Abstract
Although long considered as a disease of failed apoptosis, it is now clear that chronic lymphocytic leukemia (CLL) cells undergo extensive cell division in vivo, especially in progressive disease. Signaling via the B-cell receptor is thought to activate proliferation and survival pathways in CLL cells and also has been linked to poor outcome. Here, we have analyzed the expression of the proto-oncoprotein MYC, an essential positive regulator of the cell cycle, after stimulation of surface IgM (sIgM). MYC expression was rapidly increased after sIgM stimulation in a subset of CLL samples. The ability of sIgM stimulation to increase MYC expression was correlated with sIgM-induced intracellular calcium fluxes. MYC induction was partially dependent on the MEK/ERK signaling pathway, and MYC and phosphorylated ERK1/2 were both expressed within proliferation centers in vivo. Although stimulation of sIgD also resulted in ERK1/2 phosphorylation, responses were relatively short lived compared with sIgM and were associated with significantly reduced MYC induction, suggesting that the kinetics of ERK1/2 activation is a critical determinant of MYC induction. Our results suggest that ERK1/2-dependent induction of MYC is likely to play an important role in antigen-induced CLL cell proliferation.
Introduction
Chronic lymphocytic leukemia (CLL) is a relatively common B-cell malignancy with a very variable clinical course;1,2 some patients survive for many years, whereas others progress rapidly despite aggressive therapy. Although considered for a long time as a disease of failed apoptosis, it is now clear that increased cell division plays a major role in accumulation of CLL cells. Metabolic labeling experiments have demonstrated significant rates of cell “birth” in vivo (up to ∼ 1% of the malignant clone/day),3 and there is also evidence for telomere erosion in CLL cells4-6 indicative of extensive proliferation. Importantly, the extent of cell birth and telomere erosion is associated with poor outcome or prognostic markers, indicating that cell division is a determinant of disease progression.
Cell division occurs predominantly within proliferation centers (PCs) that are present within involved lymph nodes and to a lesser extent in the bone marrow of CLL patients. PCs are thought to be sites of antigen stimulation, implying a major role for ongoing B-cell receptor (BCR) signaling in driving cell-cycle progression in vivo, in the context of signals from soluble cytokines and supporting cells with the PC microenvironment.7,8 Signaling responses after surface IgM (sIgM) stimulation are variable in CLL samples, and retained signaling capacity is associated with markers of poorer prognosis, including unmutated (U) immunoglobulin heavy variable (IGHV) genes, ZAP-70, and CD38.9-14 For example, in our study of intracellular Ca2+ responses, the majority of U-CLL were responsive after sIgM stimulation, whereas in mutated (M)–CLL responses were more variable, with ∼ 40% retaining signaling responses.9
The molecular mechanisms that drive cell division in CLL are relatively poorly understood. Previous studies have shown that CpG-containing oligodeoxynucleotides (CpG-ODNs; with or without IL-2) regulate components of the cell-cycle machinery in CLL cells, including induction of cyclins A, D2, D3, and E and reduction in p27kip1.15-18 sIgM stimulation also has been shown to result in increased expression of cyclin D2 and cdk4 at the RNA and protein levels in CLL cells.11,14 However, what links upstream signaling pathways to these downstream effects on the cell-cycle machinery is unclear.
One candidate mediator is the proto-oncoprotein MYC, a key regulator of cell-cycle entry. MYC is a transcription factor that is activated by mitogens and that regulates the expression of proteins essential for cell-cycle progression and cell growth, including ornithine decarboxylase 1 (ODC1), cyclin D2, and cdk4.19-23 Recent immunoblotting and gene expression studies have demonstrated that expression of MYC and its target genes is increased in CLL lymph nodes compared with blood cells and that increased basal expression of MYC in circulating CLL cells is associated with progressive disease.24,25 MYC also was identified as an anti-IgM–regulated gene in CLL cells as part of a gene expression microarray study.26 However, the BCR-dependent regulation of this critical cell-cycle protein in CLL has not been studied in detail.
Here, we have analyzed the regulation of MYC after stimulation of sIgM in vitro. We demonstrate that sIgM stimulation results in induction of MYC in some CLL samples. MYC induction was partially dependent on the MEK1/2 → ERK1/2 signaling pathway, and MYC and phosphorylated ERK1/2 were both expressed within PCs in vivo. Although stimulation of sIgD also resulted in ERK1/2 phosphorylation, responses were short lived compared with sIgM and were associated with significantly reduced MYC expression, suggesting that the kinetics of ERK1/2 activation is a critical determinant of MYC induction. Our results suggest that ERK1/2-dependent induction of MYC is likely to play an important role in antigen-induced CLL proliferation.
Methods
Samples and reagents
This study was performed following ethical approval from the Southampton and South West Hampshire Research Ethics Committee. Informed consent was provided in accordance with the Declaration of Helsinki. Blood was obtained from 53 patients in total with typical CLL who attended hematology outpatient clinics at the Leicester Royal Infirmary, Portsmouth Hospital, Southampton General Hospital, the Royal Wolverhampton Hospitals NHS Trust, and the Royal Berkshire Hospital, Reading (all in the United Kingdom). Clinical details for the patients studied are given in Table 1. IGHV gene mutation status and intracellular Ca2+ responses were determined as described previously.9,27
Clinical details of samples
| Sample . | Stage* . | IGHV mutation status† . | ZAP-70, % . | CD38, % . |
|---|---|---|---|---|
| 63 | na | M | 4 | 97 |
| 189 | A | M | 12 | 1 |
| 191 | A | M | 10 | 1 |
| 232 | A | M | 30 | 33 |
| 239 | A | M | 0 | 2 |
| 268 | A | M | 4 | 0 |
| 269 | B | M | 1 | 1 |
| 273 | C | M | 0 | 3 |
| 277 | na | M | 1 | 0 |
| 318 | C | M | 2 | 5 |
| 333 | A | M | 1 | 1 |
| 353 | A | M | 2 | 39 |
| 367 | A | M | 10 | 21 |
| 368 | C | M | 74 | 2 |
| 374 | A | M | 0 | 3 |
| 379 | A | M | 0 | 1 |
| 247 | A | M | 0 | 4 |
| 290 | na | M | nd | 5 |
| 296 | na | M | nd | 3 |
| 299 | A | M | 1 | 0 |
| 351 | na | M | 17 | 57 |
| 357 | A | M | 5 | 36 |
| 370 | na | M | 3 | 1 |
| 196 | na | U | nd | 38 |
| 199 | C | U | 32 | 22 |
| 221 | A | U | 16 | 51 |
| 231 | B | U | 11 | 82 |
| 233 | A | U | 48 | 34 |
| 256 | B | U | 5 | 15 |
| 284 | B | U | 48 | 12 |
| 285 | A | U | 35 | 100 |
| 288 | A | U | 61 | 71 |
| 291 | na | U | 88 | 18 |
| 298 | C | U | 26 | 92 |
| 304 | B | U | 24 | 74 |
| 305 | C | U | 65 | 9 |
| 306 | A | U | 23 | 49 |
| 328 | A | U | 16 | 23 |
| 343 | A | U | 16 | 54 |
| 346 | B | U | 51 | 89 |
| 361 | A | U | 5 | 98 |
| 376 | A | U | 9 | 63 |
| 383 | A | U | 17 | 24 |
| 385 | A | U | 11 | 36 |
| 238 | A | U | 82 | 6 |
| 293 | A | U | 29 | 16 |
| 352 | B | U | 4 | 3 |
| 358 | na | U | 1 | 7 |
| 359 | na | U | 1 | 10 |
| 409 | B | U | 77 | 56 |
| 410 | A | U | 8 | 56 |
| 421 | B | U | 94 | 42 |
| 422 | B | U | 62 | 48 |
| Sample . | Stage* . | IGHV mutation status† . | ZAP-70, % . | CD38, % . |
|---|---|---|---|---|
| 63 | na | M | 4 | 97 |
| 189 | A | M | 12 | 1 |
| 191 | A | M | 10 | 1 |
| 232 | A | M | 30 | 33 |
| 239 | A | M | 0 | 2 |
| 268 | A | M | 4 | 0 |
| 269 | B | M | 1 | 1 |
| 273 | C | M | 0 | 3 |
| 277 | na | M | 1 | 0 |
| 318 | C | M | 2 | 5 |
| 333 | A | M | 1 | 1 |
| 353 | A | M | 2 | 39 |
| 367 | A | M | 10 | 21 |
| 368 | C | M | 74 | 2 |
| 374 | A | M | 0 | 3 |
| 379 | A | M | 0 | 1 |
| 247 | A | M | 0 | 4 |
| 290 | na | M | nd | 5 |
| 296 | na | M | nd | 3 |
| 299 | A | M | 1 | 0 |
| 351 | na | M | 17 | 57 |
| 357 | A | M | 5 | 36 |
| 370 | na | M | 3 | 1 |
| 196 | na | U | nd | 38 |
| 199 | C | U | 32 | 22 |
| 221 | A | U | 16 | 51 |
| 231 | B | U | 11 | 82 |
| 233 | A | U | 48 | 34 |
| 256 | B | U | 5 | 15 |
| 284 | B | U | 48 | 12 |
| 285 | A | U | 35 | 100 |
| 288 | A | U | 61 | 71 |
| 291 | na | U | 88 | 18 |
| 298 | C | U | 26 | 92 |
| 304 | B | U | 24 | 74 |
| 305 | C | U | 65 | 9 |
| 306 | A | U | 23 | 49 |
| 328 | A | U | 16 | 23 |
| 343 | A | U | 16 | 54 |
| 346 | B | U | 51 | 89 |
| 361 | A | U | 5 | 98 |
| 376 | A | U | 9 | 63 |
| 383 | A | U | 17 | 24 |
| 385 | A | U | 11 | 36 |
| 238 | A | U | 82 | 6 |
| 293 | A | U | 29 | 16 |
| 352 | B | U | 4 | 3 |
| 358 | na | U | 1 | 7 |
| 359 | na | U | 1 | 10 |
| 409 | B | U | 77 | 56 |
| 410 | A | U | 8 | 56 |
| 421 | B | U | 94 | 42 |
| 422 | B | U | 62 | 48 |
na indicates not available; and nd, not defined.
Binet stage at diagnosis.
M indicates mutated; and U, unmutated.
PBMCs were isolated by Lymphoprep centrifugation (Axis-Shield Diagnostics), washed and cryopreserved in RPMI-1640 (Invitrogen) supplemented with 10% (vol/vol) DMSO and 15% (vol/vol) FCS. CLL samples were thawed in complete culture medium (RPMI-1640 supplemented with 10% [vol/vol] FCS, 2mM glutamine, and 1% [wt/vol] sodium pyruvate), pelleted by centrifugation, and resuspended in complete medium. Cells were allowed to recover by incubation for 1 hour at 37°C. Cell viability by trypan blue exclusion was more than 90%. The proportion of contaminating normal CD19+CD5− B cells, as determined by flow cytometry, was less than 1.0%. BCR signaling was evaluated after cells were treated with 20 μg/mL goat F(ab′)2 anti–human IgM or IgD (Southern Biotechnology) at 37°C for various times.
Normal B cells were isolated from peripheral blood or buffy coats from healthy donors using the B Cell Isolation Kit II (Miltenyi Biotec) according to the manufacturer's protocol. CD27− cells were isolated by adding an anti-CD27 antibody to the antibody cocktail provided with the kit.
MEK1/2 inhibitor U0126 was from Sigma-Aldrich.
Cell surface staining
Thawed lymphocytes were stained for 30 minutes at 4°C with anti-CD5 PerCP-Cy5.5 and anti-CD19 APC (both from BD Biosciences) and either anti-CD38 PE (clone HB7; BD Biosciences), anti-IgM PE, or anti-IgD FITC (both Dako UK). Data were acquired on an FACSCalibur flow cytometer (BD Biosciences) with CellQuest Pro Version 3.3 software (BD Biosciences). Mean fluorescence intensity and percentage of positive staining within the CD5+CD19+ lymphocyte population were measured relative to appropriate isotype controls. For CD38 analysis, samples with greater than or equal to 30% expressing tumor cells were designated as positive.28 Determination of ZAP-70 status was performed as described; samples where greater than or equal to 20% of tumor cells expressed ZAP-70 were designated ZAP-70 positive.29
Quantitative real-time PCR
CLL cells (1 × 107) in 1 mL of complete growth medium were treated with 20 μg/mL goat F(ab′)2 anti–human IgM or IgD or isotype control antibody for 1 and 6 hours. Total RNA was isolated using the RNeasy Kit (QIAGEN) according to the manufacturer's instructions and converted to cDNA using oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase (Promega). PCR reactions were performed using a 7500 Real-Time PCR System and TaqMan Universal PCR Master Mix (Applied Biosystems) and the following TaqMan probes: Human B2M (β2-microglobulin) Endogenous Control (4333766T), ODC1 (Hs00159739_m1), CCND2 (Hs00277041_m1), CDK4 (Hs00262861_m1), and MYC (Hs00153408_m1; all Applied Biosystems). Relative RNA quantities were calculated with the equation RQ = 2−(ΔΔCT) using B2M expression as an internal control.
Immunoblot analysis
Cells were lysed on ice for 30 minutes using lysis buffer (1% [vol/vol] Nonidet P-40, 10mM Tris-HCl, pH 8.0, 150mM NaCl, and 0.02% [wt/vol] sodium azide). Samples were centrifuged, and the protein content of the supernatant was measured using the BCA Protein Assay Kit (Pierce Chemical). Immunoblotting was performed using 30 μg and 50 μg of protein lysate for analysis of ERK1/2 and MYC/MAX, respectively. The following antibodies were used: anti–T202/Y204-phosphorylated (p)ERK1/2, anti-ERK1/2, anti-p38 MAPK, anti-MAX (all from Cell Signaling Technology) and anti-MYC (clone 9E10; Cancer Research UK, Research Monoclonal Antibody Service). Secondary HRP–conjugated antibodies were from GE Healthcare. Images were collected using a Fluor-S MultiImager (Bio-Rad Laboratories) and quantified using Fluor-S software Quantity One Version 4.6.3 (Bio-Rad Laboratories Inc). All values were normalized to the relevant loading control, and relative fold-change was calculated with the isotype control antibody–treated cells taken as a 100% of expression.
PhosFlow analysis
The kinetics of ERK1/2 phosphorylation was determined using flow cytometry.30 PBMCs were thawed, washed, and resuspended in complete culture medium at 1 × 107 cells/mL. Aliquots (100 μL) were treated with F(ab′)2 anti–human IgM or IgD for various times at 37°C, fixed with BD Cytofix (BD Biosciences) according to the manufacturer's instructions, and stored at −80°C. Immediately before staining, cells were pelleted and resuspended in 90% (vol/vol) methanol and incubated on ice for 30 minutes. After washing twice with FACS buffer (BD Biosciences), the cells were labeled with 5 μL anti–pERK1/2 (pT202/pY204) AlexaFluor 488 (BD Biosciences) for 30 minutes at room temperature. For analysis of normal CD20+CD27− B cells, cells were additionally labeled with 5 μL of CD20 PerCP-Cy5.5 and 5 μL of CD27 PE (both from BD Biosciences), and AlexaFluor 488 fluorescence within the CD20+CD27− lymphocyte population was measured. Data were acquired on a FACScalibur flow cytometer and analyzed with CellQuest Pro Version 3.3 software.
Analysis of CLL cell-cycle entry
CLL cell-cycle entry was stimulated by treating cells for 3 or 48 hours with CpG-containing ODNs (7 μg/mL, ODN-2006; Invitrogen).15 S-phase entry was quantified using bromodeoxyuridine (BrdU) staining and flow cytometry (FITC BrdU Flow Kit; BD Biosciences, PharMingen). Samples were gated to exclude dead cells, and the proportion of BrdU+ cells in S-phase was determined as a proportion of all viable cells.
Immunohistochemistry
Immunohistochemical analysis was performed using formalin-fixed and paraffin-embedded lymph node tissue sections obtained from 8 cases of CLL/small lymphocytic lymphoma (SLL). Immunostaining was performed using the Bond autostainer and reagents (Leica Microsystems) and an MYC-specific monoclonal antibody (N262; Santa Cruz Biotechnology) at a dilution of 1:50 using the Bond ER1 protocol for 20 minutes or a T202/Y204 pERK1/2-specific antibody (Cell Signaling Technology) at a dilution of 1:100 using the Bond ER1 protocol for 30 minutes. A Burkitt lymphoma and colon cancer biopsy samples were used as a positive control for MYC and pERK1/2 staining, respectively.
Statistics
Statistical analyses were performed using Prism Version 4.03 software (GraphPad). All tests were 2-tailed with 95% confidence interval values.
Results
Induction of MYC protein after stimulation of sIgM in CLL cells
We performed immunoblotting to determine whether sIgM stimulation increased MYC protein expression in CLL cells (Figure 1A). We first analyzed induced MYC expression in 18 samples, all of which were considered as anti-IgM–responsive based on the ability of anti-IgM to promote intracellular Ca2+ mobilization.9 This cohort comprised 8 U-CLL samples, as well as 10 M-CLL samples representative of the subset of M-CLL that retain sIgM intracellular Ca2+ responses.9 We selected a greater than 20% increase in MYC expression as an arbitrary cut-off to separate positive and negative responses, based on consideration of the ability of immunoblotting to reproducibly detect increases. Using this cut-off, MYC expression was increased in 16/18 (89%) of these samples at 3 hours after stimulation with anti-IgM relative to control cells. On average, MYC expression was increased by 2.0-fold at this time (range, 1.0- to 4-fold) and was statistically significantly higher in anti-IgM stimulated compared with control cells (P = .0001; Figure 1B). There was no significant difference in the fold increase in MYC expression between U-CLL and M-CLL samples (P > .05; data not shown). MYC protein levels were either maintained or decreased at 6 hours after stimulation, although in the 2 cases with the highest levels of MYC induction, MYC expression continued to increase. Both the MYC1 and MYC2 isoforms, generated by alternate translation initiation, were detected and all CLL samples expressed MAX (MYC associated factor X), the obligate dimerization partner for MYC-dependent transcriptional regulation (Figure 1A).
Induction of MYC protein after sIgM stimulation in CLL samples. (A,C) CLL samples were incubated with anti-IgM for 3 or 6 hours or for 6 hours with the isotype control antibody (IC). Expression of MYC and MAX protein was analyzed by immunoblotting. Results shown are representative of those obtained from analysis of 18 intracellular Ca2+-responsive (A) and 9 intracellular Ca2+ nonresponsive (C) CLL samples. MAX expression demonstrates equal loading of protein samples. (B,D) Quantitation of the fold increase in MYC protein expression (relative to isotype-control treated cells; C) measured by densitometry analysis of immunoblots at 3 or 6 hours after stimulation with anti-IgM for intracellular Ca2+-responsive (B) and -nonresponsive (D) samples. Graphs show data for individual samples, and any statistically significant differences (Student matched paired t test) between control and anti-IgM–stimulated cells (NS indicates not significant, P > .05). (E) Comparison between MYC induction (> 20% increase compared with control cells) and positive (□) and negative (■) intracellular Ca2+ responses (Fisher exact test) in anti-IgM–treated CLL samples (n = 27).
Induction of MYC protein after sIgM stimulation in CLL samples. (A,C) CLL samples were incubated with anti-IgM for 3 or 6 hours or for 6 hours with the isotype control antibody (IC). Expression of MYC and MAX protein was analyzed by immunoblotting. Results shown are representative of those obtained from analysis of 18 intracellular Ca2+-responsive (A) and 9 intracellular Ca2+ nonresponsive (C) CLL samples. MAX expression demonstrates equal loading of protein samples. (B,D) Quantitation of the fold increase in MYC protein expression (relative to isotype-control treated cells; C) measured by densitometry analysis of immunoblots at 3 or 6 hours after stimulation with anti-IgM for intracellular Ca2+-responsive (B) and -nonresponsive (D) samples. Graphs show data for individual samples, and any statistically significant differences (Student matched paired t test) between control and anti-IgM–stimulated cells (NS indicates not significant, P > .05). (E) Comparison between MYC induction (> 20% increase compared with control cells) and positive (□) and negative (■) intracellular Ca2+ responses (Fisher exact test) in anti-IgM–treated CLL samples (n = 27).
We next studied MYC regulation in 9 samples (3 U-CLL and 6 M-CLL) all of which were considered to be sIgM intracellular Ca2+ nonresponders (Figure 1C). There was no increase in MYC expression in any of the samples at 3 hours, and MYC expression was increased by greater than 20% in only 1/9 (11%) samples at 6 hours after stimulation of sIgM (Figure 1D). On average, MYC was not differentially expressed after sIgM stimulation in these samples (P > .05). Overall, there was a very strong association between the ability of sIgM stimulation to increase MYC expression and induce intracellular Ca2+ mobilization (P = .0001, Fisher exact test; Figure 1E).
Analysis of MYC target gene expression
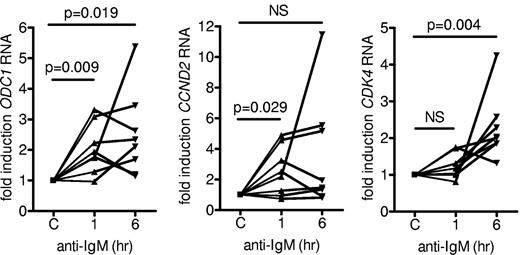
To determine whether the transcriptional activity of MYC also was increased after sIgM stimulation, we analyzed the expression of ODC1, CDK4, and CCND2 RNAs, well-characterized MYC target genes associated with proliferation.19-23 ODC1 and CCND2 RNAs were induced within 1 hour (mean induction, 2.04- and 2.54-fold, respectively), whereas CDK4 RNA was more strongly induced (mean induction, 2.3-fold) at 6 hours after stimulation with anti-IgM (Figure 2). Thus, MYC is active in sIgM-stimulated CLL cells because its induction is associated with increased MYC target gene expression.
Effect of anti-IgM on expression of MYC target genes. CLL samples (n = 8) were stimulated with anti-IgM for 1 or 6 hours or with isotype control antibody. Expression of ODC1, CDK4, and CCND2 RNA were analyzed by quantitative real-time PCR. Expression values for the isotype control antibody (C) were set to 1.0 for each time point. Graphs show the fold induction for each sample and statistically significant differences are indicated (Student matched paired t test; NS indicates not significant, P > .05).
Effect of anti-IgM on expression of MYC target genes. CLL samples (n = 8) were stimulated with anti-IgM for 1 or 6 hours or with isotype control antibody. Expression of ODC1, CDK4, and CCND2 RNA were analyzed by quantitative real-time PCR. Expression values for the isotype control antibody (C) were set to 1.0 for each time point. Graphs show the fold induction for each sample and statistically significant differences are indicated (Student matched paired t test; NS indicates not significant, P > .05).
Activation of MEK1/2 in anti-IgM–stimulated CLL cells
The MEK1/2 → ERK1/2 signaling pathway is activated after sIgM stimulation in normal B cells and in signaling responsive CLL cells,31,32 and is known to play a major role in controlling MYC expression via both transcriptional and posttranscriptional pathways.33,34 We therefore investigated the activation of ERK1/2 in CLL cells and its role in induction of MYC expression. Phosphorylation of ERK1/2 at T202/Y204 was quantified using single-cell flow cytometry in 37 CLL samples. This cohort comprised 16 M-CLL and 21 U-CLL, of which 31 samples were considered as sIgM responsive based on intracellular Ca2+ responsiveness. The kinetics of ERK1/2 activation are important in determining downstream responses;35 therefore, we investigated ERK1/2 phosphorylation for up to 45 or 60 minutes after sIgM stimulation.
ERK1/2 phosphorylation increased after sIgM stimulation in 29/31 (94%) of the intracellular Ca2+ responsive samples (Figure 3A shows representative responsive examples). ERK1/2 phosphorylation was rapidly induced reaching a peak at ∼ 5 to 15 minutes after stimulation, and there was a positive correlation between sIgM-induced signaling responses measured by increased ERK1/2 phosphorylation and intracellular Ca2+ mobilization within this cohort (Figure 3B; P = .0127; R2 = 0.16). However, there were several individual samples where intracellular Ca2+ and ERK1/2 phosphorylation responses did not seem to be closely correlated and ERK1/2 phosphorylation increased after sIgM stimulation in 5/6 (83%) of the intracellular Ca2+ nonresponsive samples.
Activation of ERK1/2 phosphorylation in sIgM-stimulated CLL B cells. (A) CLL samples were stimulated with anti-IgM for up to 60 minutes and ERK1/2 phosphorylation analyzed by flow cytometry. Graphs show the fold increase in ERK1/2 phosphorylation after stimulation with anti-IgM relative to untreated cells for 5 representative samples. (B) Correlation between the maximal percentage of cells showing increased intracellular Ca2+ and maximal fold induction of ERK1/2 phosphorylation after sIgM stimulation. Results of linear regression are shown (n = 37).
Activation of ERK1/2 phosphorylation in sIgM-stimulated CLL B cells. (A) CLL samples were stimulated with anti-IgM for up to 60 minutes and ERK1/2 phosphorylation analyzed by flow cytometry. Graphs show the fold increase in ERK1/2 phosphorylation after stimulation with anti-IgM relative to untreated cells for 5 representative samples. (B) Correlation between the maximal percentage of cells showing increased intracellular Ca2+ and maximal fold induction of ERK1/2 phosphorylation after sIgM stimulation. Results of linear regression are shown (n = 37).
After the initial peak activation of ERK1/2 phosphorylation, 2 patterns were observed in responsive CLL samples. In most samples (24/31, 77%), anti-IgM stimulation resulted in a protracted activation of ERK1/2 phosphorylation, defined here as being maintained at greater than or equal to 1.2-fold over background at 30 minutes after stimulation. The protracted activation of ERK1/2 phosphorylation was confirmed by immunoblotting in a subset of samples (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A further 5 samples showed a transient induction of ERK1/2 phosphorylation that rapidly returned to baseline (see sample 63 in Figure 3A).
Overall, these data demonstrate that there is a strong tendency for coactivation of ERK1/2 and intracellular Ca2+ responses in CLL samples after sIgM stimulation, and sIgM stimulation generally results in protracted activation of ERK1/2 phosphorylation.
Induction of MYC protein is partially dependent on activation of the ERK1/2 pathway
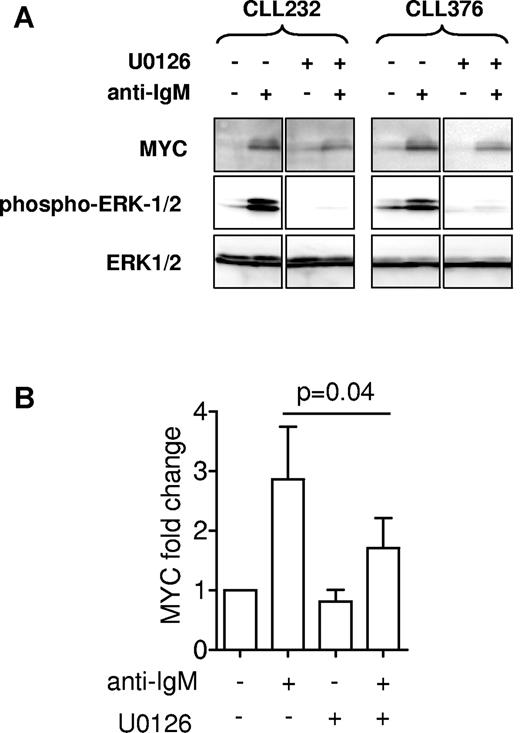
The MEK1/2 kinase mediates ERK1/2 phosphorylation and activation in response to BCR stimulation.36,37 We used the MEK1/2 inhibitor U0126 to determine whether activation of ERK1/2 was directly involved in regulation of MYC expression. Analysis of ERK1/2 phosphorylation confirmed that MEK1/2 was effectively inhibited in U0126-treated cells. The induction of MYC protein expression by anti-IgM was significantly reduced (by ∼ 50%) in cells pretreated with U0126 (Figure 4A-B). Therefore, MEK1/2 is required for optimal MYC expression in CLL cells after activation of sIgM, although other pathways seem to contribute.
Effect of U0126 on MYC induction. Cells were stimulated with anti-IgM for 3 hours in the presence of U0126 (10μM) or DMSO as a control. (A) Expression of MYC and phosphorylated and total ERK1/2 was analyzed by immunoblotting. Two representative samples are shown of a total of 5 samples analyzed. Note that intervening lanes were removed for clarity, and the complete blot is shown in supplemental Figure 1D. (B) Quantitation of MYC expression. Graphs show mean (± SEM) MYC expression relative to untreated cells (set to 1.0), derived from 5 independent experiments. The reduction in MYC expression in cells treated with anti-IgM and U0126 was statistically significant compared with cells treated with anti-IgM alone (Student matched pairs t test).
Effect of U0126 on MYC induction. Cells were stimulated with anti-IgM for 3 hours in the presence of U0126 (10μM) or DMSO as a control. (A) Expression of MYC and phosphorylated and total ERK1/2 was analyzed by immunoblotting. Two representative samples are shown of a total of 5 samples analyzed. Note that intervening lanes were removed for clarity, and the complete blot is shown in supplemental Figure 1D. (B) Quantitation of MYC expression. Graphs show mean (± SEM) MYC expression relative to untreated cells (set to 1.0), derived from 5 independent experiments. The reduction in MYC expression in cells treated with anti-IgM and U0126 was statistically significant compared with cells treated with anti-IgM alone (Student matched pairs t test).
MEK1/2 activity is required for optimal MYC expression and cell-cycle entry in CLL cells treated with CpG-ODN
We examined the effects of U0126 on MYC expression and cell- cycle entry in cells treated with CpG-ODN, a well-studied model for cell-cycle entry in CLL cells.15,16 Our analysis focused on U-CLL samples, because previous studies have demonstrated that CpG-ODN predominantly induce a proliferative response in these cells, whereas CpG-ODN generally promote apoptosis in M-CLL.15,38,39 Consistent with this, we found that CpG-ODN stimulation of U-CLL samples for 48 hours resulted in a greater than or equal to 2-fold increase in the proportion of BrdU-positive cells in all 6 samples analyzed, although the proportion of cells entering cell cycle varied considerably between samples (Figure 5A-B). Treatment with CpG-ODN also slightly reduced levels of spontaneous cell death; the average proportion of dead cells in these 6 samples was 21% in untreated and 15% in CpG-ODN stimulated cases (data not shown).
Effect of U0126 on CpG-ODN–treated CLL cells. CLL cells (n = 6) were pretreated with 10μM U0126 or DMSO for 15 minutes, before stimulation with CpG-ODN (7μg/mL) for 3 or 48 hours. (A) Representative analysis of BrdU and 7AAD staining in untreated (top) and CpG-ODN (bottom)–treated CLL cells (48 hours). S-phase and dead cells are gated in the bottom panel. (B) Quantitation of S-phase (48 hours, in the presence or absence of CpG-ODN ± U0126. (C) Immunoblot analysis of MYC, phosphorylated ERK1/2, and β-actin expression at 3 hours.
Effect of U0126 on CpG-ODN–treated CLL cells. CLL cells (n = 6) were pretreated with 10μM U0126 or DMSO for 15 minutes, before stimulation with CpG-ODN (7μg/mL) for 3 or 48 hours. (A) Representative analysis of BrdU and 7AAD staining in untreated (top) and CpG-ODN (bottom)–treated CLL cells (48 hours). S-phase and dead cells are gated in the bottom panel. (B) Quantitation of S-phase (48 hours, in the presence or absence of CpG-ODN ± U0126. (C) Immunoblot analysis of MYC, phosphorylated ERK1/2, and β-actin expression at 3 hours.
Stimulation with CpG-ODN increased ERK1/2 phosphorylation and MYC expression in all samples tested (Figure 5C). Side-by-side comparison of MYC expression in 4 samples demonstrated that, on average, the levels of MYC induced by CpG-ODN were slightly higher than those induced by anti-IgM (2.1 ± 0.3-fold and 1.6 ± 0.1-fold, respectively [mean ± SD], Student t test, P = .027). Pretreatment with U0126 partially suppressed the induced MYC expression paralleling the effects of sIgM stimulation (Figure 4B). For CpG-ODN stimulation, it also was possible to detect an inhibitory effect of U0126 on S-phase entry (Figure 5B-C).
Effect of sIgD stimulation on ERK1/2 phosphorylation and MYC expression in CLL cells
CLL cells generally express both sIgM and sIgD. In contrast to sIgM, sIgD is not down-modulated by antigen engagement in vivo, and the majority of CLL samples retain signaling responses in vitro to sIgD stimulation.9 We therefore compared the effects of sIgM and sIgD in individual samples of CLL. We analyzed the effects of sIgD stimulation on ERK1/2 phosphorylation using the same 31 sIgM-responsive samples, all of which were considered sIgD-responsive as assessed by intracellular Ca2+ mobilization. Rapid increases in ERK1/2 phosphorylation were detected in 29/31 (93%) of these samples (Figure 6A). However, in contrast to sIgM, sIgD responses were almost always transient and rapidly returned to background. Using the cut-off of greater than or equal to 1.2-fold increase at 30 minutes, only 3/31 (10%) samples showed protracted ERK1/2 responses (see supplemental Figure 1B for a direct comparison of sIgM and sIgD responses). The transient responses to sIgD stimulation were confirmed by immunoblotting in a subset of samples (supplemental Figure 1A). The difference in the proportion of transient or protracted responses after stimulation of sIgM or sIgD was highly significant (Fisher exact test, P = .0001; Figure 6B). We also investigated ERK1/2 phosphorylation in 6 samples that were considered sIgM nonresponsive but that retained responsiveness to anti-IgD. Similar to the retained intracellular Ca2+ responsiveness, 5/6 (83%) of these samples also demonstrated a greater than 1.2-fold increase in ERK1/2 phosphorylation after sIgD stimulation (data not shown). The response was transient in 4/5 responding samples.
Activation of ERK1/2 phosphorylation and MYC expression in sIgD-stimulated CLL and normal B cells. (A) CLL samples were stimulated with anti-IgD for up to 60 minutes and analyzed for ERK1/2 phosphorylation by flow cytometry. Graphs show the fold increase in ERK1/2 phosphorylation after stimulation with anti-IgD relative to untreated cells for 4 representative samples. Note that the same samples are shown in Figure 3A (after sIgM stimulation), and direct comparison of sIgM and sIgD responses is shown in supplemental Figure 1B. (B) Comparison between protracted/transient ERK1/2 responses after stimulation of sIgM (□) or sIgD (■; Fisher exact test, P = .0001; n = 37). (C) Quantitation of MYC protein induction after stimulation of sIgM or sIgD, relative to isotype antibody-treated controls (n = 18). Note values for anti-IgM–treated cells are the same as those shown in Figure 1B and are shown again here to allow direct comparison with anti-IgD–treated cells. See supplemental Figure 1C for side-by-side immunoblot analysis of MYC expression in sIgM- or sIgD-stimulated CLL cells. (D) Normal B cells were stimulated with anti-IgM (□) or anti-IgD (■) for up to 60 minutes and ERK1/2 phosphorylation analyzed by flow cytometry. Graph shows mean fold increase in ERK1/2 phosphorylation for CD20+CD27− cells. Data are mean values ± SEM (n = 8). (E) CD19+CD27− B cells isolated from healthy donors were stimulated with anti-IgM or anti-IgD for 3 or 6 hours or for 6 hours with the isotype control. MYC and MAX expression was analyzed by immunoblotting. Results are shown for cells isolated from 2 separate donors.
Activation of ERK1/2 phosphorylation and MYC expression in sIgD-stimulated CLL and normal B cells. (A) CLL samples were stimulated with anti-IgD for up to 60 minutes and analyzed for ERK1/2 phosphorylation by flow cytometry. Graphs show the fold increase in ERK1/2 phosphorylation after stimulation with anti-IgD relative to untreated cells for 4 representative samples. Note that the same samples are shown in Figure 3A (after sIgM stimulation), and direct comparison of sIgM and sIgD responses is shown in supplemental Figure 1B. (B) Comparison between protracted/transient ERK1/2 responses after stimulation of sIgM (□) or sIgD (■; Fisher exact test, P = .0001; n = 37). (C) Quantitation of MYC protein induction after stimulation of sIgM or sIgD, relative to isotype antibody-treated controls (n = 18). Note values for anti-IgM–treated cells are the same as those shown in Figure 1B and are shown again here to allow direct comparison with anti-IgD–treated cells. See supplemental Figure 1C for side-by-side immunoblot analysis of MYC expression in sIgM- or sIgD-stimulated CLL cells. (D) Normal B cells were stimulated with anti-IgM (□) or anti-IgD (■) for up to 60 minutes and ERK1/2 phosphorylation analyzed by flow cytometry. Graph shows mean fold increase in ERK1/2 phosphorylation for CD20+CD27− cells. Data are mean values ± SEM (n = 8). (E) CD19+CD27− B cells isolated from healthy donors were stimulated with anti-IgM or anti-IgD for 3 or 6 hours or for 6 hours with the isotype control. MYC and MAX expression was analyzed by immunoblotting. Results are shown for cells isolated from 2 separate donors.
MYC induction was analyzed by immunoblotting in the 18 samples shown in Figure 1B, all of which were considered as sIgD responsive in terms of intracellular Ca2+ responses. In contrast to sIgM responses, some of these samples did not show any evidence of increased MYC protein expression after stimulation of sIgD at any time point, and in those cases that did, the increase was clearly lower than after sIgM stimulation (Figure 6C and supplemental Figure 1C). Overall, the induction of MYC protein at both 3 and 6 hours was significantly lower following stimulation of sIgD compared with sIgM (Student t test, P = .002 and P = .003, respectively; Figure 6C). Thus, in contrast to sIgM, sIgD engagement generally triggers a transient activation of ERK1/2 that is associated with a relatively weak induction of MYC protein expression.
ERK1/2 phosphorylation and MYC expression in normal B cells
Because responses to sIgM and sIgD stimulation differed in CLL, we also analyzed responses in normal B cells to determine whether these differences are CLL specific. We focused on naive (CD27−) B cells as a comparator because these cells, unlike CD27+ cells, coexpress sIgM and sIgD at relatively high homogeneous levels and are thought to represent the normal counterpart of U-CLL.40 In contrast to CLL cells, stimulation of either sIgM or sIgD on normal CD27− B cells induced protracted ERK1/2 phosphorylation responses and equivalent induction of MYC protein (Figure 6D-E). Thus, reduced induction of MYC in sIgD stimulation CLL cells seems to be a specific feature of these cells.
Expression of MYC and phosphorylated ERK1/2 in vivo
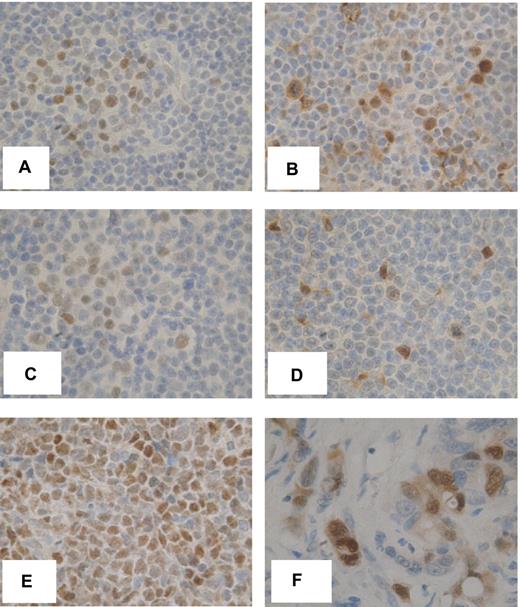
To confirm the relevance of our findings, we analyzed MYC expression in vivo by immunohistochemistry in lymph node biopsies from 8 patients with CLL/SLL. MYC expression was detected within the malignant cells in 7 (88%) of these samples (Figure 7 illustrates 2 representative samples). MYC-positive cells were mostly confined to PCs that were visible as areas comprising larger, CD5+, CD23+ CLL cells (Figure 7A,C). Typically 20% to 50% of cells in the PCs expressed MYC. Expression patterns were similar to that of the proliferation marker Ki67 (data not shown). Phosphorylated ERK1/2 also was detected in CLL cells in PCs (Figure 7). Because PCs are considered the likely site of antigen engagement, these data are consistent with the idea that induction of MYC downstream of sIgM and ERK1/2 is a key proliferation-promoting pathway in CLL.
Expression of phosphorylated ERK1/2 and MYC in vivo. Immunohistochemical analysis of MYC (A,C) and phospho-ERK1/2 (B,D) in 2 CLL/SLL lymph node biopsy samples. The images show expression within representative PCs comprising larger, less densely stained cells. (E-F) MYC expression in Burkitt lymphoma and phospho-ERK1/2 expression in colon cancer, respectively. Original magnification ×600.
Expression of phosphorylated ERK1/2 and MYC in vivo. Immunohistochemical analysis of MYC (A,C) and phospho-ERK1/2 (B,D) in 2 CLL/SLL lymph node biopsy samples. The images show expression within representative PCs comprising larger, less densely stained cells. (E-F) MYC expression in Burkitt lymphoma and phospho-ERK1/2 expression in colon cancer, respectively. Original magnification ×600.
Discussion
Recent in vivo labeling studies and analysis of telomeres have challenged the long-held view that CLL is predominantly a disease of failed apoptosis and demonstrated a key role for cell proliferation in driving disease progression.3-6 It is critical therefore to identify the pathways that control cell-cycle entry in CLL because this will provide novel opportunities for therapeutic targeting. The key observation in this work is that activation of sIgM signaling pathways leads to induction of the MYC proto-oncoprotein, an essential positive regulator of cell-cycle entry.
Several recent studies have investigated MYC expression in CLL. MYC was identified as a sIgM-regulated gene, as part of a global gene expression array analysis reported by Gribben and colleagues.26 We have confirmed this and have now shown that MYC also is induced at the protein level. Similar to intracellular Ca2+, it is likely that MYC induction in vitro may occur only in a subset of the malignant clone. However, the absence of high-quality antibodies has prevented us from investigating this directly. We also have shown that MYC is transcriptionally active because its induction is associated with increased expression of downstream target genes ODC1, CDK4, and CCDN2, required for cell-cycle entry. Consistent with our findings, a previous immunoblotting study revealed increased expression of MYC in lymph nodes tissues, compared with circulating CLL cells.25 Importantly, we studied MYC expression in situ and demonstrated activation of MYC (as well as ERK1/2 phosphorylation) specifically within the PCs of CLL/SLL lymph nodes, sites of malignant cell proliferation and probably antigen engagement and sIgM stimulation.
Overall, the ability of sIgM stimulation to increase MYC expression was associated with intracellular Ca2+ mobilization, another readout of signaling capacity that is linked to poor prognostic markers.9 Thus, MYC is one part of a program of downstream events linked to sIgM stimulation and one that is likely to play a critical role in cell-cycle entry. However, there was variation between intracellular Ca2+ responses and ERK1/2 phosphorylation responses in some individual samples. Heterogeneity within sIgM signaling responses has been described previously,31 and it will be important to further investigate the functional and clinical significance of these variable signaling responses. Because MYC plays a direct role in proliferation, its induction may provide a useful marker of functionally relevant responses to antigen engagement, and hence of clinical behavior.
Our results demonstrate that MEK1/2 → ERK1/2 signaling plays an important role in MYC induction, in both anti-IgM and CpG-ODN–treated cells. This is consistent with the finding that the MYC regulating transcription factor Elk1 is phosphorylated and activated downstream of ERK1/2 after pre-B cell receptor activation, where transcriptional induction of MYC downstream of ERK1/2 has been shown to be critical for the expansion of early B cells.41 However, effects of U0126 were partial, consistent with the idea that additional pathways are involved in MYC induction, acting either in parallel or in series. MYC induction after sIgM stimulation of normal mature mouse B cells in the presence of lipopolysaccharide is partly dependent on NFKB1 and REL,42 and treatment of CLL cells with BAFF also has been shown to increase MYC expression via canonical NF-κB signaling.24 Because sIgM stimulation induces NF-κB activation, NF-κB also may contribute to optimal MYC expression.43,44 Recent studies also have demonstrated that inhibition of PI3Kδ by CAL-101 interferes with sIgM-induced ERK1/2 activation,45 suggesting that proliferation promoting effects of PI3K activation15 may be at least partly mediated via ERK1/2 → MYC signaling. Future studies will focus on uncovering the molecular circuitry linking these signaling molecules, their downstream pathways and MYC expression in CLL cells.
Although anti-IgM stimulation enhances MYC expression, this does not seem to be sufficient to promote efficient CLL cell S-phase entry or division. For this reason, we focused on a cell-cycle model whereby CLL cells are treated with CpG-containing ODN to activate TLR9. This model has been used previously to investigate pathways of cell-cycle control and responses are linked to important clinical parameters, including time to treatment and overall survival.16,17,38 Using this approach, we confirmed that MEK1/2 activity was required for optimal MYC induction and S-phase entry. The modestly higher levels of MYC induced by CpG-ODN compared with anti-IgM may contribute to effective cell-cycle entry in CpG-ODN– but not anti-IgM–stimulated cells. However, it is also likely that other pathways contribute to determine proliferative responses. In CLL cells, anti-IgM has been shown to increase expression of cyclin D2 and cdk4 but not to substantially decrease the negative cell-cycle regulator p27kip1.14 Additional signals from supporting immunocytes or stromal cells are presumably required to down-modulate p27kip1 and other negative regulators, supporting efficient sIgM-induced cell-cycle entry in vivo.14
Interestingly, consequences of stimulation of sIgD and sIgM were distinct in CLL cells. Whereas sIgM stimulation generally led to protracted ERK1/2 phosphorylation and MYC induction in responsive samples, sIgD stimulation generally triggered transient ERK1/2 phosphorylation with relatively modest effects on MYC. These observations suggest it is the kinetics of ERK1/2 activation that is critical for regulation of MYC and are consistent with findings from other systems where protracted ERK1/2 activation is required for effective MYC induction and cell-cycle entry. Transient ERK1/2 activation leads to transcriptional activation of the MYC gene but does not effectively increase MYC protein expression because it fails to prevent MYC proteolysis,33,34 whereas protracted ERK1/2 activation lead to increased MYC gene transcription and stabilization of MYC via phosphorylation.35,46 Interestingly, similar differences in the kinetics of signaling responses have been observed for intracellular Ca2+ fluxes, at least in CD38 positive CLL cells, where sIgM and sIgD stimulation induced protracted and transient intracellular Ca2+ fluxes, respectively.13 These observations also may explain why retained sIgM responsiveness but not sIgD correlates with prognostic markers and outcome in CLL; although signal competent, sIgD stimulation does not effectively engage downstream pathways driving cell-cycle progression. Although sIgM responses were similar between responsive CLL samples and normal CD27− B cells, sIgD responses were clearly much weaker in CLL cells compared with normal CD27− B cells, indicating that this is a specific feature of CLL cells.
In summary, our results demonstrate that sIgM activation leads to ERK1/2-dependent induction of MYC expression in CLL cells. MYC induction seems to be dependent on protracted ERK1/2 activation because MYC was not induced in cells treated with anti-IgD, which induced transient ERK1/2 phosphorylation responses. Pharmacologic inhibition of signaling pathways activated by sIgM and leading to induction of MYC, including MEK1/2, may be an attractive therapeutic strategy, especially in progressive disease.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are very grateful to Drs Andrew Duncombe, Vlad Malykh, Helen McCarthy, Abe Jacob, Ben Kennedy, and Henri Grech and to Richard Palmer and colleagues for providing CLL samples and associated data and to the patients who donated clinical samples. They are also very grateful for the help of Isla Henderson for characterization of CLL samples, the support of Professor Christian Ottensmeier, and the helpful comments of Dr Andrew Steele.
This work was supported by the Kay Kendall Leukaemia Fund, Cancer Research UK, the Southampton Experimental Cancer Medicine Center, and Tenovus Solentside.
Authorship
Contribution: S.K., S.D., A.P., C.I.M., K.N.P., and K.-A.S. performed the research and analyzed data; S.K., K.N.P., M.A.-K., F.K.S., and G.P. designed the research and analyzed data; S.K. and G.P. wrote the initial draft of the manuscript, and all authors contributed to the modification of the draft and approved the final submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Sergey Krysov, Cancer Research UK Centre, Somers Cancer Research Building (MP824), Cancer Sciences Unit, University of Southampton School of Medicine, Southampton General Hospital, Tremona Road, Southampton SO16 6YD, United Kingdom; e-mail: s.krysov@soton.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal