Abstract
Exogenous HIV-1 matrix protein p17 was found to deregulate biologic activities of many different immune cells that are directly or indirectly involved in AIDS pathogenesis after binding to unknown cellular receptor(s). In particular, p17 was found to induce a functional program in monocytes related to activation and inflammation. In the present study, we demonstrate that CXCR1 is the receptor molecule responsible for p17 chemokine–like activity on monocytes. After CXCR1 binding, p17 was capable of triggering rapid adhesion and chemotaxis of monocytes through a pathway that involved Rho/ROCK. Moreover, CXCR1-silenced primary monocytes lost responsiveness to p17 chemoattraction, whereas CXCR1-transfected Jurkat cells acquired responsiveness. Surface plasmon resonance studies confirmed the capacity of p17 to bind CXCR1 and showed that the p17/CXCR1 interaction occurred with a low affinity compared with that measured for IL-8, the physiologic CXCR1 ligand. In all of its activities, p17 mimicked IL-8, the natural high-affinity ligand of CXCR1. Recent studies have highlighted the role of IL-8 and CXCR1 in HIV-1 replication and AIDS pathogenesis. Our findings herein call for an exploration of the therapeutic potential of blocking the p17/IL-8/CXCR1 axis in HIV-1 infection.
Introduction
Extensive changes in monocyte phenotype, differentiation, and function, which perturb their important role in innate immunity, have been reported in HIV-1 patients. Monocyte trafficking is increased in HIV-1–infected individuals, leading to enhanced homing of these cells to sites of HIV-1 infection, where they promote and sustain inflammatory processes that are major precipitating events in AIDS pathogenesis.1 Monocytes usually harbor integrated virus at a low frequency, so many of the effects that HIV-1 has on monocytes in vivo are indirect, resulting from the action of viral proteins or disruption of host immunologic networks. The hypothesis that mechanisms other than active viral replication are involved in monocyte dysfunction is further reenforced by the evidence that the blocking of HIV-1 replication is not linked to their functional recovery.2
Different HIV-1 proteins are known to promote an activated immunologic environment,3,4 and a direct influence of HIV-1 viral gene products in triggering monocyte activation and migration has been long postulated. The HIV-1 matrix protein p17 is continuously released in the extracellular space from HIV-1–infected cells. In fact, exogenous p17 is easily detected in the plasma of HIV-1 patients5 and in tissue specimens such as those from the brain6 and lymph nodes,7 even in patients successfully treated with highly active antiretroviral therapy (HAART).7 Extracellular p17 has been found to deregulate the biologic activity of many different immune cells, and this occurs after the interaction of its NH2-terminal region with an unidentified receptor(s) (p17R).8,9 Experiments performed on primary human monocytes have shown that the p17/p17R interaction selectively activates the transcriptional factor AP-1,10 whereas more recent data emphasized the Rho/ROCK pathway as a major target of p17-mediated signaling.11 Rho is known to play a crucial role in monocyte migration,12 and its effector, ROCK, a serine/threonine kinase, is involved in the regulation of actin organization.13 Therefore, it was possible to hypothesize a link between Rho activation and monocyte migratory activity.
In light of this evidence, we have investigated in the present study the potential direct chemotactic effect of p17 on human monocytes. We demonstrate that the chemokine receptor CXCR1 is the cellular receptor responsible for p17 chemokine–like activity on human monocytes. After CXCR1 binding, p17 chemoattracts monocytes through a signaling pathway that involves Rho/ROCK activation. Because CXCR1 is the receptor for IL-8, we also highlight the capability of p17 to display important IL-8–like activities.
Methods
Recombinant HIV-1 p17 protein and mAb to p17
Purified endotoxin-free recombinant HIV-1 matrix protein p17 (in its monomeric form) and glutathione S-transferase (GST) were produced as described previously.11 The absence of endotoxin contamination (< 0.25 endotoxin U/mL) in protein preparations was assessed with the Limulus Amoebocyte Assay (Associates of Cape Cod). MBS-3, a mAb to p17 that neutralizes the p17/p17R interaction,9 was produced in our laboratory.
Cell cultures
The Jurkat leukemic T-cell line was obtained from the American Type Culture Collection (Manassas, VA). Human umbilical vein endothelial cells (HUVECs) were isolated and cultured as described previously,14 and all of the experiments were performed on third- to fifth-passage HUVECs. When reported, HUVECs were pretreated for 2 hours at 37°C with 15 μ/mL of heparinase II or III.
Isolation of human monocytes and neutrophils
Blood was collected from healthy donors who gave informed consent for this research according to the Declaration of Helsinki. Human monocytes and neutrophils were isolated from whole blood, as described previously,15 and resuspended in adhesion buffer (PBS, 1mM CaCl2, 1mM MgCl2, and 10% FCS, pH 7.2) or plated in complete medium (RPMI containing 2mM glutamine and 10% FCS). Monocytes were pretreated, when indicated, with an isotype-matched mAb, a neutralizing mAb to CXCR-1 (MAb330), CXCR2 (MAb331), and CXCR4 (MAb171; R&D Systems) with PTx (Sigma-Aldrich) or the ROCK inhibitor Y-27632 (Alexis).
Static adhesion assay
Monocytes were suspended at 5 × 106/mL in adhesion buffer and 20 μL of cell suspension was added to 18-well glass slides (Thermo Scientific) coated overnight at 4°C with human fibrinogen (1 μg/mL; Sigma-Aldrich). Cells were then stimulated for 2 minutes at 37°C with 5 μL of the agonists or for 10 minutes with 5 μL of phorbol 12-myristate 13-acetate (500 ng/mL). After washing, adherent cells were fixed in 1.5% glutaraldehyde. Computer-assisted enumeration of cells in 4 high-power fields by light microscopy was performed. Results are expressed as the -fold increase compared with control.
Chemotaxis assays
Neutrophil and monocyte migration was assessed using 3-μm pore-size Transwells (BD Biosciences), and Jurkat cell migration was assessed using 8-μm pore-size Transwells. Cells were suspended at 2 × 106/mL in adhesion buffer. Cell suspension (100 μL) was added to the top well and medium containing agonists (600 μL) was added to the bottom well. Chemotaxis was performed for 90 minutes at 37°C, and then filters were removed. After fixation with 1.5% paraformaldehyde, migrated cells were counted in 5 high-power fields by light microscopy at a 10× magnification.
Adhesion assays on endothelial cells
HUVECs were allowed to reach a confluent monolayer in collagen-coated 48-well plates. Monocytes (5 × 105/well) were seeded on the HUVEC monolayer and stimulated with PBS, GST (1μM), formyl-methionyl-leucyl-phenylalanine (fMLP; 0.05μM), or p17 (1μM) for 30 minutes at 37°C. Unbound monocytes were removed by washing, and cells were then fixed for 15 minutes with 100% cold methanol and stained with Diff Quick (Merz-Dade). Monocytes bound to HUVECs were counted with a calibrated eyepiece in 4 different fields at a 20× magnification. Results are expressed as the -fold increase compared with control.
Transmigration assay
HUVECs (105 cells) were seeded on collagen-coated 24-well Transwell culture inserts (3 μm; BD Biosciences) and cultured for 3 days to form a monolayer. Monocytes (2 × 105/100 μL of adhesion buffer) were then seeded on the HUVEC monolayer before Transwell immersion. Adhesion buffer (600 μL) alone or containing GST (50nM), fMLP (10nM), or p17 (50nM) was added in the lower chamber. After 4 hours at 37°C, monocytes migrated to the bottom compartment were counted in 5 high-power fields by light microscopy at a 10× magnification.
Measurement of monocyte accumulation in skin
Male C57BL/6N mice (Charles River Laboratories) were maintained under standard conditions according to our institutional guidelines. The Animal Care and Use Committee of the University of Brescia gave approval for this study. Three mice/group were shaved and skin at the injection site was surgically excised 4 hours after intradermal injection of 50 μL of PBS or p17 (1 μg). Biopsies were fixed in 10% formalin for 48-72 hours and embedded in paraffin. For morphological analysis, multiple 4-μm sections were obtained from paraffin blocks and stained with H&E.
For immunohistochemistry, slides were deparaffinized, serially rehydrated, and stained according to routine procedures using a rabbit polyclonal Ab to Iba1 (1:300; Wako). Reactivity was revealed with the Dako EnVision rabbit-HRP followed by diaminobenzidine. Nuclei were counterstained with H&E. Cells in cutaneous and subcutaneous infiltration were counted in 3 high-power fields. Sections were photographed using the DP-70 Olympus digital camera mounted on an Olympus BX60 microscope, and the digital pictures (each corresponding to 0.028 mm2) were used for cell counting. Values are expressed as the means ± SD of 9 determinations. Sections were photographed using the DP-70 Olympus digital camera mounted on an Olympus BX60 microscope (magnification ×100, numerical aperture of the objective lens 0.40), and the digital pictures obtained with Cell*F software (Olympus Soft Imaging Solutions GmBH; each corresponding to 0.028 mm2) were used for cell counting.
siRNA technique
Nucleoporation of monocytes (Lonza) was performed using the Amaxa Nucleofector (Amaxa Biosystems). siRNAs (100nM) were added to 3 × 106 cells resuspended in 100 μL of nucleofection buffer. CXCR1 silencing was performed using 4 distinct siRNAs targeting 4 different regions of CXCR1 receptor (Dharmacon). Fluorescein-labeled irrelevant (scr) siRNAs (Invitrogen) were used as a negative control and to evaluate the efficiency of siRNA nucleoporation by flow cytometry. The efficacy of CXCR1 siRNA was evaluated by real-time PCR analysis and by flow cytometry using a mAb to CXCR1 and APC-conjugated secondary Ab (Caltag Laboratories).
Real-time PCR for gene-expression analysis
Total RNA was isolated from monocytes (1 × 106 cells) using the RNeasy Mini Kit (QIAGEN). After retrotranscription, 50 ng of cDNA was mixed with sterile water and the SYBR Green qPCR Master Mix (Promega) and amplified using the following PCR primers (0.2μM each; Primm): CXCR1, 5′-TGG GAA ATG ACA CAG CAA AA-3′ (forward) and 5′-AGT GTA CGC AGG GTG AAT CC-3′ (reverse); human β actin, 5′-GGCACCCAGCACAATGAAG-3′ (forward), and 5′-GCTGATCCACATCTGCTGG-3′ (reverse). Quantification of CXCR1 cDNA was normalized in each reaction according to the internal β-actin control. Results are expressed as a percentage of control.
Transfections
Jurkat cells were plated at 3 × 105/mL and, after 24 hours, nucleofected with 2 μg of the endotoxin-free plasmids pEGFP-N3 or pEGFP-N3 CXCR1 (a kind gift from Dr Ingrid U. Schraufstatter, Department of Vascular Biology, University of California-San Diego, La Jolla, CA). The vector pEGFP-N3 expressing a red-shifted variant of wild-type green fluorescent protein (GFP) was from Clontech. After transfection, Jurkat cells were incubated for 24 hours at 37°C, and transfection efficiency was evaluated as enhanced GFP expression by flow cytometry. Cell-surface expression of CXCR1 was evaluated by flow cytometry using a mAb to CXCR1 and APC-conjugated secondary Ab.
SPR binding assay
Surface plasmon resonance (SPR) measurements were performed on a BIAcore X instrument (GE Healthcare). Anti-GST Ab was immobilized on a CM5 surface (GE Healthcare) using standard amine-coupling chemistry. Recombinant human CXCR1 with a C-terminal GST tag (10 μg/mL in running buffer composed of 50mM HEPES, pH 7.0, 0.01% CHS, 0.1% CHAPS, and 0.33mM synthetic phospholipid blend [dioleoyl] DOPC:DOPS [7:3 wt/wt; Avanti polar lipids])16 was injected over the anti-GST surface at a flow rate of 5 μL/min, allowing the immobilization of approximately 1600 RU (equal to approximately 0.025 pmoles). A sensor chip coated with anti-GST Ab alone was used as a negative control and for blank subtraction. The immobilization of the receptor to the sensor chip was confirmed by injecting increasing concentrations of mAb to CXCR1 in running buffer containing 0.1 mg/mL of BSA at a flow rate of 30 μL/min and evaluating its binding to CXCR1. IL-8, p17, and SDF-1α were injected over the CXCR1 or control surfaces as described for the CXCR1 mAb. Kinetic parameters were obtained from the sensorgram overlays using the nonlinear fitting (single site model) software package BIAevaluation Version 3.2 software (BiaCore). Only sensorgrams providing values of χ2 close to 10 were used.17
Superoxide anion production
Superoxide anion (O2−) release was evaluated with a microplate spectrometry reader for 5 minutes by measuring at 550 nm the reduction of cytochrome c induced by O2−. The O2− production by 2 × 105 neutrophils suspended in HBSS, pH 7.4, was triggered by adding the stimulus (fMLP 100nM) in 96-well plates. In addition, the priming effect of GST, IL-8, and p17 on O2− production in response to fMLP was tested by pretreating neutrophils with GST, IL-8 (5nM), and p17 (5nM and 25nM) for 15 minutes at 37°C before fMLP treatment.
Statistical analysis
Data obtained from multiple independent experiments are expressed as the means ± SD. Data were analyzed for statistical significance using a paired 2-tail Student t test or the 1- and 2-way ANOVA when appropriate. The Bonferroni posttest was used to compare data. Differences were considered significant at P < .05. Statistical tests were performed using Prism Version 5 software (GraphPad).
Results
P17 induces monocyte adhesion and migration
Chemokines signal through G-protein–linked receptors, which trigger intracellular signaling events controlling rapid integrin-dependent adhesion on endothelial cells and chemotaxis of leukocytes in tissue microenvironment. To determine a possible role of p17 as a chemokine-like protein, we performed monocyte adhesion and migration assays in response to the viral protein.
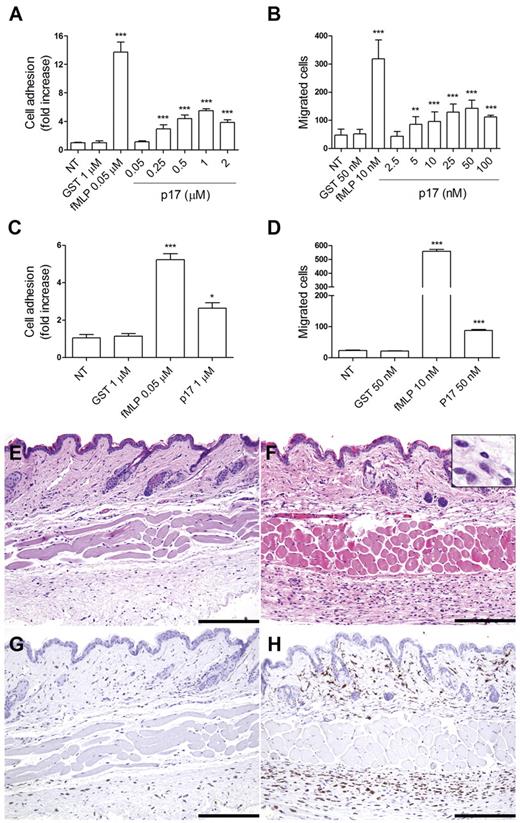
Figure 1A shows that p17 triggered a rapid (2 minutes) concentration-dependent adhesion of primary human monocytes to fibrinogen. Adhesion induced by p17 occurred at a protein concentration 0.25μM and reached a peak at 1μM. As shown in Figure 1B, p17 was also able to induce monocyte migration in a Transwell chemotaxis assay. The viral protein exhibited a statistically significant chemoattraction of monocytes at a concentration as low as 5nM, with a peak activity at 50nM. As expected, a strong induction of monocyte adhesion (Figure 1A) and migration (Figure 1B) was obtained using fMLP (0.05μM and 10nM, respectively) as a reference chemoattractant, whereas adhesion and migration were not promoted by GST (1μM and 50nM, respectively), which was used as an irrelevant protein. Our data showing that monocyte adhesion required 2-log more p17 concentration than that required for chemotaxis can be explained by the finding by Campbell et al,18 that adhesion required the simultaneous occupancy of many receptors (ie, a high agonist concentration), whereas chemotaxis occurred at a low agonist concentration. This phenomenon is common to chemokines, because requirements for triggering adhesion and chemotaxis are distinct, thus permitting their independent regulation.18
P17 induces the adhesion, migration, and transmigration of isolated human monocytes. (A) Static adhesion assay on fibrinogen. Monocytes were stimulated for 2 minutes at 37°C with PBS (NT), GST, fMLP, or p17 at the indicated concentrations. Bars represent the means ± SD of 3 independent experiments performed in triplicate. Statistical analysis was performed by 1-way ANOVA and the Bonferroni posttest was used to compare data. ***P < .001 compared with NT. (B) Transwell migration assay of monocytes in response to PBS (NT), GST, fMLP, or p17 at the indicated concentrations. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by 1-way ANOVA and the Bonferroni posttest was used to compare data. **P < .01 and ***P < .01 compared with NT. (C) Static adhesion assay on HUVECs. Monocytes were stimulated for 30 minutes at 37°C with PBS (NT), GST, fMLP, or p17. Bars represent the means ± SD of 4 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. *P < .05 and ***P < .001 compared with NT. (D) Transwell migration assay of monocytes through HUVEC monolayer in response to GST, fMLP, or p17. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .01 compared with NT. (E-H) Serial sections obtained from PBS-injected (left panels) or p17-injected (right panels) mice and stained for H&E (E-F) or anti-Iba1 (G-H). Skin biopsies obtained from p17-injected mice contain a greater number of infiltrating cells compared with mice injected with PBS. Most of the infiltrating cells correspond to large mononuclear cells (F insert), as defined by the expression of the monocyte/macrophage marker Iba1 (H). Scale bar indicates 200 μm.
P17 induces the adhesion, migration, and transmigration of isolated human monocytes. (A) Static adhesion assay on fibrinogen. Monocytes were stimulated for 2 minutes at 37°C with PBS (NT), GST, fMLP, or p17 at the indicated concentrations. Bars represent the means ± SD of 3 independent experiments performed in triplicate. Statistical analysis was performed by 1-way ANOVA and the Bonferroni posttest was used to compare data. ***P < .001 compared with NT. (B) Transwell migration assay of monocytes in response to PBS (NT), GST, fMLP, or p17 at the indicated concentrations. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by 1-way ANOVA and the Bonferroni posttest was used to compare data. **P < .01 and ***P < .01 compared with NT. (C) Static adhesion assay on HUVECs. Monocytes were stimulated for 30 minutes at 37°C with PBS (NT), GST, fMLP, or p17. Bars represent the means ± SD of 4 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. *P < .05 and ***P < .001 compared with NT. (D) Transwell migration assay of monocytes through HUVEC monolayer in response to GST, fMLP, or p17. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .01 compared with NT. (E-H) Serial sections obtained from PBS-injected (left panels) or p17-injected (right panels) mice and stained for H&E (E-F) or anti-Iba1 (G-H). Skin biopsies obtained from p17-injected mice contain a greater number of infiltrating cells compared with mice injected with PBS. Most of the infiltrating cells correspond to large mononuclear cells (F insert), as defined by the expression of the monocyte/macrophage marker Iba1 (H). Scale bar indicates 200 μm.
Monocyte adhesion and migration assays were also performed on HUVECs. As expected, fMLP increased the adhesion of monocytes on HUVECs compared with unstimulated or GST-treated monocytes (Figure 1C). In the same assay, monocytes stimulated with p17 at a 1μM concentration showed a 2.3-fold increase in cell adhesion to HUVECs compared with control monocytes (Figure 1C). Moreover, p17 was able to induce migration across endothelial cells, with p17-stimulated monocytes approximately 3.3-fold more active in performing transendothelial migration compared with control monocytes (Figure 1D).
Because p17 is known to be a heparin/heparan sulfate–binding protein,19 we assessed whether p17 binding to heparan sulfate proteoglycans (HSPGs) expressed on HUVECs could play a part in monocyte adhesion and transmigration. Treatment of HUVECs with heparinase II or III to remove HSPGs did not influence the ability of p17 to induce monocyte adhesion to or transmigration through HUVECs (data not shown), thus excluding a role of HSPGs expressed on HUVECs in p17 chemokine activity.
The effect of p17 was also analyzed in vivo by intradermal injection of protein (1 μg/50 μL). As shown in Figure 1E and F, a higher number of infiltrating immune cells was observed in the skin of p17-injected mice compared with PBS-injected mice (55.63 ± 10.2 vs 19.64 ± 8.8; P < .001). Skin-infiltrating cells in the p17-injected mice were represented by more rare polymorphonuclear cells and by a significant fraction (75%) of monocytes (Iba1+; Figure 1G-H).
PTx treatment inhibits p17-induced adhesion and migration
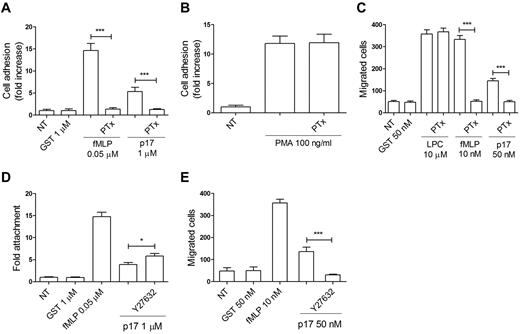
Chemokines bind 7 transmembrane receptors coupled with the Gi class of heterodimeric G proteins. Pertussis toxin (PTx) is known to prevent Gi proteins from interacting with G protein–coupled receptors, thus blocking intracellular communication. To determine the nature of the p17 receptor, monocytes were pretreated with 500 ng/mL of PTx for 2 hours at 37°C, then stimulated with p17 and tested for their capability to adhere and migrate. P17-triggered monocyte adhesion and migration (Figure 2A-C) were completely inhibited by PTx pretreatment. In the same experiment, adhesion and chemotaxis (Figure 2A-C) induced by fMLP was inhibited, as expected, by PTx.20 The inhibitory effect of PTx was not attributable to a generic toxic effect of PTx pretreatment, because adhesion of monocytes was retained after 10 minutes of phorbol 12-myristate 13-acetate stimulation (100 ng/mL; Figure 2B) and cell migration occurred in response to L-α-lysophosphatidylcholine, palmitoyl C16:0 (Sigma-Aldrich; 10μM; Figure 2C), which agrees with previous observations.15,21 PTx pretreatment of monocytes was also found to inhibit both p17-induced adhesion on and transendothelial migration through HUVECs (data not shown). These data show that p17 induces monocyte adhesion and migration through a signaling pathway dependent on PTx-sensitive heterotrimeric GTP-binding proteins.
P17-induced adhesion and migration of monocytes involve a PTx-sensitive and Rho/Rock–dependent signaling pathway. (A-B) Static adhesion of monocytes on fibrinogen. Monocytes pretreated with 500 ng/mL of PTx for 2 hours at 37°C were stimulated for 2 minutes at 37°C with PBS (NT), GST, fMLP, p17 (A) or for 10 minutes with phorbol 12-myristate 13-acetate (PMA; B). Bars represent the means ± SD of 3 independent experiments performed in triplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001. (C) Transwell migration assay of monocytes in response to the indicated treatments. Monocytes pretreated for 2 hours at 37°C with 500 ng/mL of PTx were stimulated for 90 minutes at 37°C with PBS (NT), GST, L-α-lysophosphatidylcholine palmitoyl C16:0 (LPC), fMLP, or p17. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001. (D) Static adhesion of monocytes on fibrinogen. Monocytes were pretreated for 20 minutes at 37°C with 25μM of the ROCK inhibitor Y27632 and then stimulated for 2 minutes at 37°C with PBS (NT), GST, fMLP, or p17. Bars represent the means ± SD of 3 independent experiments performed in triplicate. Statistical analysis was performed by paired 2-tail Student t test. *P < .05. (E) Transwell migration assay of monocytes in response to the indicated treatments. Monocytes pretreated for 20 minutes at 37°C with 25μM of the ROCK inhibitor Y27632 were stimulated for 90 minutes at 37°C with PBS (NT), GST, fMLP, or p17 (50nM). Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001.
P17-induced adhesion and migration of monocytes involve a PTx-sensitive and Rho/Rock–dependent signaling pathway. (A-B) Static adhesion of monocytes on fibrinogen. Monocytes pretreated with 500 ng/mL of PTx for 2 hours at 37°C were stimulated for 2 minutes at 37°C with PBS (NT), GST, fMLP, p17 (A) or for 10 minutes with phorbol 12-myristate 13-acetate (PMA; B). Bars represent the means ± SD of 3 independent experiments performed in triplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001. (C) Transwell migration assay of monocytes in response to the indicated treatments. Monocytes pretreated for 2 hours at 37°C with 500 ng/mL of PTx were stimulated for 90 minutes at 37°C with PBS (NT), GST, L-α-lysophosphatidylcholine palmitoyl C16:0 (LPC), fMLP, or p17. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001. (D) Static adhesion of monocytes on fibrinogen. Monocytes were pretreated for 20 minutes at 37°C with 25μM of the ROCK inhibitor Y27632 and then stimulated for 2 minutes at 37°C with PBS (NT), GST, fMLP, or p17. Bars represent the means ± SD of 3 independent experiments performed in triplicate. Statistical analysis was performed by paired 2-tail Student t test. *P < .05. (E) Transwell migration assay of monocytes in response to the indicated treatments. Monocytes pretreated for 20 minutes at 37°C with 25μM of the ROCK inhibitor Y27632 were stimulated for 90 minutes at 37°C with PBS (NT), GST, fMLP, or p17 (50nM). Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001.
Inhibition of ROCK increases p17-induced adhesion and inhibits p17-induced migration of monocytes
Rho GTPase signals are responsible for monocyte migration activity.12 This occurs through the RhoA effector ROCK, a Ser/Thr kinase that is involved in the regulation of actin organization.13 Recently, the RhoA/ROCK pathway was also identified as a major target of p17-mediated signaling,11 prompting us to investigate possible links between Rho activation and monocyte recruitment after p17 stimulation. Monocytes were pretreated for 20 minutes at 37°C with the highly selective ROCK inhibitor Y-27632, and then stimulated with p17 at concentrations of 1μM and 50nM to promote cell adhesion and migration, respectively. GST was used as an irrelevant protein and fMLP as a reference chemoattractant. Inhibition of ROCK significantly increased the rapid, p17-induced adhesion of monocytes to fibrinogen (Figure 2D). Conversely, inhibition of ROCK significantly inhibited chemotaxis of monocytes induced within 90 minutes by p17 (Figure 2E). These results agree with previous observations showing that ROCK activity has a biphasic role. Initially, inhibition of ROCK is required to allow cellular attachment, and then, reactivation of ROCK is required for maturation of adhesion complexes and subsequent migration.22,23 Our results show that p17 activates the Rho/ROCK-signaling pathway, thus promoting monocyte adhesion and migration.
The chemokine activity of p17 is mediated by CXCR1
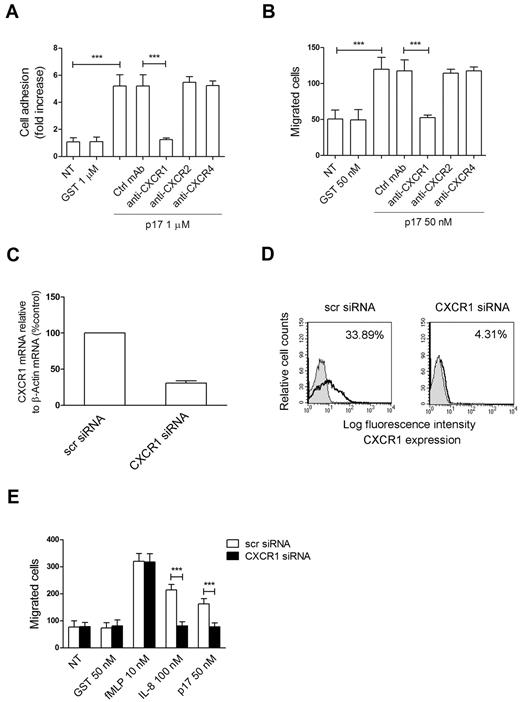
Human monocytes express an array of chemokine 7-pass receptors, among which CXCR1, CXCR2, or CXCR4 may play a role in p17 chemokine–like activity. Indeed, CXCR1 is known to mediate monocyte arrest on HUVECs24 and to predominantly promote cell migration in response to IL-8.25 Moreover, CXCR1 is known to be involved in the Rho/ROCK-signaling pathway, because inhibition of ROCK by Y27632 makes cells refractory to CXCR1 migration by IL-8.26 In addition, CXCR2 and CXCR4 are G-coupled receptors involved in monocyte recruitment, acting independently or through the RhoA/ROCK signaling pathway.22,26 Therefore, we investigated whether monocyte rapid adhesion and migration could be ascribed to p17 interaction with CXCR1, CXCR2, or CXCR4. We performed experiments with monocytes pretreated for 1 hour at 37°C with neutralizing mAbs to CXCR1, CXCR2, or CXCR4 or with a control isotype-matched mAb. As shown in Figure 3, mAb to CXCR1 suppressed both p17-dependent rapid adhesion (Figure 3A) and chemotaxis (Figure 3B) to control levels. No interference with p17 activity was observed in monocytes pretreated with the control mAb or with neutralizing mAbs to CXCR2 or CXCR4. These results show that the chemokine activity of p17 is specifically mediated by CXCR1.
P17 chemokine–like activity is mediated specifically by the IL-8 receptor CXCR1. (A) Static adhesion of monocytes on fibrinogen. Monocytes were stimulated for 2 minutes at 37°C with PBS (NT) or GST or pretreated for 1 hour at 37°C with 50 μg/mL of mAb to CXCR1, CXCR2, or CXCR4 or with an unrelated mAb (Ctrl mAb) and then stimulated for 2 minutes with p17 (1μM). Bars represent the means ± SD of 3 independent experiments performed in triplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001. (B) Transwell migration assay of monocytes in response to the indicated treatments. Monocytes were stimulated for 90 minutes at 37°C with PBS (NT) or GST or pretreated for 1 hour at 37°C with 50 μg/mL of mAb to CXCR1, CXCR2, or CXCR4 or with Ctrl mAb and then stimulated with p17 (50nM). Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001. (C) Analysis of CXCR1 gene expression performed using quantitative real-time PCR. Monocytes were nucleoporated with scr siRNAs used as a negative control or with a pool of 4 distinct siRNAs specific for 4 distinct regions of CXCR1. Analysis of real-time PCR data were performed with the 2−ΔΔCt method using relative quantitation study software. Quantification of CXCR1 mRNA was normalized in each reaction according to the internal β-actin control. Bars represent the means ± SD of 3 independent experiments performed in triplicate. (D) Effect of CXCR1 silencing on surface-receptor expression. Monocytes nucleofected with scr siRNA or CXCR1 siRNA were incubated with isotype control Ab (solid histogram) or mAb to CXCR1 (open histogram), and then stained with APC-conjugated secondary Ab and analyzed by flow cytometry. (E) Transwell migration assay of monocytes nucleoporated with CXCR1 siRNAs or with scr siRNAs in response to the indicated treatments. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001.
P17 chemokine–like activity is mediated specifically by the IL-8 receptor CXCR1. (A) Static adhesion of monocytes on fibrinogen. Monocytes were stimulated for 2 minutes at 37°C with PBS (NT) or GST or pretreated for 1 hour at 37°C with 50 μg/mL of mAb to CXCR1, CXCR2, or CXCR4 or with an unrelated mAb (Ctrl mAb) and then stimulated for 2 minutes with p17 (1μM). Bars represent the means ± SD of 3 independent experiments performed in triplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001. (B) Transwell migration assay of monocytes in response to the indicated treatments. Monocytes were stimulated for 90 minutes at 37°C with PBS (NT) or GST or pretreated for 1 hour at 37°C with 50 μg/mL of mAb to CXCR1, CXCR2, or CXCR4 or with Ctrl mAb and then stimulated with p17 (50nM). Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001. (C) Analysis of CXCR1 gene expression performed using quantitative real-time PCR. Monocytes were nucleoporated with scr siRNAs used as a negative control or with a pool of 4 distinct siRNAs specific for 4 distinct regions of CXCR1. Analysis of real-time PCR data were performed with the 2−ΔΔCt method using relative quantitation study software. Quantification of CXCR1 mRNA was normalized in each reaction according to the internal β-actin control. Bars represent the means ± SD of 3 independent experiments performed in triplicate. (D) Effect of CXCR1 silencing on surface-receptor expression. Monocytes nucleofected with scr siRNA or CXCR1 siRNA were incubated with isotype control Ab (solid histogram) or mAb to CXCR1 (open histogram), and then stained with APC-conjugated secondary Ab and analyzed by flow cytometry. (E) Transwell migration assay of monocytes nucleoporated with CXCR1 siRNAs or with scr siRNAs in response to the indicated treatments. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by paired 2-tail Student t test. ***P < .001.
CXCR1 silencing inhibits p17-induced monocyte migration
The involvement of CXCR1 in p17-induced cell migration was also investigated by silencing CXCR1 expression on primary monocytes using Amaxa nucleofection technology to deliver CXCR1 siRNAs. The efficiency of nucleofection was determined by flow cytometric analysis, and showed an average of 87% ± 8% nucleoporated monocytes (data not shown). The effects of siRNA on CXCR1 expression were evaluated by real-time PCR analysis and by flow cytometry. As shown in Figure 3C, approximately 75% inhibition of CXCR1 transcripts was observed in monocytes 24 hours after nucleofection with CXCR1 siRNAs compared with monocytes nucleofected with scr siRNA. Consequently, the expression of CXCR1 on monocytes nucleofected with CXCR1 siRNA was strongly impaired (4.31%) compared with cells nucleofected with scr siRNA (33.89%; Figure 3D). The biologic consequence of CXCR1 silencing was evident in monocytes collected 24 hours after nucleofection, which showed a significant inhibition in p17-induced migration of CXCR1-silenced monocytes compared with monocytes nucleofected with scr siRNAs (Figure 3E). CXCR1 silencing also resulted in a significant reduction of monocyte migratory activity to IL-8 (Figure 3E). Specificity of the reaction was further confirmed by the finding that CXCR1 silencing did not interfere with the activity of fMLP (Figure 3E).
The p17-induced migratory activity is sustained by CXCR1
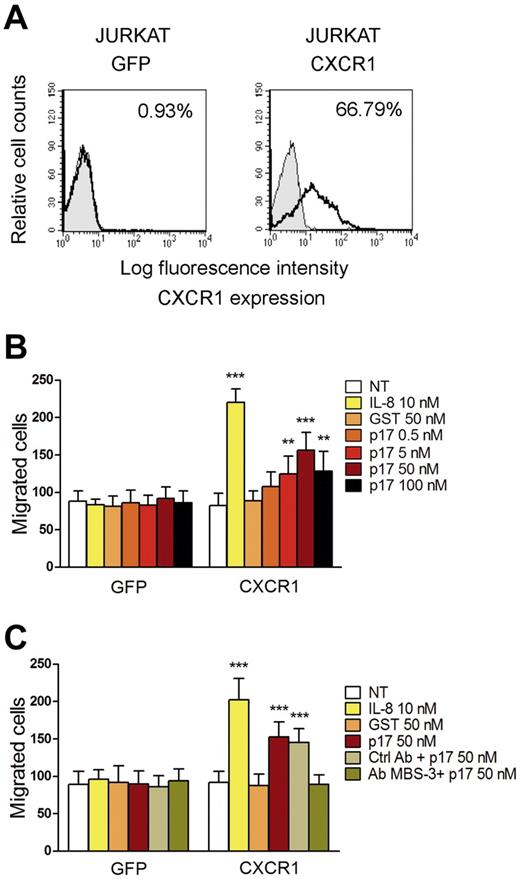
CXCR1 is considered the major factor responsible for the chemotactic response of cells to IL-8.25 Therefore, we investigated whether the de novo expression of CXCR1 was responsible for p17 chemotactic activity. Jurkat cells were used to express CXCR1 because they do not express this receptor, but do possess the necessary signaling machinery to support cell migration mediated by CXCR1.27 Cells were nucleofected with pEGFP-N3 or pEGFP-N3 expressing human CXCR1 (pEGFP-N3-CXCR1). The efficiency of nucleofection was determined by flow cytometry and found to be 65% ± 6% in 3 different experiments (data not shown). Flow cytometric analysis showed that a high percentage (66.79%) of cells transfected with pEGFP-N3-CXCR1 expressed the receptor on their surface, compared with cells transfected with pEGFP-N3, which were CXCR1 negative (Figure 4A). The migratory behavior of Jurkat cells expressing EGFP or CXCR1 after IL-8 or p17 stimulation was examined with a Transwell chemotaxis assay. Figure 4B shows that Jurkat cells nucleofected with the pEGFP-N3 did not migrate in response to GST, p17, or IL-8. Conversely, CXCR1-expressing Jurkat cells were able to migrate in response to IL-8 and p17 only (Figure 4B). The migratory activity induced by p17 in cells expressing CXCR1 was dose dependent, with a peak of p17 activity at a protein concentration of 50nM. Moreover, p17-induced migration was inhibited by the presence of anti-p17 mAb MBS-3 (Figure 4C). Therefore, our results show that CXCR1 expression in Jurkat cells is sufficient to mediate chemotaxis in response to p17. The blocking effect of mAb MBS-3 provides evidence for the specificity of the p17/CXCR1 interaction and the consequent p17 chemotactic activity.
CXCR1 expression in Jurkat cells is sufficient to mediate chemotaxis in response to p17. (A) Surface expression of CXCR1 on Jurkat cells transfected with the control vector pEGFP-N3 or with pEGFP-N3 expressing CXCR1. Cells were incubated with isotype control mAb (solid histogram) or mAb to CXCR1 (open histogram), and then stained with APC-conjugated secondary Ab and analyzed by flow cytometry. (B-C) Transwell migration assays of Jurkat cells transfected with pEGFP-N3 or pEGFP-N3-CXCR1 in response to the indicated treatments. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by 2-way ANOVA. The Bonferroni posttest was used to compare data. **P < .01; ***P < .001. (B) Transfected Jurkat cells were stimulated for 90 minutes at 37°C with PBS (NT), IL-8, GST, or p17. (C) Jurkat cells, pre-incubated for 30 minutes at 37°C with 50 μg/mL of Ctrl mAb or mAb to p17 (MBS-3), were subsequently stimulated for 90 minutes at 37°C with PBS (NT), IL-8, GST, or p17.
CXCR1 expression in Jurkat cells is sufficient to mediate chemotaxis in response to p17. (A) Surface expression of CXCR1 on Jurkat cells transfected with the control vector pEGFP-N3 or with pEGFP-N3 expressing CXCR1. Cells were incubated with isotype control mAb (solid histogram) or mAb to CXCR1 (open histogram), and then stained with APC-conjugated secondary Ab and analyzed by flow cytometry. (B-C) Transwell migration assays of Jurkat cells transfected with pEGFP-N3 or pEGFP-N3-CXCR1 in response to the indicated treatments. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by 2-way ANOVA. The Bonferroni posttest was used to compare data. **P < .01; ***P < .001. (B) Transfected Jurkat cells were stimulated for 90 minutes at 37°C with PBS (NT), IL-8, GST, or p17. (C) Jurkat cells, pre-incubated for 30 minutes at 37°C with 50 μg/mL of Ctrl mAb or mAb to p17 (MBS-3), were subsequently stimulated for 90 minutes at 37°C with PBS (NT), IL-8, GST, or p17.
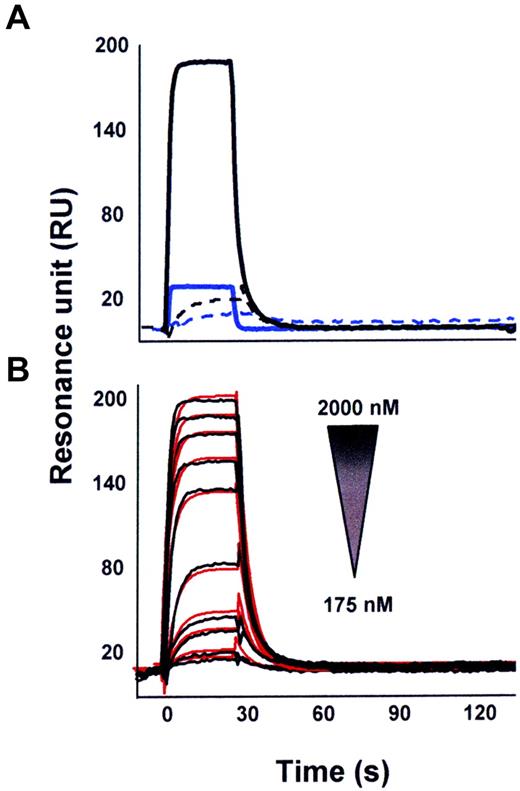
Characterization of the p17/CXCR1 interaction by SPR
SPR was used to assess the capacity of p17 to bind CXCR1 immobilized on a BIAcore sensor chip coated with a lipid bilayer, a model shown to ensure structural stability and proper tridimensional conformation of G-coupled 7-transmembrane domain receptors.17,28 The proper conformation of immobilized CXCR1 was demonstrated by the observation that the mAb to CXCR1 still retained the capacity to recognize the immobilized receptor (Table 1) and that IL-8 bound to immobilized-CXCR1 with a dissociation constant (Kd) comparable to that already measured under different experimental conditions29 (Table 1). As shown in Figure 5, p17 was capable of binding to CXCR1 immobilized on the sensor chip. Specificity of the binding was demonstrated by the lack of p17 binding to sensor chip–immobilized anti-GST mAb. Moreover, SDF-1α, a CXCR1-unrelated chemokine, did not show any binding to the receptor (Figure 5A). Finally, the p17/CXCR1 interaction was found to be dose dependent (Figure 5B). The interaction of p17 with CXCR1 occurred with a very rapid dissociation rate (Table 1), and thus with a low affinity compared with that measured for IL-8, the physiologic CXCR1 ligand (Kd = 1.85μM and 8nM, respectively; Table 1).
Binding parameters of the interaction of p17, IL-8, SDF-1α, and anti-CXCR1 mAb by real-time SPR
| . | CXCR1 . | ||
|---|---|---|---|
| Association rate (Kon), 1/Ms . | Dissociation rate (Koff), 1/s . | Dissociation constant (Kd), M . | |
| Anti-CXCR1 Ab | 2.9 × 105 | 1.8 × 10−3 | 6.1 × 10−9 |
| p17 | 5.8 × 104 | 0.107 | 1.8 × 10−6 |
| IL-8 | 1.7 × 103 | 1.4 × 10−5 | 8.0 × 10−9 |
| SDF-1α | ND | ND | ND |
| . | CXCR1 . | ||
|---|---|---|---|
| Association rate (Kon), 1/Ms . | Dissociation rate (Koff), 1/s . | Dissociation constant (Kd), M . | |
| Anti-CXCR1 Ab | 2.9 × 105 | 1.8 × 10−3 | 6.1 × 10−9 |
| p17 | 5.8 × 104 | 0.107 | 1.8 × 10−6 |
| IL-8 | 1.7 × 103 | 1.4 × 10−5 | 8.0 × 10−9 |
| SDF-1α | ND | ND | ND |
The association rate (Kon) and dissociation rate (Koff) are reported and the dissociation constant (Kd) was derived from the Koff/Kon ratio. The results are representative of 2 independent experiments with similar results. Kd values were also calculated independently from binding kinetics by performing Scatchard plot analysis of the equilibrium binding data. The correlation coefficients of the linear regression of these analyses were always higher than 0.8 and yielded Kd values similar to those calculated from the Koff/Kon ratio (data not shown).
ND indicates not determinable.
SPR analysis of the p17/CXCR1 and p17/CXCR2 interaction. (A) P17 (straight lines) or SDF-1α (dashed lines, both at 1.25μM) were injected over BIAcore CM5 sensor chips coated with a mAb to GST (blue lines) or in the presence of CXCR1 recombinant protein with GST tag (black lines). (B) Overlay of blank-subtracted sensorgrams resulting from the injection of increasing concentrations of p17 over the GST-CXCR1 surface. Black lines represent the experimental data. Red lines represent the fits.
SPR analysis of the p17/CXCR1 and p17/CXCR2 interaction. (A) P17 (straight lines) or SDF-1α (dashed lines, both at 1.25μM) were injected over BIAcore CM5 sensor chips coated with a mAb to GST (blue lines) or in the presence of CXCR1 recombinant protein with GST tag (black lines). (B) Overlay of blank-subtracted sensorgrams resulting from the injection of increasing concentrations of p17 over the GST-CXCR1 surface. Black lines represent the experimental data. Red lines represent the fits.
P17 displays IL-8–like activity on neutrophils
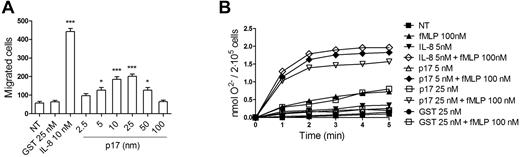
Neutrophils express the highest levels of CXCR1 among CXCR1-expressing cells. Therefore, we verified on neutrophils the capability of p17 to exert those IL-8–like activities known to be primarily (chemotaxis) or exclusively (respiratory burst) mediated by CXCR1.25,30 We performed migration assays with a 3-μm Transwell chemotaxis system in response to p17, IL-8, or GST. The resulting data showed that p17 was chemotactic for neutrophils at concentrations ranging from 10nM to 25nM, revealing a typical bell-shaped chemokine dose–response range (Figure 6A).
P17 exerts some IL-8–like activities on neutrophils. (A) Transwell migration assay of neutrophils in response to the indicated concentration of p17. IL-8 was used as a positive control and GST as an irrelevant protein. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by 1-way ANOVA, and the Bonferroni posttest was used to compare data. ***P < .001 compared with NT. (B) Priming effect of p17 on fMLP-stimulated neutrophil oxidative burst. Cells were untreated or treated with IL-8, p17, or GST for 15 minutes at 37°C, and then stimulated or not with fMLP (100nM). Results are expressed as nanomoles of O2− production/2 × 105 cells. Results are representative of 4 different experiments with similar results.
P17 exerts some IL-8–like activities on neutrophils. (A) Transwell migration assay of neutrophils in response to the indicated concentration of p17. IL-8 was used as a positive control and GST as an irrelevant protein. Bars represent the means ± SD of 3 independent experiments performed in duplicate. Statistical analysis was performed by 1-way ANOVA, and the Bonferroni posttest was used to compare data. ***P < .001 compared with NT. (B) Priming effect of p17 on fMLP-stimulated neutrophil oxidative burst. Cells were untreated or treated with IL-8, p17, or GST for 15 minutes at 37°C, and then stimulated or not with fMLP (100nM). Results are expressed as nanomoles of O2− production/2 × 105 cells. Results are representative of 4 different experiments with similar results.
In terms of duration and intensity, the respiratory burst induced by IL-8 is considerably weaker than that observed with other chemoattractant. IL-8 does not directly activate NADPH oxidase, but is able to elicit its rapid and transient activation, as well as to potentiate O2− production induced by stimulants such as fMLP via a priming mechanism.31
The enzyme kinetic of O2− production in response to fMLP, p17, IL-8, or GST is presented in Figure 6B. Incubation of neutrophils with fMLP (100nM) induced a rapid but weak production of O2−, whereas incubation with IL-8 (5nM), p17 (5nM and 25nM), or GST (5nM and 25nM) did not. A 15-minutes preexposure of neutrophils to IL-8 (5nM) or p17 (5nM and 25nM) enhanced both the initial and maximum rates of O2− production subsequently induced by fMLP, whereas GST preexposure did not. Similar to IL-8, the priming effect of p17 (5nM and 25nM) on O2− production appeared at 1 minute and reached a plateau 3 minutes after fMLP stimulation (Figure 6B). Our data show that, like IL-8,31 p17 exerts a priming effect on the O2− production induced by fMLP on neutrophils.
Discussion
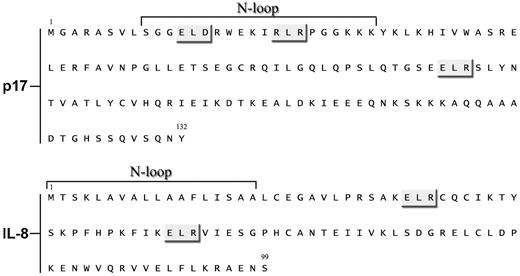
In the present study, we demonstrate that CXCR1 is the receptor responsible for p17 chemokine–like activity on human primary monocytes. Although p17 does not share any primary amino acid sequence identity with IL-8, and its affinity for CXCR1 is considerably lower than IL-8, p17 was a surprisingly efficacious ligand. The chemokine/chemokine receptor system in general is intricately complex, and the structural and thermodynamic basis for ligand-binding affinity, receptor activation, and downstream signaling are not yet completely understood. The monomeric form of IL-8 is known to be the high-affinity ligand for CXCR1, with N-loop residues interacting with the receptor N-terminal domain residues (site I).32 Site I binding results in conformational changes that are essential for the receptor exoloops and transmembrane residues (site II)33 to interact with the IL-8 N-terminal Glu-Leu-Arg (ELR) residues and trigger downstream signaling events for function.32 The p17 domain responsible for receptor interaction resides in a partially unfolded α-helix forming a loop within the N-terminus of viral protein34 (Figure 7). Moreover, an ELR motif is included between aa 74-76, whereas 2 other ELR-like motifs, namely ELD (aa 12-14) and RLR (aa 20-22) reside within the p17 N-loop (Figure 7). Because sequence analysis reveals that the CXCR1 N-domain is highly negatively charged and shows characteristics of an unstructured or minimally structured domain, it can bind the p17 N-loop by the “fly-casting” mechanism,35 making possible the interaction of the p17 ELR or ELR-like motifs with the CXCR1 extracellular domain.
Comparison of p17 and IL-8 amino acid sequences. Sequences are represented by the single-letter amino acid code. ELR-like sequences are indicated with a gray box and N-loops of the proteins are shown.
Comparison of p17 and IL-8 amino acid sequences. Sequences are represented by the single-letter amino acid code. ELR-like sequences are indicated with a gray box and N-loops of the proteins are shown.
The finding of a strong IL-8–like activity despite p17's low affinity to CXCR1 is not surprising, because Ahuja et al reported previously that chemokine potency and high-affinity binding can be separable functions, with low-affinity sites capable of mediating efficient receptor activation.36 It has also been shown that it is common for viral cytokine or chemokine homologs to act as agonists, even displaying a lower affinity for a receptor than their mammalian counterparts. For example, this has been shown to be the case for the EBV-encoded IL-10 homolog37 and for poxvirus homologs of the epidermal growth factor (EGF).38 Interestingly, the viral EGF homologs, which have 10- to 1000-fold lower affinity than endogenous EGF, are more potent mitogens than their mammalian counterparts because of attenuation of receptor degradation, which leads to sustained signal transduction. We recently showed the presence of naturally occurring p17 variants with mutations within the p17 primary sequence, which generate signaling pathways that lead to a more potent and prolonged activation of the transcriptional factor AP-1.10 Therefore, it is possible that variant p17 proteins may possess different affinities and potencies toward CXCR1.
To further increase the complexity of the system, p17, like IL-8, displays the ability to interact with HSPGs.29,39 Such an interaction may allow a better presentation of the viral protein to CXCR1, setting up synergistic and/or cooperative interactions leading to increased ligand-binding affinity, as demonstrated previously for many heparin-binding chemokines.40
Recent data have provided compelling evidence for p17 as a key modulator of viral fitness, because it played a key role in simian immunodeficiency virus adaptation to the new human host.41 The finding that p17 has the capability of binding CXCR1 is extremely interesting and leads to a hypothesis that it may represent a refined method of modulating viral fitness under host-specific selection pressure. A recent study highlighted the role of CXCR1 in HIV-1 replication and AIDS pathogenesis.42 That study showed that a haplotype of the human gene was protective against rapid disease progression in HIV-1 patients, probably acting through reduced CXCR1-mediated signals in HIV-1 replication. It will be interesting to examine the functional responses of monocytes expressing the variant CXCR1 molecule to p17 stimulation compared with cells expressing the wild-type receptor. This finding enforces the hypothesis that p17 plays an essential role in AIDS pathogenesis and highlights the need to block the biologic activity of the exogenous viral protein. We reported previously that p17 is capable of triggering monocytes to produce MCP-110 ; therefore, p17 may play both a direct and an indirect MCP-1–mediated role in monocyte extravasation and subsequent tissue damage in AIDS patients. This is particularly important in brain tissue, where p17 accumulation has been found to be predominantly associated with mature macrophages and microglia of fully developed neurodegenerative lesions.6 Conversely, the presence of p17 in tissue would allow the attraction of activated monocytes toward virus-producing cells, thus favoring a rapid spread of infection. This could have a particular relevance in lymphoid organs, a major site of HIV-1 replication, where p17 is known to accumulate and persist for years even in patients undergoing successful HAART.7 In addition, it has been shown that p17 enhances HIV-1 infection by promoting a pro-inflammatory microenvironment that is favorable to HIV-1 replication and spread.9,43 According to these data, it is possible to delineate a “vicious cycle” in which p17, inflammatory mediators, and eventually other viral products help each other to sustain an in situ inflammation and HIV-1 replication.
Several studies have shown the involvement of IL-8 in HIV-1 replication and AIDS pathogenesis, revealing that: (1) IL-8 secretion is enhanced in the serum, plasma, CNS, and lymphoid tissue of HIV-1 patients44-47 ; (2) IL-8 is induced by HIV-1 infection48 and by HIV-1 proteins49 ; (3) IL-8 stimulates HIV-1 replication and spread42,46 ; and (4) levels of IL-8 are increased in the brains of HIV-1 patients suffering with HIV-1–associated dementia, and this chemokine synergistically enhances MCP-1–mediated monocyte migration.49
At present, the p17 protein is the target of a therapeutic vaccine aimed at boosting and better directing the immune response against the functional AT20 epitope involved in the p17/receptor interaction.8 At the same time, inhibitors of IL-8 and CXCR1 are major targets for drug development.50 Our identification of p17, IL-8, and CXCR1 as important players in AIDS pathogenesis calls for an exploration of the therapeutic potential of blocking the p17/IL-8/CXCR1 axis in HIV-1 infection and AIDS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cristina Rossini for her skillful technical assistance.
This work was supported by the Italian Ministry of University and Scientific Research (Programmi di Ricerca di Rilevante Interesse Nazionale) and Istituto Superiore di Sanita (AIDS grants 40G.16 to A.C. and 40H.51 to M.R.) and in part by the Bonino-Pulejo Foundation (Messina, Italy). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: C.G. and A.C. conceived the study, designed the experiments, performed the research, and wrote the manuscript; C.G. and A.K.M. performed the adhesion and migration assays on monocytes and the migration and respiratory burst assays on neutrophils, analyzed the data, and performed the statistical analyses; F.C. and S.M. performed and analyzed the data on monocyte silencing for CXCR1 and Jurkat cell transfections; A.B. and M.R. characterized the p17/CXCR1 interaction by SPR and analyzed the data; W.V. performed the in vivo experiments; S.F. performed the flow cytometric analyses; and A.C. and M.R. contributed reagents, materials, and analytical tools.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arnaldo Caruso, Section of Microbiology, Department of Experimental and Applied Medicine, School of Medicine, University of Brescia, Piazzale Spedali Civili 1, 25123 Brescia, Italy; e-mail: caruso@med.unibs.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal