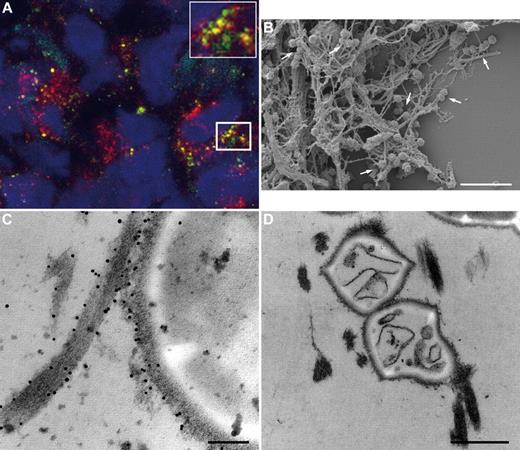
Colocalization of M1 protein and the fXIII cross-linking site in human biopsies. (A) Tissue biopsies from patients with streptococcal necrotizing fasciitis were sectioned and immunofluorescently stained for M1 protein (green) in combination with anti–N-ϵ-γ-glutamyl-lysine (red). Confocal microscopy revealed colocalization of both antibodies, seen at higher magnification in the inset figure. Cell nuclei are stained in blue with DAPI. (B) Scanning electron microscopy showing bacteria entrapped in the fibrin network (arrows) in a biopsy from a patient with streptococcal necrotizing fasciitis. Scale bar indicates 5 μm. (C) Transmission electron micrograph displaying fXIII-mediated cross-linking of bacterial surface proteins to fibrin by detection of the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine. The scale bar indicates 100 nm. (D) Transmission electron microscopy shows dead bacteria inside the fibrin network in a biopsy from a patient with streptococcal necrotizing fasciitis. The scale bar indicates 0.5 μm.
Colocalization of M1 protein and the fXIII cross-linking site in human biopsies. (A) Tissue biopsies from patients with streptococcal necrotizing fasciitis were sectioned and immunofluorescently stained for M1 protein (green) in combination with anti–N-ϵ-γ-glutamyl-lysine (red). Confocal microscopy revealed colocalization of both antibodies, seen at higher magnification in the inset figure. Cell nuclei are stained in blue with DAPI. (B) Scanning electron microscopy showing bacteria entrapped in the fibrin network (arrows) in a biopsy from a patient with streptococcal necrotizing fasciitis. Scale bar indicates 5 μm. (C) Transmission electron micrograph displaying fXIII-mediated cross-linking of bacterial surface proteins to fibrin by detection of the gold-labeled antibody against N-ϵ-γ-glutamyl-lysine. The scale bar indicates 100 nm. (D) Transmission electron microscopy shows dead bacteria inside the fibrin network in a biopsy from a patient with streptococcal necrotizing fasciitis. The scale bar indicates 0.5 μm.
Local treatment with fXIII dampens systemic bacterial spreading in infected mice
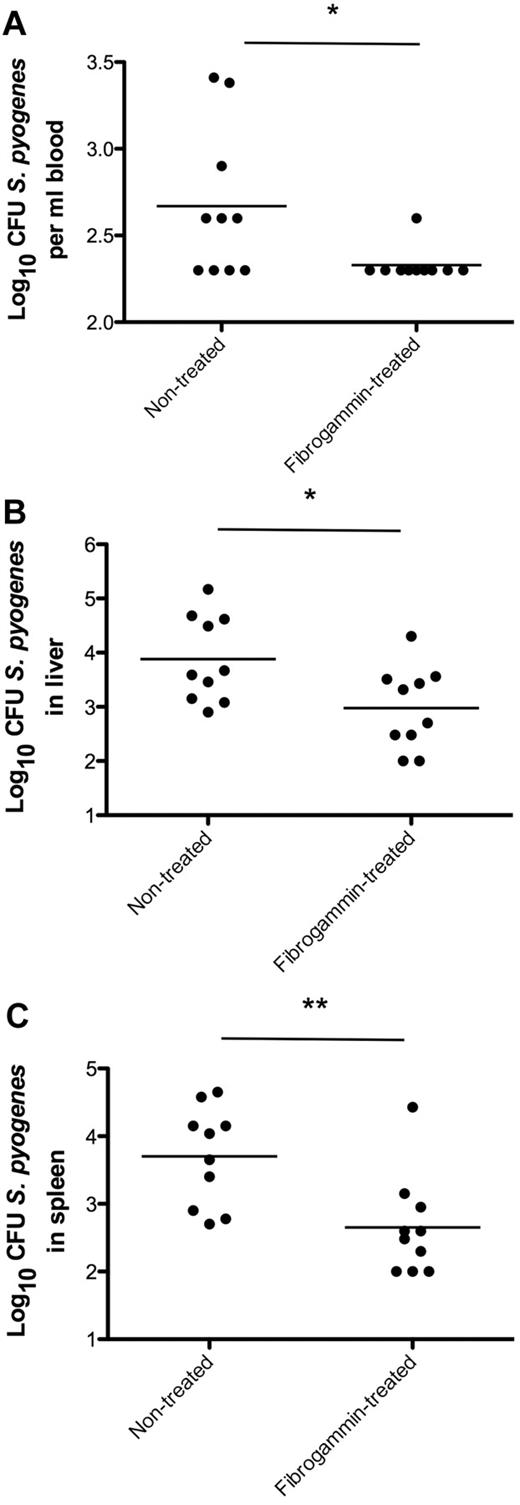
To determine whether treatment with fXIII is able to prevent bacterial spreading in an animal model of infection, wild-type mice were subcutaneously infected with S pyogenes. Three hours after challenge, half of the mice were treated with FibrogamminP, a human plasma fXIII concentrate that was injected into the site of infection. A dose of 200 U/kg body weight was chosen, which is well tolerated in mice and gives rise to approximately 250% of total fXIII activity when injected intravenously.23 Mice infected with S pyogenes but without FibrogamminP treatment served as controls. Infected animals were killed after 24 hours of infection, and bacterial loads in the blood, liver, and spleen were determined. As depicted in Figure 7, FibrogamminP treatment resulted in decreased bacterial loads in the blood, liver, and spleen of the treated mice, suggesting that fXIII dampens the systemic dissemination of S pyogenes in the infected animals.
fXIII-Treatment of wild-type mice and bacterial dissemination. Mice received a subcutaneous injection of S pyogenes and were treated with FibrogamminP 3 hours after infection. Nontreated mice served as controls. Twenty-four hours after infection, mice were killed and bacterial loads in the blood (A), liver (B), and spleen (C) were determined. Data are presented as means of 10 mice per group and were obtained from 3 independent experiments. *P < .05; **P < .01.
fXIII-Treatment of wild-type mice and bacterial dissemination. Mice received a subcutaneous injection of S pyogenes and were treated with FibrogamminP 3 hours after infection. Nontreated mice served as controls. Twenty-four hours after infection, mice were killed and bacterial loads in the blood (A), liver (B), and spleen (C) were determined. Data are presented as means of 10 mice per group and were obtained from 3 independent experiments. *P < .05; **P < .01.
The results presented in this study support the concept that fXIII has an important role in the early immune response to bacterial infections. We have shown that fXIII triggers an immobilization of bacteria within the fibrin network at the local focus of infection, which is combined with an induction of plasma-derived antimicrobial activity and subsequent bacterial killing. The 2 mechanisms work in concert and may together diminish early bacterial dissemination and down-regulate the inflammatory response.
Discussion
Sensing the first signs of inflammation and rapid elimination of an invading microorganism are key features of the early immune response to infection. In particular, potential ports of microbial entry are at great risk and therefore need special protection. Therefore, the immune system has developed mechanisms that allow an efficient clearance of inhaled (eg, with the help of mannose-binding lectin24 ) or swallowed (eg, by the action of intestinal mucins25 ) pathogens. Wounds present another port of entry and are associated with a great risk of promoting infections and allowing microorganisms to enter the circulatory system. To prevent their dissemination and eventual systemic complications, it is of great importance that the host's defense system is activated as soon as wound sealing begins. It therefore appears likely that coagulation plays an important role in these very early processes. However, the extent and underlying mechanisms of this contribution to immunity are poorly understood.
In the present study, we show for the first time that, in addition to its proinflammatory role, coagulation plays an active role in the containment and elimination of bacteria in infections caused by S pyogenes. Our data support a model based on 2 separate mechanisms involving a fXIII-triggered covalent immobilization of microorganisms inside the fibrin network and the generation of antimicrobial activity. We found that clotting is activated at the bacterial surface via the intrinsic pathway of coagulation also referred to as the contact system or kallikrein/kinin system. Apart from bacteria,26 fungi27 and viruses28 have also been reported to interact with the contact system, supporting the notion that contact activation is subject to the principles of pattern recognition.29 The system is activated within seconds and leads to the release of antimicrobial peptides21,30 and inflammatory mediators,31 further supporting its role in early innate immunity. In addition to the generation of antimicrobial peptides due to activation of the intrinsic pathway of coagulation, processing of thrombin has recently been shown to release host defense peptides with a broad specificity.32 However, the extent to which theses peptides contribute to the antimicrobial activity seen in the present study and the ability of these peptides to kill other bacterial species need to be clarified.
The in vivo data presented herein show that the lack of fXIII evokes pathologic inflammatory reactions, which is illustrated by a massive neutrophil influx to the site of infection and subsequent tissue destruction as seen in the infected mice. The inability to immobilize bacteria in a fibrin network leads to a dramatic increase of the intrinsic-driven clotting time in these animals, which is an indication of more severe systemic infection in the knockout compared with wild-type mice. These findings are in agreement with a recent report by Sun et al,33 who used mice deficient in factor V or fibrinogen to show that local thrombosis/fibrin deposition limits the survival and dissemination of group A streptococci.33 Many bacterial pathogens are able to activate plasminogen at their surface using different modes of action.34 This mechanism would allow bacteria to escape their entrapment in a fibrin network. For example, it has been reported for Yersinia pestis that mutant strains lacking the plasminogen activator Pla failed to cause an otherwise systemic infection when tested in an subcutaneous murine infection model.35
Human plasma fXIII is fully active in mice,23 and as a proof of concept we administered the human protein in a murine infection model. When wild-type mice were treated with human plasma fXIII, dissemination of S pyogenes was significantly reduced compared with nontreated mice. Recent findings showing that fXIII also cross-links surface proteins from other bacterial species, such as E coli and S aureus bacteria,9 imply that the mechanism described herein is an important part of the early immune response. Our results underline the importance of fXIII in the early defense against S pyogenes and suggest that fXIII is an interesting target for the development of novel antimicrobial therapies.
Clotting has been previously implicated in immunity in invertebrate models, where its immune function is more visible because of the lack of redundancy with adaptive effector mechanisms. One of the best-studied examples is the clotting system of the horseshoe crab, which is triggered by minute amounts of bacterial elicitors such as lipopolysaccharide. This leads to the production of antimicrobial activity and communication with other effector systems. Similarly, there may be cross-talk between complement and blood clotting, for example, via the binding of ficolin to fibrin/fibrinogen.36 The picture that emerges from evolutionary comparisons is that proteolytic cascades and their constituent proteases are used as flexible modules that can be triggered by endogenous and exogenous microbial elicitors.37 Even the same proteolytic event can be activated by distinct elicitors in different contexts. One such example is the cleavage of the Drosophila protein Spätzle, which can act as a key signal both during development and in the immune system. In both cases, cleaved Spätzle binds to Toll, the founding member of the TLR family. We show here that blood clotting, which has so far been mostly been studied in the context of its physiologic hemostatic function, plays a key role in immunity both as an effector mechanism and by communicating with other branches of the immune system. This leads to fast and efficient instant immune protection, which keeps infections localized and leaves additional time for other effector mechanisms to be activated.5
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Monica Heidenholm and Maria Baumgarten for excellent technical assistance and Rita Wallén and Eric Hallberg for help with electron microscopy.
This work was supported in part by the foundations of Alfred Österlund, Crafoord, Greta and Johan Kock, the German Research Foundation (DFG grant LO1620/1-1 to T.G.L.), King Gustav V's 80th Anniversary Foundation, Hansa Medical AB, the Medical Faculty at Lund University, and the Swedish Research Council (project 7480). L.J. was supported by the Swedish Society for Medical Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: T.G.L. performed the research, analyzed the data, and wrote the paper; M.M. contributed analytic tools and performed the research; L.J., S.O., and A.I.O. performed the research; G.D. and A.N.-T. contributed analytic tools; and U.T. and H.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: CSL Behring GmbH (Marburg, Germany) and Hansa Medical AB (Lund, Sweden) are in the process of filing a patent application on fXIII, for which G.D., H.H., M.M., T.G.L., and U.T. are listed as inventors. The remaining authors declare no competing financial interests.
Correspondence: Heiko Herwald, Department of Clinical Sciences, Section for Clinical and Experimental Infection Medicine, Lund University, BMC B14, Tornavägen 10, S-221 84 Lund, Sweden; e-mail: Heiko.Herwald@med.lu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal