Abstract
Any role for reduced-intensity conditioning (RIC) before hematopoietic cell transplantation (HCT) from a human leukocyte antigen (HLA)–haploidentical donor remains to be defined. We therefore assessed 83 patients (age, 16-70 years): 68 with acute leukemia (including 34 in remission and 34 with refractory disease) and 15 patients with myelodysplastic syndrome, in HCT trials using RIC with busulfan, fludarabine, and antithymocyte globulin. The HLA-haploidentical donors, offspring (n = 38), mothers (n = 24), or siblings (n = 21) of patients, underwent leukapheresis after receiving granulocyte colony-stimulating factor, and donated cells were transplanted without further manipulation. Cyclosporine and methotrexate were given for GVHD prophylaxis. The cumulative incidences of neutrophil engraftment, grade 2 to 4 acute GVHD, chronic GVHD, and transplantation-related mortality after HCT, were 92%, 20%, 34%, and 18%, respectively. After a median follow-up time of 26.6 months (range, 16.8-78.8 months), the event-free and overall survival rates were 56% and 45%, respectively, for patients with acute leukemia in remission; 9% and 9%, respectively, for patients with refractory acute leukemia; and 53% and 53%, respectively, for patients with myelodysplastic syndrome. HCT from an HLA-haploidentical family member resulted in favorable outcomes when RIC containing antithymocyte globulin was performed. This study is registered at www.clinicaltrials.gov as #NCT00521430 and #NCT00732316.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) from a human leukocyte antigen (HLA)-matched donor from a patient's family or an unrelated donor registry is curative for a significant proportion of patients with acute leukemia and myelodysplastic syndrome (MDS). However, a suitable HLA-matched hematopoietic cell donor is available for only approximately 60% of patients who require allogeneic HCT; the values for patients from ethnic minorities are lower.1 Among other potential graft sources for patients who lack an HLA-matched donor are HLA-haploidentical familial donors,2-4 HLA partially matched unrelated donors,5 and unrelated umbilical cord blood.6 HLA-haploidentical familial donors are almost always available, allowing the graft to be obtained without undue delay. Further, such persons can make additional donations for repeat HCT, donor lymphocyte infusion, or investigational cell therapy.7,8
During allogeneic HCT, especially using traditional myeloablative conditioning, the extent of HLA mismatch between donor and recipient is a barrier to successful donor cell engraftment.3,4 Although consistent engraftment was achieved after HCT from related donors with fully matched HLA antigens or a single mismatch at the HLA-A, -B, or -DR locus,9 the rate of graft failure increased after HCT from related donors with 2 or 3 HLA antigen mismatches.2,3 HLA-mismatched HCT also caused the incidences of acute and chronic GVHD and delayed immune recovery to rise, and increased the rate of transplantation-related mortality (TRM).2-4,10
In animal HCT models,11-13 as well as in clinical HCT settings,14-18 donor hematopoietic cells can engraft across a haplotype mismatch in the major histocompatibility complex (MHC) after reduced-intensity conditioning (RIC); the success rates of engraftment ranges to > 90%.13,14,17,18 Most RIC regimens feature the use of monoclonal13,15 or polyclonal antibodies14,18 against T cells; alternatively, RIC is sometimes followed by early post-transplantation administration of cyclophosphamide.16,17 Further, such grafts are not depleted of T cells ex vivo. Remarkably, after HLA-haploidentical HCT using such conditioning regimens, the rates of grade 2 to 4 acute GVHD (16%-43%), chronic GVHD (14%-30%), and TRM (9%-15%) were relatively low.14,16-18 Therefore, there are needs to investigate the role of RIC in HLA-haploidentical HCT further.
In a previous study involving 29 patients with acute leukemia or MDS,18 we showed that HCT from an HLA-haploidentical family donor was feasible without ex vivo T-cell depletion after RIC with busulfan, fludarabine, and antithymocyte globulin (ATG). We have extended our work with HLA-haploidentical HCT using the same approach in 54 additional patients. We therefore describe our experience with HLA-haploidentical HCT in our entire cohort of 83 patients with acute leukemia or MDS.
Methods
Patients and hematopoietic cell donors
The 83 included patients consisted of 47 males and 36 females, of median age 40 years (range, 16-70 years) who underwent HLA-haploidentical HCT between April 2004 and December 2009. Of these patients, 71 were unable to find a suitable HLA-matched donor in their families or donor registries; whereas in 12 patients, who had undergone allogeneic HCT previously, HLA-haploidentical HCT was performed as a salvage treatment. All patients were aged 15 to 75 years old and had been diagnosed with high-risk acute leukemia, including acute myelogenous leukemia (AML) in the first complete remission (CR1) but with high-risk chromosomal changes,19 acute lymphoblastic leukemia (ALL) in CR1 with chromosomal changes of t(9;22) or t(4;11), or acute leukemia past CR1; or had been diagnosed with high-risk MDS (refractory anemia with excess blasts-1, -2, or chronic myelomonocytic leukemia); or low-risk MDS (refractory anemia or refractory cytopenia with multilineage dysplasia) with failure to respond or recurrent cytopenia after treatment with ATG or hypomethylating agents. Other eligibility criteria included a Karnofsky performance score more than or equal to 70 and adequate organ function (total bilirubin < 2.0 mg/dL, aspartate aminotransferase < 120 U/L, creatinine < 2.0 mg/dL), and a cardiac ejection fraction > 45% (by a multigated blood pool scan). After December 2008, patient-eligibility criteria were expanded to include AML patients in CR1 with initial intermediate-risk chromosomal findings19 or with comorbidities precluding high-dose cytosine arabinoside consolidation chemotherapy, as well as ALL patients in CR1 regardless of initial chromosomal findings. All patients had at least 1 family member, 70 years of age or younger, who was mismatched at 1 to 3 of the 6 HLA-A, -B, and -DR loci. The HCT treatment protocols were approved by the institutional review boards of the Asan Medical Center and Pusan National University Hospital, and were listed on the National Institutes of Health clinical trial registry at www.clinicaltrials.gov as #NCT00521430 and #NCT00732316. All patients and donors participating in the study provided written informed consent in accordance with the Declaration of Helsinki.
Transplantation procedures
The RIC therapy consisted of busulfan (Busulfex, Otsuka Pharmaceuticals) 3.2 mg/kg per day intravenously on days −7 and −6 of HCT (day 0 being the first day of donor cell infusion), fludarabine 30 mg/m2 per day intravenously on days −7 to −2, and rabbit ATG (thymoglobulin, Genzyme Transplant) 3 mg/kg per day on days −4 to −1. Two patients transplanted in 2005 received horse ATG (Lymphoglobulin, Genzyme Transplant) 15 mg/kg per day instead of rabbit ATG. Seven patients transplanted after September 2009 received thymoglobulin on days −3 to −1 only (total dose, 9 mg/kg).
Starting on day −3, each hematopoietic cell donor was given granulocyte colony-stimulating factor (G-CSF) 10 μg/kg per day subcutaneously for 4 to 5 days. Starting on the fourth day (day 0 of HCT) of G-CSF administration, donor mononuclear cells were harvested by large-volume leukapheresis, with the goal of collecting at least 5 × 106 CD34+ cells per kilogram of recipient body weight. The collected cells were administered on the same day to the designated patient through a central venous catheter. Sixty-eight donors (82%) required cell collections on 2 days (days 0 and 1), 13 (16%) required 3 collections, and 1 each required 4 and 5 collections.
GVHD prophylaxis and supportive care
For GVHD prophylaxis, patients were given cyclosporine 1.5 mg/kg intravenously every 12 hours starting on day −1, and subsequently switched to a 1.5- to 2-fold greater oral dose of cyclosporine. The blood concentrations of cyclosporine were monitored at least once weekly and were referenced to 100 to 300 ng/mL. In addition, patients received methotrexate 15 mg/m2 intravenously 1 day and 10 mg/m2 3, 6, and 11 days after the last donor cell infusion. The dose of cyclosporine was halved if serum creatinine concentration increased to twice the baseline level and was discontinued if the concentration rose to 3 times the baseline level. The dose of methotrexate was halved if serum creatinine concentration increased to twice the baseline level or if a patient developed a grade 2 hepatic toxicity or stomatitis, and was withheld if serum creatinine increased to 3 times the baseline level or if a patient developed grade 3 hepatic toxicity or stomatitis. Starting 30 to 60 days after HCT, the cyclosporine dose was decreased by 10% every 2 to 4 weeks in patients with no evidence of GVHD. In 7 patients who were transplanted after September 2009, the last dose of methotrexate was omitted.
Supportive care for patients included intravenous diphenylhydantoin during busulfan administration, oral ciprofloxacin, and antifungal agents (oral fluconazole or intravenous echinocandin) from the start of conditioning therapy, and intravenous G-CSF 450 μg/day starting on day 5 of HCT. Patients were given ciprofloxacin, antifungal agents, and G-CSF until absolute neutrophil counts (ANCs) recovered to 3000/μL. γ-Globulin 0.5 g/kg was administered intravenously on days −7 or 7 of HCT, biweekly until day 91, and monthly until day 180. Patients were monitored weekly until day 100 for cytomegalovirus (CMV) using the pp65 antigenemia assay; those who were positive for CMV received preemptive treatment with ganciclovir. After February 2008, patients were tested for Epstein-Barr virus (EBV) DNA in blood by quantitative PCR every 2 weeks until day 100.
Patient monitoring
The first 2 consecutive days on which ANCs were ≧ 500/μL and the first 7 consecutive days of unsupported platelet counts ≧ 20 000/μL were recorded as the times of neutrophil and platelet engraftment, respectively. Bone marrow was biopsied on the day before conditioning therapy and 4 to 6 weeks after HCT. CR was defined as the recovery of peripheral blood ANCs to > 1000/μL and evidence of donor cell regeneration in a bone marrow aspirate, with 5% or fewer blasts. Recurrence was defined as > 5% leukemic blasts in a bone marrow sample after a previously documented CR. In patients with persistent leukemia after HCT, the day of progression was defined as the day on which leukemia blasts reappeared in peripheral blood, with bone marrow examination showing > 5% persistent blasts. Toxic effects of RIC (ie, stomatitis, nausea/vomiting, and diarrhea) were graded according to the Common Toxicity Criteria Version 2.0 (National Cancer Institute). Hepatic veno-occlusive disease20 and acute21 and chronic GVHD22 were diagnosed and graded as described. Hematopoietic chimerism after HCT was analyzed using a PCR-based procedure assaying short tandem repeats in DNA. Posttransplantation immune reconstitution was estimated by counting CD4+ lymphocytes, CD8+ lymphocytes, and NK cells in peripheral blood at initiation of conditioning therapy and 1, 2, 3, 6, and 12 months after HCT.
Statistical analyses
Outcome included donor cell engraftment, occurrence of GVHD, disease progression/recurrence, graft failure, TRM, and event-free and overall patient survival. Time to engraftment, acute and chronic GVHD, progression/recurrence of underlying disease, graft failure, and TRM were measured from the time of transplantation, estimated using the cumulative incidence function23 and compared using Gray method.24 Death and progression/recurrence of underlying disease were considered as competing risks in analyses of engraftment, GVHD, and graft failure. TRM and progression/recurrence were considered to be mutually competing risks. Event-free survival was measured from HCT to disease progression/recurrence, TRM, or last follow-up. Overall survival was measured from HCT to death or last follow-up. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test.
To identify pretransplantation variables that might predict outcomes of HLA-haploidentical HCT, the following variables were analyzed in univariate analysis: age (≤ 50 vs > 50 years), gender, disease status at HCT (standard vs high risk), previous allogeneic HCT, prior HCT (allogeneic or autologous), ATG doses (3 vs 4), donor type (offspring vs mothers vs siblings), donor age (≤ 40 vs > 40 years), donor gender, donor-patient HLA-mismatch number by antigen (≤ 2 of 6 vs 3 of 6), donor-patient HLA mismatches by allele (≤ 3 of 8 vs 4 of 8), donor-patient NK-cell alloreactivity,25 graft mononuclear cell dose (≤ 7 vs > 7 × 108/kg), CD34+ cell dose (≤ 5 vs > 5 × 106/kg), and CD3+ cell dose (≤ 5 vs > 5 × 108/kg). For multivariate analysis, each variable with a P < .2, as determined by univariate analysis, was considered for entry into the model selection process, based on the Fine and Gray model (GVHD, disease progression/recurrence, and TRM),26 or Cox proportional hazards model (event-free and overall survival figures).
Results
Patient and donor characteristics
The demographic characteristics of patients and donors are summarized in Table 1. Of the 83 patients, 52 had AML, 16 ALL, 9 high-risk MDS, and 6 low-risk MDS. Of the 68 patients with acute leukemia, 15 were in CR1 status at the time of HCT, 18 in the second CR (CR2), 1 in the third CR (CR3), and 34 had refractory disease. Of the 15 patients with acute leukemia in CR1, 12 had AML; of these, 6 showed normal initial bone marrow cytogenetic findings; 4 had high-risk cytogenetic changes (including 2 with complex abnormalities and 1 each with del3q and t11;19, respectively); and 1 had a favorable-risk cytogenetic changes of t(8;21). Initial bone marrow cytogenetic data were not available for one patient. The patient with the initial cytogenetic change of t(8;21) had mild, chronic azotemia, was not thought to be a candidate for high-dose cytosine arabinoside consolidation chemotherapy, and was therefore enrolled in the HLA-haploidentical HCT protocol. At the time of transplantation, 54 patients (65%) were deemed to be unsuitable candidates for myeloablative conditioning for HCT: 23 because of age ≧ 50 years, 17 because of previous HCT (12 allogeneic and 5 autologous), 11 because of immediate prior exposure to intensive and/or multiple cycles of salvage chemotherapy, 2 because of poor general condition, and 1 because of a comorbidity.
Patient and donor characteristics
| Characteristics . | No. of patients . |
|---|---|
| Median patient age, y (range) | 40 (16-70) |
| Sex | |
| Male | 47 |
| Female | 36 |
| Underlying disease | |
| AML (CR1/CR2/CR3/REF) | 52 (12/15/1/24) |
| ALL (CR1/CR2/REF) | 16 (3/3/10) |
| MDS (RA/RARS/RCMD) | 6 (1/1/4) |
| MDS (RAEB/CMMoL) | 9 (7/2) |
| Previous HCT | |
| HLA-matched sibling HCT | 7 |
| UD-HCT | 5 |
| Autologous HCT | 5 |
| Donors (of patients) | |
| Offspring | 38 |
| Mothers | 24 |
| Siblings | 21 |
| Donor sex | |
| Male | 45 |
| Female | 38 |
| Median donor age, y (range) | 36 (3-68) |
| No. of donor-patient HLA-A, -B, and -DR mismatch (by antigen) | |
| GVHD direction, 0/1/2/3 | 1/10/32/40 |
| Rejection direction, 1/2/3 | 13/32/38 |
| No. of donor patient HLA-A, -B, -C, and DRB1 mismatch (by allele) | |
| GVH direction, 1/2/3/4/unknown | 1/6/20/32/24 |
| Rejection direction, 1/2/3/4/unknown | 2/5/18/34/24 |
| Donor-patient NK-cell alloreactivity | |
| Yes/no/unknown | 10/49/24 |
| CMV IgG serology of patients | |
| Positive | 82 |
| Negative | 1 |
| CMV IgG serology of donors | |
| Positive | 80 |
| Negative | 3 |
| Characteristics . | No. of patients . |
|---|---|
| Median patient age, y (range) | 40 (16-70) |
| Sex | |
| Male | 47 |
| Female | 36 |
| Underlying disease | |
| AML (CR1/CR2/CR3/REF) | 52 (12/15/1/24) |
| ALL (CR1/CR2/REF) | 16 (3/3/10) |
| MDS (RA/RARS/RCMD) | 6 (1/1/4) |
| MDS (RAEB/CMMoL) | 9 (7/2) |
| Previous HCT | |
| HLA-matched sibling HCT | 7 |
| UD-HCT | 5 |
| Autologous HCT | 5 |
| Donors (of patients) | |
| Offspring | 38 |
| Mothers | 24 |
| Siblings | 21 |
| Donor sex | |
| Male | 45 |
| Female | 38 |
| Median donor age, y (range) | 36 (3-68) |
| No. of donor-patient HLA-A, -B, and -DR mismatch (by antigen) | |
| GVHD direction, 0/1/2/3 | 1/10/32/40 |
| Rejection direction, 1/2/3 | 13/32/38 |
| No. of donor patient HLA-A, -B, -C, and DRB1 mismatch (by allele) | |
| GVH direction, 1/2/3/4/unknown | 1/6/20/32/24 |
| Rejection direction, 1/2/3/4/unknown | 2/5/18/34/24 |
| Donor-patient NK-cell alloreactivity | |
| Yes/no/unknown | 10/49/24 |
| CMV IgG serology of patients | |
| Positive | 82 |
| Negative | 1 |
| CMV IgG serology of donors | |
| Positive | 80 |
| Negative | 3 |
REF indicates refractory disease; RA, refractory anemia; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RAEB, refractory anemia with excess blasts; CMMoL, chronic myelomonocytic leukemia; UD, unrelated donor; and NK, natural killer.
The 83 HLA-haploidentical family donors were offspring (median age, 26 years; range, 3-42 years), mothers (median age, 48 years; range, 40-68 years), or siblings (median age, 37 years; range, 21-51 years) of the patients. Between donors and patients, there was a mean of 2.23 mismatches of the 6 HLA-A, -B, and -DR antigens in the graft-versus-host direction and a mean of 2.30 mismatches of the 6 antigens in the rejection direction. When classified according to killer immunoglobulin-like receptor ligand incompatibility,25 10 of the 59 donor-patient pairs (17%) exhibited NK-cell alloreactivity. The median number of mononuclear cells transplanted was 8.1 × 108/kg of recipient body weight (range, 1.8-17.0 × 108/kg of recipient body weight), the median number of CD34+ cells transplanted was 7.5 × 10/kg6 of recipient body weight (range, 0.273.5 × 106/kg of recipient body weight), and the median number of donor CD3+ cells infused was 4.8 × 108/kg of recipient body weight (range, 0.5-10.0 × 108/kg of recipient body weight). Positive IgG serology for CMV was observed in 99% of patients and 96% of donors.
Engraftment and GVHD
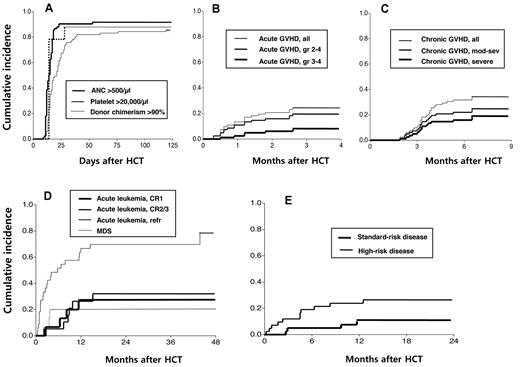
The clinical outcomes of HCT relative to disease status at the time of transplantation are shown in Table 2. The conditioning regimen of reduced-dose busulfan, fludarabine, and ATG was well tolerated, with 5%, 10%, and 19% of patients experiencing grade 3 or 4 stomatitis, nausea/vomiting, or diarrhea, respectively. Hepatic veno-occlusive disease occurred in 5 patients (6%; 1 mild and 4 moderate). Seventy-six recipient achieved initial donor cell engraftment with ANCs more than or equal to 500/μL at a median of 13.5 days (range, 9-53 days) after HCT (cumulative incidence, 92%; 95% confidence interval [CI], 86%-98%; Figure 1A). The remaining 7 patients experienced early disease progression (n = 4) or TRM (n = 3). No recipient experienced primary engraftment failure. Seventy-two patients achieved platelet counts more than or equal to 20 000/μL at a median of 17 days (range, 11-119 days) after HCT (cumulative incidence, 87%; 95% CI, 80%-94%).
Outcomes of HLA-haploidentical hematopoietic cell transplantation according to the disease status
| Outcomes . | Acute leukemia . | MDS (n = 15) . | ||
|---|---|---|---|---|
| CR1 (n = 15) . | CR2/CR3 (n = 19) . | REF (n = 34) . | ||
| Engraftment, ANCs > 500/μL | ||||
| Yes | 15 | 19 | 28 | 14 |
| Censored | — | — | 6 | 1 |
| Disease progression | — | — | 4 | 1 |
| TRM | — | — | 2 | — |
| Median days (range) | 12 (11-18) | 13 (11-18) | 14 (9-23) | 16 (11-53) |
| Engraftment, platelets > 20 000/μL | ||||
| Yes | 15 | 19 | 25 | 13 |
| Median days (range) | 15 (11-119) | 17 (11-27) | 22 (12-76) | 25 (13-105) |
| Acute GVHD | ||||
| All | 4 | 4 | 7 | 5 |
| Grades 2-4 | 3 | 3 | 6 | 4 |
| Grade 3 or 4 | 1 | 1 | 2 | 2 |
| Chronic GVHD | ||||
| All | 8 | 8 | 5 | 7 |
| Moderate to severe | 6 | 4 | 4 | 6 |
| Severe | 5 | 2 | 2 | 6 |
| Secondary graft failure | — | — | 2 | 2 |
| Positive CMV antigenemia | 11 | 17 | 22 | 8 |
| CMV diseases | 1 | 2 | 1 | — |
| Colitis | 1 | 2 | — | — |
| Esophagitis | — | — | 1 | — |
| EBV reactivation (/evaluated) | 9/14 | 8/11 | 9/15 | 4/9 |
| EBV-PTLD | 1 | — | 1 | 1 |
| Progression/recurrence of leukemia/MDS | 4 | 6 | 24 | 3 |
| TRM | 2 | 2 | 8 | 4 |
| Death | 6 | 9 | 30 | 7 |
| Causes of death | ||||
| Progression/recurrence of leukemia/MDS | 4 | 7 | 22 | 3 |
| Acute GVHD | — | 1 | 2 | 1 |
| Secondary graft failure | — | — | 1 | 2 |
| EBV-related PTLD | 1 | — | 1 | — |
| CMV colitis | — | 1 | — | — |
| Idiopathic interstitial pneumonitis | — | — | 1 | — |
| Pulmonary hemorrhage | — | — | — | 1 |
| H1N1 influenza pneumonia | 1 | — | — | — |
| Acute meningoencephalitis | — | — | 1 | — |
| Pneumonia | — | — | 1 | — |
| Sepsis | — | — | 1 | — |
| Outcomes . | Acute leukemia . | MDS (n = 15) . | ||
|---|---|---|---|---|
| CR1 (n = 15) . | CR2/CR3 (n = 19) . | REF (n = 34) . | ||
| Engraftment, ANCs > 500/μL | ||||
| Yes | 15 | 19 | 28 | 14 |
| Censored | — | — | 6 | 1 |
| Disease progression | — | — | 4 | 1 |
| TRM | — | — | 2 | — |
| Median days (range) | 12 (11-18) | 13 (11-18) | 14 (9-23) | 16 (11-53) |
| Engraftment, platelets > 20 000/μL | ||||
| Yes | 15 | 19 | 25 | 13 |
| Median days (range) | 15 (11-119) | 17 (11-27) | 22 (12-76) | 25 (13-105) |
| Acute GVHD | ||||
| All | 4 | 4 | 7 | 5 |
| Grades 2-4 | 3 | 3 | 6 | 4 |
| Grade 3 or 4 | 1 | 1 | 2 | 2 |
| Chronic GVHD | ||||
| All | 8 | 8 | 5 | 7 |
| Moderate to severe | 6 | 4 | 4 | 6 |
| Severe | 5 | 2 | 2 | 6 |
| Secondary graft failure | — | — | 2 | 2 |
| Positive CMV antigenemia | 11 | 17 | 22 | 8 |
| CMV diseases | 1 | 2 | 1 | — |
| Colitis | 1 | 2 | — | — |
| Esophagitis | — | — | 1 | — |
| EBV reactivation (/evaluated) | 9/14 | 8/11 | 9/15 | 4/9 |
| EBV-PTLD | 1 | — | 1 | 1 |
| Progression/recurrence of leukemia/MDS | 4 | 6 | 24 | 3 |
| TRM | 2 | 2 | 8 | 4 |
| Death | 6 | 9 | 30 | 7 |
| Causes of death | ||||
| Progression/recurrence of leukemia/MDS | 4 | 7 | 22 | 3 |
| Acute GVHD | — | 1 | 2 | 1 |
| Secondary graft failure | — | — | 1 | 2 |
| EBV-related PTLD | 1 | — | 1 | — |
| CMV colitis | — | 1 | — | — |
| Idiopathic interstitial pneumonitis | — | — | 1 | — |
| Pulmonary hemorrhage | — | — | — | 1 |
| H1N1 influenza pneumonia | 1 | — | — | — |
| Acute meningoencephalitis | — | — | 1 | — |
| Pneumonia | — | — | 1 | — |
| Sepsis | — | — | 1 | — |
REF indicates refractory disease; —, not applicable; and PTLD, posttransplantation lymphoproliferative disorder.
Engraphment, GVHD, disease progression/recurrence, and TRM observed in the study. Cumulative incidence of (A) donor cell engraftment, (B) acute GVHD, and (C) chronic GVHD after HLA-haploidentical HCT in patients who received a conditioning regimen containing reduced-dose busulfan, fludarabine, and ATG. Cumulative incidence of (D) progression/recurrence of acute leukemia/MDS and (E) TRM relative to disease status at HCT.
Engraphment, GVHD, disease progression/recurrence, and TRM observed in the study. Cumulative incidence of (A) donor cell engraftment, (B) acute GVHD, and (C) chronic GVHD after HLA-haploidentical HCT in patients who received a conditioning regimen containing reduced-dose busulfan, fludarabine, and ATG. Cumulative incidence of (D) progression/recurrence of acute leukemia/MDS and (E) TRM relative to disease status at HCT.
Posttransplantation hematopoietic chimerism was assessed in peripheral blood mononuclear cells at 2, 4, 6, 8, 12, and 24 weeks after HCT. Conversion to donor chimerism occurred rapidly, with 65 patients achieving > 90% donor chimerism at 2 weeks and 8 achieving chimerism at 4 weeks after HCT (cumulative incidence, 89%; 95% CI, 83%-96%).
Acute GVHD occurred in 20 patients (cumulative incidence, 24%; 95% CI, 17%-36%) at a median of 0.9 months after HCT (range, 0.3-2.6 months; Figure 1B). Of these, 16 patients (80%) had grade 2 to 4 acute GVHD (cumulative incidence, 20%; 95% CI, 13%-30%) and 6 (30%) had grade 3 or 4 acute GVHD (cumulative incidence, 7%; 95% CI, 3%-16%).
Chronic GVHD occurred in 28 patients (cumulative incidence, 34%; 95% CI, 25%-46%) at a median of 3.3 months after HCT(range, 1.9-6.5 months; Figure 1C), with 20 patients experiencing moderate to severe chronic GVHD (cumulative incidence, 24%; 95% CI, 17%-36%) and 15 severe chronic GVHD (cumulative incidence, 18%; 95% CI, 12%-29%). Of the 34 patients who survived for longer than 1 year after transplantation without progression/recurrence of underlying disease, 4 were receiving systemic immunosuppressive therapy for active chronic GVHD at one year. Of the 31 survivors in the study, 2 had bronchiolitis obliterans syndrome. Otherwise, no surviving patients had a major morbidity that precluded normal daily activities.
Disease progression/recurrence, TRM, and secondary graft failure
Surviving patients were followed up for a median of 26.6 months (range, 16.8-78.8 months). Four of 15 patients with acute leukemia in CR1 at the time of HCT and 6 of 19 patients in CR2/CR3 experienced leukemia recurrence (cumulative incidences, 27% and 32%, respectively, Figure 1D). Of the 34 patients with refractory acute leukemia at the time of HCT, 32 had active leukemia in the marrow or leptomeninges at that time. Of these 32 patients, 16 achieved CR after HLA-haploidentical HCT. Eventually, however, 24 of the 34 patients with refractory acute leukemia developed progressive/recurrent leukemia (cumulative incidence, 79%). Of the 15 patients with MDS, 3 had recurrent disease (cumulative incidence, 20%).
Of the 83 patients, 16 died without progression/recurrence of underlying leukemia or MDS, resulting in a cumulative TRM incidence of 18% (95% CI, 12%-29%). In addition, 1 patient died suddenly at home 16.3 months after HCT, with normal physical and laboratory findings 4 weeks earlier; thus, this patient was not considered to show TRM. Fourteen patients died within 1 year after HCT (1-year TRM, 17%; 95% CI, 11%-28%). The causes of TRM are listed in Table 2. Four patients experienced secondary graft failure 1.5 to 5.9 months after HLA-haploidentical HCT (cumulative incidence, 5%; 95% CI, 2%-13%).
Event-free and overall survival
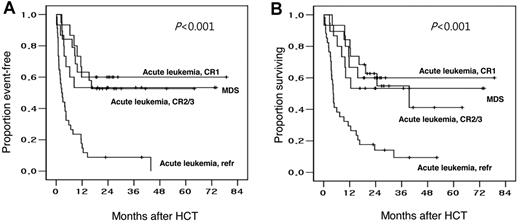
The Kaplan-Meier estimates of event-free and overall survival were 60% and 60%, respectively, for patients with acute leukemia in CR1; 53% and 41%, respectively, for patients with acute leukemia in CR2/CR3; 9% and 9%, respectively, for patients with refractory acute leukemia; and 53% and 53%, respectively, for patients with MDS, respectively (Table 2; Figure 2).
Event-free and overall survival of patients observed in the study. Kaplan-Meier plots of (A) event-free and (B) overall survival, relative to disease status at HCT, after HLA-haploidentical HCT in patients receiving a conditioning therapy containing reduced-dose busulfan, fludarabine, and ATG.
Event-free and overall survival of patients observed in the study. Kaplan-Meier plots of (A) event-free and (B) overall survival, relative to disease status at HCT, after HLA-haploidentical HCT in patients receiving a conditioning therapy containing reduced-dose busulfan, fludarabine, and ATG.
Univariate and multivariate analyses
Univariate analysis showed that the donor age and donor-patient HLA-allele disparity were correlated with grade 2 to 4 acute GVHD (Table 3). None of the variables analyzed correlated with chronic GVHD. The disease status at HCT showed a significant correlation with disease progression/recurrence, TRM, event-free survival, and overall survival. The cumulative incidences of TRM for patients with standard- and high-risk disease at the time of HCT were 10% (95% CI, 4%-25%), and 24% (95% CI, 14%-41%), respectively (Figure 1E). A history of previous allogeneic HCT showed significant correlations with disease progression/recurrence and event-free survival.
Univariate and multivariate analyses
| Variables . | Categories . | Grades 2-4 acute GVHD . | Leukemia/MDS progression/recurrence . | TRM . | Event-free survival . | Overall survival . |
|---|---|---|---|---|---|---|
| Univariate analyses (no. of patients with an event) | ||||||
| Disease status* | Standard vs high risk | NS | P < .001 (10/40 vs 27/43) | P = .035 (4/40 vs 12/43) | P < .001 (15/40 vs 39/43) | P < .001 (15/40 vs 37/43) |
| Previous allogeneic HCT | No vs yes | NS | P = .018 (28/71 vs 9/12) | NS | P = .015 (43/71 vs 11/12) | NS |
| Donor age, years | ≤ 40 vs > 40 | P = .048 (7/53 vs 9/30) | NS | NS | NS | NS |
| Donor-patient HLA allele mismatch | 3/8 or less vs 4/8 | P = .012 (1/27 vs 9/23) | NS | NS | NS | NS |
| Multivariate analysis [hazard ratio (95% CI)] | ||||||
| Disease status | Standard vs high risk | NS | P < .001 [3.56 (1.81-7.00)] | P = .040 [3.17 (1.05-9.57)] | P < .001 [5.53 (2.99-10.21)] | P < .001 [4.66 (2.52-8.61)] |
| Donor age, years | ≤ 40 vs > 40 | P = .048 [2.64 (1.01-6.94)] | NS | NS | NS | NS |
| Previous allogeneic HCT | No vs yes | NS | NS | NS | P = .012 [2.41 (1.22-4.78)] | NS |
| Variables . | Categories . | Grades 2-4 acute GVHD . | Leukemia/MDS progression/recurrence . | TRM . | Event-free survival . | Overall survival . |
|---|---|---|---|---|---|---|
| Univariate analyses (no. of patients with an event) | ||||||
| Disease status* | Standard vs high risk | NS | P < .001 (10/40 vs 27/43) | P = .035 (4/40 vs 12/43) | P < .001 (15/40 vs 39/43) | P < .001 (15/40 vs 37/43) |
| Previous allogeneic HCT | No vs yes | NS | P = .018 (28/71 vs 9/12) | NS | P = .015 (43/71 vs 11/12) | NS |
| Donor age, years | ≤ 40 vs > 40 | P = .048 (7/53 vs 9/30) | NS | NS | NS | NS |
| Donor-patient HLA allele mismatch | 3/8 or less vs 4/8 | P = .012 (1/27 vs 9/23) | NS | NS | NS | NS |
| Multivariate analysis [hazard ratio (95% CI)] | ||||||
| Disease status | Standard vs high risk | NS | P < .001 [3.56 (1.81-7.00)] | P = .040 [3.17 (1.05-9.57)] | P < .001 [5.53 (2.99-10.21)] | P < .001 [4.66 (2.52-8.61)] |
| Donor age, years | ≤ 40 vs > 40 | P = .048 [2.64 (1.01-6.94)] | NS | NS | NS | NS |
| Previous allogeneic HCT | No vs yes | NS | NS | NS | P = .012 [2.41 (1.22-4.78)] | NS |
NS indicates not significant.
Standard risk was defined as patients with acute leukemia in CR1, CR2, or CR3 at the time of transplantation or with low-risk MDS (ie, refractory anemia, refractory anemia with ring sideroblasts, or refractory cytopenia with multilineage dysplasia). Otherwise, patients were defined as high risk.
Multivariate analyses showed that donor age > 40 years was an independent predictor of grade 2 to 4 acute GVHD (Table 3), and high-risk disease status at the time of HCT was an independent predictor of disease progression/recurrence, TRM, and shorter event-free and overall survival. In addition, previous allogeneic HCT was an independent predictor of shorter event-free survival.
Posttransplantation infections and immune recovery
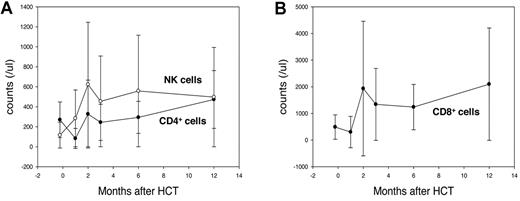
Fifty-eight of 72 evaluated patients (81%) had at least 1 positive assay result for CMV pp65 antigenemia, with 37 of these patients requiring preemptive treatment with ganciclovir. Four patients developed CMV disease (3 with colitis and 1 with esophagitis). One of these patients survived, whereas the other 3 died of CMV colitis per se or of other causes. Thirty of 49 monitored patients (61%) were positive for EBV DNA in blood; of these, 3 developed posttransplantation lymphoproliferative disorder. Two of these 3 patients died because of progressive EBV infection, and the third died of leukemia progression after EBV infection was cleared by treatment with rituximab. In the remaining 27 patients positive for EBV-DNA, such DNA became undetectable without specific treatment between 47 and 167 days after HCT. Other late infections observed in the study patients included cutaneous Varicella zoster virus infection (n = 12), pulmonary tuberculosis (n = 2), and Nocardia muscle abscess (n = 1). Despite the use of ATG, a long-acting T-cell depletion agent, patients experienced early reconstitution of their lymphocyte counts (Figure 3).
Posttransplantation immune reconstitution measured by lymphocyte subsets. Mean (A) CD4+ lymphocyte, NK cell, and (B) CD8+ lymphocyte counts after HLA-haploidentical HCT in patients receiving a conditioning therapy containing reduced-dose busulfan, fludarabine, and ATG. Data from patients who survived at least 6 months without progression/recurrence of underlying disease were analyzed, including data obtained from 48 patients before conditioning therapy and at 1, 2, and 3 months after HCT; and from 44 and 27 patients at 6 and 12 months, respectively. Among the lymphocyte subsets, NK-cell counts recovered earliest, exceeding pretransplantation counts at 1 month, with > 90% of patients maintaining NK-cell counts > 100/μL beginning 3 months after HCT. CD8+ lymphocyte counts exceeded pretransplantation levels at 2 months, and > 90% of patients maintained counts > 200/μL beginning 3 months after HCT. CD4+ lymphocyte counts recovered more slowly, with only approximately half of all patients showing CD4+ lymphocyte counts > 200/μL at 2 to 6 months. However, 12 months after HCT, however, 24 of 27 patients (89%) had CD4+ lymphocyte counts more than 200/μL. Error bars represent ± 1 SD.
Posttransplantation immune reconstitution measured by lymphocyte subsets. Mean (A) CD4+ lymphocyte, NK cell, and (B) CD8+ lymphocyte counts after HLA-haploidentical HCT in patients receiving a conditioning therapy containing reduced-dose busulfan, fludarabine, and ATG. Data from patients who survived at least 6 months without progression/recurrence of underlying disease were analyzed, including data obtained from 48 patients before conditioning therapy and at 1, 2, and 3 months after HCT; and from 44 and 27 patients at 6 and 12 months, respectively. Among the lymphocyte subsets, NK-cell counts recovered earliest, exceeding pretransplantation counts at 1 month, with > 90% of patients maintaining NK-cell counts > 100/μL beginning 3 months after HCT. CD8+ lymphocyte counts exceeded pretransplantation levels at 2 months, and > 90% of patients maintained counts > 200/μL beginning 3 months after HCT. CD4+ lymphocyte counts recovered more slowly, with only approximately half of all patients showing CD4+ lymphocyte counts > 200/μL at 2 to 6 months. However, 12 months after HCT, however, 24 of 27 patients (89%) had CD4+ lymphocyte counts more than 200/μL. Error bars represent ± 1 SD.
Discussion
In our prospective series of 83 patients with acute leukemia or MDS, which is the largest study to date reporting outcomes of HLA-haploidentical HCT from a family donor after RIC, we found that a conditioning regimen consisting of reduced-dose busulfan, fludarabine, and ATG, one of the more widely used and relatively less toxic conditioning regimens, induced sufficient immunosuppression in the host to allow donor hematopoietic cells to consistently engraft across the HLA haplotype barrier. Further, while achieving consistent donor cell engraftment, the incidences of acute and chronic GVHD and TRM were not excessive. The cumulative incidence rates of TRM were 19% in the overall patient cohort and only 10% in patients with standard-risk disease.
Successful engraftment of allogeneic hematopoietic cells across the MHC haplotype barrier after RIC has been well documented in studies of HCT in mice11 and large animals.12,13 In swine leukocyte antigen-haploidentical HCT models,13 2 transplantation approaches were compared: grafting of mobilized peripheral blood cells after RIC with total body irradiation (TBI; 100 cGy) plus anti-CD3 immunotoxin, and grafting of bone marrow cells after myeloablative conditioning with TBI (1150 cGy) plus cyclophosphamide 50 mg/kg. Remarkably, the results favored the former approach with all 9 animals in this group achieving sustained donor cell engraftment, whereas the latter approach failed in 5 of 9 animals. Further, whereas only one animal in the RIC group developed acute GVHD (grade 2), all 4 engrafted animals in the myeloablative conditioning group developed grade 3 or 4 acute GVHD. Therefore, these swine HCT models demonstrated that RIC containing anti–T-cell monoclonal antibody could achieve consistent engraftment across the major MHC haplotype barrier, with a low rate of GVHD.
Several clinical studies have shown the feasibility of RIC before HLA-haploidentical HCT in adult patients with hematologic disease. For example, RIC consisting of TBI (200 cGy), fludarabine 150 mg/m2, and cyclophosphamide 29 mg/kg, was used in 68 patients with hematologic malignancies or bone marrow failure undergoing HLA-haploidentical HCT; bone marrow grafts were used without ex vivo depletion of T cells.16 Three to 4 days after transplantation, patients were given cyclophosphamide 50 to 100 mg/kg. Rates of engraftment, grade 2 to 4 acute GVHD, and 1-year TRM were 87%, 34%, and 15%, respectively. An RIC consisting of cyclophosphamide 2 g/m2, fludarabine 120 mg/m2, and alemtuzumab 100 mg was given to 49 patients undergoing HLA-haploidentical HCT from family donors who gave G-CSF-mobilized peripheral blood cells.15 Although 7 patients experienced graft failure, only 16% and 14% of patients developed grade 2 to 4 acute GVHD and chronic GVHD, respectively. However, 31% of patients experienced TRM, mostly attributable to infections. Finally, an RIC regimen consisting of orally administered busulfan 8 mg/kg, fludarabine 80 mg/m2, and rabbit ATG 8 mg/kg, before HCT from HLA-haploidentical donors, given to 26 patients with various hematologic malignancies, resulted in engraftment in 25 of 26 patients and complete donor chimerism (25 at 2 weeks).14 The incidences of grade 2 to 4 acute GVHD and chronic GVHD were 20% and 25%, respectively.
The high engraftment rates, as well as the low GVHD rates, that we and others14,16,17 have observed after HLA-haploidentical HCT after RIC are intriguing and contrast with the traditional concept of allogeneic HCT. An increase in HLA disparity has been considered as one of the most important determinants of increased graft failure, as well as a rise in GVHD.2-4 Results from animal and clinical studies on RIC, before major MHC gene haploidentical HCT, strongly indicate that disparities in the major MHC genes may vary in significance, depending on the intensity and nature of the conditioning regimen. The lower tissue damage resulting from RIC, compared with myeloablative conditioning, may reduce the release of tissue antigens and proinflammatory cytokines, thereby favoring a post-transplantation host environment in which triggering of acute GVHD is decreased. Further, the rate and kinetics of conversion from the host to donor chimerism may differ in patients who undergo RIC and myeloablative conditioning. Finally, changes in the conditioning regimen given before allogeneic HCT can increase the level and functions of regulatory T cells, resulting in development of a regulatory rather than a stimulating immune environment after HCT.27 Within our patients, the numbers of HLA antigen or allele mismatches between donor and recipient were not independent predictors of the transplantation outcomes, such as GVHD, disease progression/recurrence, TRM, graft failure (data not shown), or patient survival; similar findings were observed in another retrospective study.28 Likewise, in the setting of unrelated donor HCT, the use of RIC regimens containing anti–T-cell antibodies resulted in the absence of adverse effects of HLA disparity on all of engraftment, GVHD, and TRM.5,29 Together, the results indicate that the roles played by disparities in major MHC genes as determinants of adverse outcomes of HCT may be less important when an RIC regimen containing anti–T-cell agents is used.
It should be noted that the favorable outcomes of HLA-haploidentical HCT in our study were observed in Korean patients, an ethnic population with a low level of HLA-gene heterogeneity30 and that experiences less GVHD after HLA-matched HCT.31,32 Hence, further clinical studies are necessary to ascertain whether our results can be extended to patients of other countries.
Prolonged immune suppression, as well as excessive patient mortality caused by infections, was of concern in patients who underwent HLA-haploidentical HCT, especially after ex vivo-T cell depletion.4,33 However, among our patients, no obvious increase in the incidence of late infections causing undue mortality after HCT was evident. Moreover, immune recovery occurred promptly after HCT, as shown by the fact that mean NK cell and CD8+ lymphocyte counts exceeded pretransplantation levels at 1 and 2 months, respectively, after HCT. More than 90% of patients maintained NK-cell counts > 100/μL and CD8+ lymphocyte counts > 200/μL beginning 3 months after HCT. Among various pretransplantation patient- and donor-related variables, disease status was an independent predictor of disease progression/recurrence, TRM, and event-free and overall survival. Disease progression/recurrence remains the predominant cause of treatment failure after HLA-haploidentical HCT, especially in patients with refractory acute leukemia.
The use of RIC is a new concept in HLA-haploidentical HCT. Therefore, further prospective studies are warranted to investigate the effects of various RIC regimens on transplantation outcomes. For example, HLA-haploidentical HCT after an RIC regimen of TBI (200 cGy), fludarabine, and post-transplant cyclophosphamide resulted in low rates of GVHD (grade 2-4 acute, 34%; extensive chronic, 5%-25%), low rate of infection-related death (5%), but high rate of recurrence of myeloid leukemia.16 On the other hand, HLA-haploidentical HCT after RIC of cyclophosphamide, fludarabine, and alemtuzumab resulted in low rates of acute and chronic GVHD, but high rates of graft failure (14%) and infection-related death (22%).15 In addition, prospective studies are needed to compare the outcomes of allogeneic HCT according to the various graft sources (HLA-matched siblings, HLA-fully or partially matched unrelated donors, and HLA-haploidentical familial donors), especially in patients with acute leukemia in remission or MDS. The effect of donor-patient NK cell alloreactivity, shown to be associated with a decrease in the recurrence of AML after T cell–depleted HLA-haploidentical HCT,34 should be evaluated in the setting of T cell–replete HLA-haploidentical HCT, using RIC. The ethnic backgrounds of donor-patient pairs should be considered because of variations in the expression patterns of killer immunoglobulin-like receptor genes among different ethnic populations.35 Finally, despite the use of RIC in our current study, a recurrence of underlying disease did not seem to be excessive in patients with acute leukemia in remission and MDS. Further studies are necessary to determine whether a stronger graft-versus-leukemia effect would result from HLA-haploidentical HCT compared with HLA-matched HCT, as has been suggested in a murine model.36
In conclusion, we have shown that HCT from an HLA-haploidentical familial donor was feasible after RIC with busulfan, fludarabine, and ATG. This transplantation approach produced consistent donor cell engraftment with low rates of GVHD and TRM. HLA-haploidentical HCT using our approach may be used when a suitable HLA-matched donor is not available or when allogeneic HCT is needed urgently. Further, because of the less intense nature of our conditioning regimen, elderly patients, patients with comorbidities, and patients who have been extensively treated, particularly with prior HCT can also undergo HLA-haploidentical HCT.
Presented at the 52nd Annual Meeting of the American Society of Hematology in Orlando, FL, December 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Koen van Besien (University of Chicago) for helpful review of the manuscript and insightful comments.
Authorship
Contribution: K.-H.L. designed the study, analyzed the data, and wrote the manuscript; Je-Hwan Lee, Jung-Hee Lee, D.-Y.K., S.-H.K., and H.-J.S. provided patient care, analyzed the data, and reviewed the manuscript critically; M.S., Y.-S.L., Y.-A.K., M.J., H.-J.H., and A.-R.J. collected the data; and S.-C.Y. performed the statistical analyses and reviewed the manuscript critically.
Conflict-of-interest disclosure: K.-H.L. has served as a consultant for Otsuka Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Kyoo-Hyung Lee, Department of Internal Medicine, Asan Medical Center, 88 Olympic-ro-43-gil, Songpa-gu, Seoul 138–736, Korea; e-mail: khlee2@amc.seoul.kr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal