Abstract
CLEC9A is a recently discovered C-type lectin receptor involved in sensing necrotic cells. In humans, this receptor is selectively expressed by BDCA3+ myeloid dendritic cells (mDCs), which have been proposed to be the main human cross-presenting mDCs and may represent the human homologue of murine CD8+ DCs. In mice, it was demonstrated that antigens delivered with antibodies to CLEC9A are presented by CD8+ DCs to both CD4+ and CD8+ T cells and induce antitumor immunity in a melanoma model. Here we assessed the ability of CLEC9A to mediate antigen presentation by human BDCA3+ mDCs, which represent < 0.05% of peripheral blood leukocytes. We demonstrate that CLEC9A is only expressed on immature BDCA3+ mDCs and that cell surface expression is lost after TLR-mediated maturation. CLEC9A triggering via antibody binding rapidly induces receptor internalization but does not affect TLR-induced cytokine production or expression of costimulatory molecules. More importantly, antigens delivered via CLEC9A antibodies to BDCA3+ mDCs are presented by both MHC class I (cross-presentation) and MHC class II to antigen-specific T cells. We conclude that CLEC9A is a promising target for in vivo antigen delivery in humans to increase the efficiency of vaccines against infectious or malignant diseases.
Introduction
Dendritic cells (DCs) are central players in the induction of adaptive immune responses.1 They reside in peripheral tissues, where they are positioned to capture antigens. In the immature state, DCs continuously sample their environment by receptor-mediated endocytosis, pinocytosis, and phagocytosis. Once DCs also encounter danger signals, such as those present in pathogens, endogenous danger molecules, inflammatory cytokines, or immune complexes, they become activated and migrate to the lymph nodes and differentiate into mature DCs, which is accompanied by stabilization of peptide-MHC complexes on the cell surface, up-regulation of costimulatory molecules, and cytokine release. These alterations contribute to optimal antigen presentation to T lymphocytes. DCs have the unique capacity to process extracellular antigens for cross-presentation via MHC class I. This feature allows DCs to induce CD8 T-cell responses against dying cells, tumor cells, and viruses that do not replicate in DCs.
In human peripheral blood, 2 main populations of DCs can be distinguished: CD11c-positive myeloid DCs (mDCs) and CD11c-negative plasmacytoid DCs (pDCs).2,3 Human mDCs can be subdivided further on the basis of differential surface expression of BDCA1 (CD1c), CD16, and BDCA3 (CD141).4,5 Because the frequency of circulating mDCs in human blood is very low (< 2% of the peripheral blood leukocytes), many studies exploit in vitro–generated monocyte-derived DCs (moDCs) as “surrogate mDCs” because of the relative ease of obtaining large quantities of these cells. DC subsets are heterogeneous in the expression of cell surface markers and pathogen-recognition receptors, cytokine production after stimulation, as well as in their ability to stimulate T lymphocytes.4-7 The heterogeneity in human mDCs most likely reflects their functional specialization. Recently, it has been proposed that BDCA3+ mDCs may represent the main cross-presenting human myeloid DCs.8-10 Because of their cross-presenting abilities, as well as their expression profiles, it has been hypothesized that BDCA3 mDCs resemble mouse CD8+ DCs, which are the main murine cross-presenting DCs.8,11-13
To sense danger, DCs are equipped with a set of pattern recognition receptors (PRRs), which recognize molecular patterns that are conserved among microbial species. TLRs and C-type lectin receptors (CLRs) are 2 main PRR families expressed on DC membranes. CLRs recognize pathogens via unique branching and positioning of carbohydrate residues.14 In contrast to most PRRs, which are key receptors for the induction of intracellular signaling cascades, most CLRs function as antigen uptake receptors. Antigens internalized via CLRs are usually loaded onto MHC class II molecules; however, several CLRs are able to cross-present their captured antigens onto MHC class I molecules.15-17
CLEC9A (C-type lectin domain family 9A, DNGR-1) is a recently discovered CLR involved in the sensing and presentation of antigens derived from necrotic cells.11,18-20 Human CLEC9A is selectively expressed on BDCA3+ mDCs, although low-level staining was shown to be present on B cells and monocytes.11,18,19 CLEC9A-expressing BDCA3 mDCs reside in human blood, spleen, lymph nodes, tonsils, and BM.9,10 CLEC9A is an endocytic receptor.18-20 In murine CD8+ DCs, antigens delivered via anti-CLEC9A antibodies are presented on both MHC class II and class I.11,19,21-23 In vivo, targeted delivery of tumor antigens via anti-CLEC9A antibodies in combination with adjuvant induced antitumor immunity in a murine melanoma model.19
The ability of CLRs to internalize antigens and deliver them to endocytic compartments for processing and antigen presentation has been one of the main reasons that CLRs are targeted for the induction of tumor-specific immune responses.24 In particular, the role of CLEC9A in targeting tumor antigens to DCs has recently gained a lot of interest in the field of tumor immunology because CLEC9A is selectively expressed on BDCA3+ mDCs, the main cross-presenting human mDC subset. However, because of the low abundance of BDCA3+ mDCs in human blood, thus far, functional studies on CLEC9A have been limited to either mouse CD8+ DCs, cell lines transfected with human CLEC9A, or in vitro–expanded cord blood DCs.10,11,18-23 In this study, we show that CLEC9A expression on human mDCs is restricted to immature BDCA3+ mDCs and that antigens delivered via CLEC9A antibodies to freshly isolated blood BDCA3+ mDCs are (cross-) presented via both MHC class I and II to antigen specific CD4+ and CD8+ T cells. Our findings show that CLEC9A is a potent candidate receptor for targeting antigens to human BDCA3 myeloid DCs.
Methods
DC isolation and culturing
DCs were isolated or generated from buffy coats and leukapheresis products. Buffy coats were obtained from healthy volunteers according to institutional guidelines. Peripheral blood mononuclear cells (PBMCs) were purified from buffy coats via Ficoll density gradient centrifugation (Lucron Bioproducts). To obtain peripheral blood leukocytes, monocytes were depleted from PBMCs via their adhering to plastic culture flasks. Leukapheresis products were obtained from patients with melanoma who were participating in ongoing DC clinical trials. These trials were approved by the Dutch Medical Ethical Committee, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. moDCs were differentiated from monocytes that adhered to plastic culture flasks with IL-4 (300 U/mL) and GM-CSF (450 U/mL; both from Cellgenix) in X-VIVO 15 culture medium (Lonza Walkersville) supplemented with 2% human serum (Sanquin). Immature moDCs were harvested at day 6.
CD1c+ mDCs and CD16+ mDCs were isolated from PBMCs with a CD1c+ DC isolation kit and CD16+ monocyte isolation kit, respectively. BDCA3 myeloid DCs were isolated from peripheral blood leukocytes by selection for BDCA3+ cells with a CD141 (BDCA3) isolation kit (all Miltenyi Biotec). mDC purity was routinely up to 95%, as assessed by double staining CD11c+/CD1c+ for CD1c-mDCs, CD11c+/CD16+ for CD16-mDCs, and CD11c+/BDCA3+ for BDCA3+ mDCs (all Miltenyi Biotec). The yield from 1 buffy coat (∼ 500 × 106 PBMCs) was approximately 0.15 × 106 for BDCA3+ mDCs, 5 × 106 for CD1c+ mDCs, and 10 × 106 for CD16+ mDCs. Freshly isolated immature myeloid DCs were activated overnight at 37°C with 4 μg/mL imidazoquinoline resiquimod (R848; Axxora) and 2 μg/mL polyinosinic:polycytidylic acid (poly I:C; Sigma-Aldrich) in X-VIVO 15 culture medium supplemented with 2% HS.
Phenotype
The phenotype of mDC subsets was analyzed by flow cytometry. The following primary monoclonal antibodies and appropriate isotype controls were used: anti–human CLEC9A18 ; anti–human DEC205 (eBioscience); anti–human DC-SIGN17 ; anti–human DCIR (R&D Systems); anti–human mannose receptor (BD pharmingen); mIgG1-PE, mIgG1-APC, anti–HLA-ABC-PE (W6/32), anti–HLA-DR/DP-FITC (Q5/13), anti–CD80-PE, and anti–CD86-APC (all BD Bioscience Pharmingen); and anti–CD40-PE (Beckman Coulter). Alexa 488–conjugated goat anti–mouse IgG was used as a secondary labeled antibody (Invitrogen). Flow cytometric analysis was performed with a FACSCalibur (BD Biosciences). FACS data were analyzed with CellQuest Pro (BD Biosciences).
Cytokine and chemokine detection
Specific combinations of the following cytokines and chemokines in DC and DC/T-cell supernatants were measured with Flowcytomix kits; IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, TNF-α, CXCL10, MIP-1α, and RANTES (Bender MedSystems) according to the manufacturer's instructions. IL-12p70 and IFN-γ production were measured with standard sandwich ELISA (both Pierce Biotechnology).
Internalization assays
Freshly isolated or overnight-activated BDCA3 mDCs were incubated with mouse monoclonal anti–human CLEC9A (10 μg/mL)18 or mouse monoclonal anti–human DEC205 (10 μg/mL; eBioscience) in medium on ice for 30 minutes. After we washed away the unbound antibodies, receptors were allowed to internalize at 37°C for various time points or kept on ice as a control. The amount of antibody complexes remaining on the cell surface was then determined by FACS analysis after staining cells with Alexa 488–conjugated goat anti–mouse IgG (Invitrogen). The percentage of CLEC9A and DEC205 surface expression was determined over time ([mean fluorescent intensity at 37°C/mean fluorescent intensity at 4°C] × 100).
To detect internalized CLEC9A by confocal microscopy, freshly isolated BDCA3 mDCs were allowed to adhere to fibronectin-coated coverslips (Roche Diagnostics). After blocking the cells with human IgG (Jackson ImmunoResearch Laboratories), cells were incubated with polyclonal rabbit anti–human CLEC9A (10 μg/mL; Abcam) at 4°C. After being washed, CLEC9A was allowed to internalize at 37°C in X-VIVO 15 + 2% HS for 45 minutes. BDCA3 mDCs were fixed with 2% paraformaldehyde and incubated with mouse anti–human HLA-DR/DP (Q5/13). Cells were permeabilized with 0.1% Triton X-100, fixed, and blocked with human IgG. Staining of internalized anti-CLEC9A antibodies was reinforced with biotinylated goat anti–rabbit (Vector Laboratories). CLEC9A and HLA-DR/DP were visualized with Alexa 488–conjugated streptavidin (Molecular Probes) and Alexa 647–conjugated goat anti–mouse IgG (Invitrogen), respectively. Coverslips were mounted on Mowiol. Images were acquired with an Olympus FV1000 Confocal laser scanning microscope. Isotype controls rabbit IgG (Jackson ImmunoResearch Laboratories) and mouse IgG2a (Miltenyi Biotec) did not show any staining. Pictures were analyzed with ImageJ Version 1.45a software.
CLEC9A triggering
Purified immature BDCA3 mDCs were incubated overnight at 37°C with purified mouse IgG (5 μg/mL; Jackson ImmunoResearch Laboratories) or mouse anti–human polyclonal CLEC9A IgG (5 μg/mL; Abcam) either in the presence or absence of 2 μg/mL poly I:C (Sigma-Aldrich) and 4 μg/mL R848 (Axxora). After 16 hours, cells were harvested, DC phenotype was analyzed by flow cytometry, and cytokine and chemokine production was measured with Flowcytomix kits (Bender MedSystems).
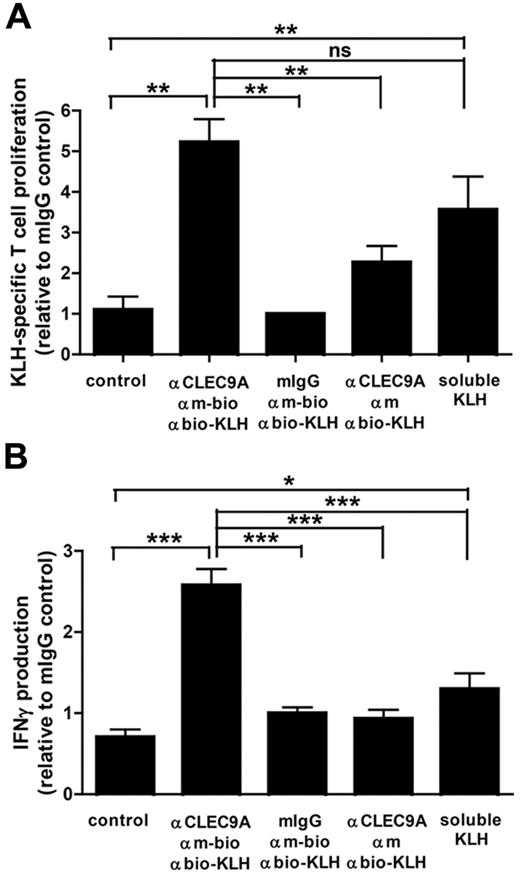
KLH-specific CD4+ T-cell responses
In our ongoing DC vaccination trials in melanoma patients, DCs are pulsed with the protein keyhole limpet hemocyanin (KLH) to provide T-cell help, as well as to monitor the immune response.25 To study KLH-specific T-cell activation, BDCA3+ mDCs and CD4+ T lymphocytes were isolated from leukapheresis products of patients with melanoma participating in one of these ongoing DC vaccination trials. CD4+ T lymphocytes were isolated from the BDCA3-negative fraction by the use of anti-CD4–conjugated magnetic microbeads (Miltenyi Biotec). KLH was delivered to freshly isolated immature BDCA3+ mDCs via CLEC9A in a 3-step procedure on ice. First, BDCA3 mDCs were incubated with mouse polyclonal anti-CLEC9A on ice (Abcam). After washing away unbound antibodies, KLH was delivered to CLEC9A via subsequent incubation on ice with biotinylated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) and KLH coupled to antibiotin molecules (10 μg/mL). As control, mouse-purified IgG (Jackson ImmunoResearch Laboratories) was used as first labeling step.
To test the specific binding of antibiotin molecules, unlabeled goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) was used as second labeling step. After washing away unbound antibodies and KLH, KLH delivered via CLEC9A was allowed to internalize at 37°C for 45 minutes before the addition of CD4+ T cells. BDCA3+ mDCs and autologous CD4+ T lymphocytes were plated in a 96-well tissue culture microplate in a ratio 1:10 in the presence of 2 μg/mL poly I:C (Sigma-Aldrich) and 4 μg/mL R848 (Axxora). As positive control, DCs and T cells were cultured in the continuous presence of soluble KLH (10 μg/mL; Immucothel, Biosyn Arzneimittel GmbH). IFN-γ production was measured in the supernatants 24 to 66 hours after the addition of T cells. After 4 days of culture, 1 μCi/well of tritiated thymidine was added for 16 hours, and incorporated thymidine was measured in a β-counter. T-cell proliferation data were normalized to account for intraexperimental differences.
Gp100-specific CD8+ T-cell responses (cross-presentation)
Polyethylene glycol–coated poly(lactic-co-glycolic acid) [PLGA] nanoparticles encapsulating Atto-488 and gp100:272-300 long peptide (30 μg peptide/mg PLGA nanoparticle) coated with protein A and rabbit polyclonal anti-CLEC9A (Abcam) or rabbit IgG isotype control were produced as described previously.26 The physicochemical properties of anti-CELC9A– and RbIgG-coated nanoparticles are summarized in Table 1. BDCA3+ mDCs were incubated for 30 to 60 minutes at 37°C with rabbit anti-CLEC9A– or rabbit IgG-coated nanoparticles containing gp100:272-300 (50 μg/mL PLGA corresponds to 0.5μM gp100:272-300).
Physicochemical properties of anti-CLEC9A– and RbIgG-coated PLGA nanoparticles
| Samples . | Nanoparticles diameter, mn, ± SD . | Polydispersity index, ± SD . | Zeta potential, mV, ± SD . | gp100, μg/mg PLGA . | Antibodies, μg/mg PLGA . |
|---|---|---|---|---|---|
| αCLEC9A-NP | 243.4 ± 7.2 | 0.210 ± 0.048 | −16.4 ± 1.7 | 30 | 20 |
| RbIgG-NP | 247.5 ± 9.8 | 0.259 ± 0.029 | −17.2 ± 1.6 | 30 | 22 |
| Samples . | Nanoparticles diameter, mn, ± SD . | Polydispersity index, ± SD . | Zeta potential, mV, ± SD . | gp100, μg/mg PLGA . | Antibodies, μg/mg PLGA . |
|---|---|---|---|---|---|
| αCLEC9A-NP | 243.4 ± 7.2 | 0.210 ± 0.048 | −16.4 ± 1.7 | 30 | 20 |
| RbIgG-NP | 247.5 ± 9.8 | 0.259 ± 0.029 | −17.2 ± 1.6 | 30 | 22 |
PLGA nanoparticles with gp100:272-300 long peptide and Atto488 coated with αCLEC9A or RbIgG via protein A were characterized by DLS and ζ potential measurements. Nanoparticles diameter data represent the mean value ± SD and the polydispersity index as measured by DLS. Zeta potential data represent the mean value ± SD of five readings. Loading efficiency of gp100:272-300 long peptide was measured by Coomassie dye protein assay. The conjugation efficiency of αCLEC9A and RbIgG was determined by Coomassie dye protein assay.
PLGA indicates poly(lactic-co-glycolic acid).
As controls, BDCA3 mDCs were incubated either with irrelevant peptide (tyrosinase:369-376; 10μM), gp100 short peptide (gp100:280-288; 10μM), or gp100 long peptide (gp100:272-300; 10μM). Gp100 cross-presentation was assessed by coculturing 7 × 103 nanoparticles or peptide-loaded BDCA3+ mDCs with 5 × 104 allogeneic CD8+ T cells expressing the gp100:280-288 TCR27,28 in the presence of 2 μg/mL poly I:C (Sigma-Aldrich) and 4 μg/mL R848 (Axxora). After overnight incubation, nanoparticle uptake by BDCA3+ mDCs was analyzed by flow cytometry. CD69 expression on the T cells was measured by flow cytometry by the use of PerCp-conjugated anti–human CD69 and allophycocyanin-conjugated anti–human CD4 and CD8 (all BD Biosciences). IFN-γ production after overnight incubation was measured with standard sandwich ELISA (Pierce Biotechnology).
Statistical analysis
Data were analyzed with 1-way ANOVA followed by Student-Newman-Keuls test. Statistical significance was defined as P < .05.
Results
CLEC9A is selectively expressed by human BDCA3+ mDCs
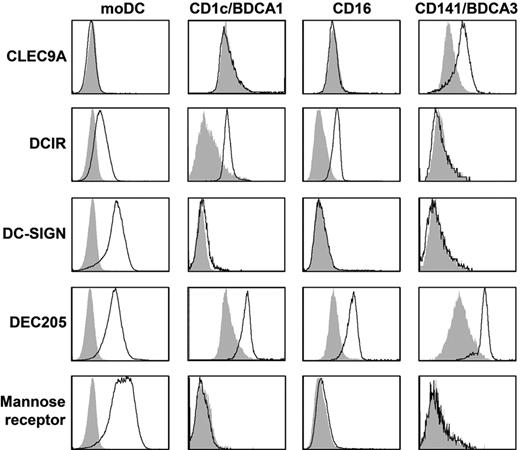
To study C-type lectin expression on human myeloid DC subsets, day 6 immature moDCs and freshly isolated CD1c-mDC, CD16-mDC, and BDCA3-mDC were stained with anti-CLEC9A, anti-DCIR, anti–DC-SIGN, anti-DEC205, and anti–mannose receptor antibodies and analyzed by flow cytometry (Figure 1, Table 2, and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All mDC subsets expressed DEC205. In line with previous reports,4,29,30 expression of DC-SIGN and mannose receptor was detected only on moDCs. DCIR and CLEC9A demonstrated complementary expression as DCIR was expressed by all mDC subsets except BDCA3+ mDCs, whereas CLEC9A expression was restricted to BDCA3+ mDCs. Because murine studies suggested that CLEC9A is an attractive target for antigen delivery to DCs in vivo,11,19,22 we studied CLEC9A expression and function on freshly isolated human BDCA3+ mDCs in more detail.
Expression of CLRs on mDC subsets. Expression of CLEC9A, DCIR, DC-SIGN, DEC205, and mannose receptor was analyzed by flow cytometry on day 6 immature monocyte-derived DCs or freshly isolated BDCA1/CD1c+ mDCs, CD16+ mDCs, or BDCA3/CD141+ mDCs. Representative examples of 3 independent experiments are shown. Filled gray histograms represent isotype controls, open black histograms represent cells stained with specific antibodies. See supplemental Figure 1 for dot plots and gates.
Expression of CLRs on mDC subsets. Expression of CLEC9A, DCIR, DC-SIGN, DEC205, and mannose receptor was analyzed by flow cytometry on day 6 immature monocyte-derived DCs or freshly isolated BDCA1/CD1c+ mDCs, CD16+ mDCs, or BDCA3/CD141+ mDCs. Representative examples of 3 independent experiments are shown. Filled gray histograms represent isotype controls, open black histograms represent cells stained with specific antibodies. See supplemental Figure 1 for dot plots and gates.
Expression of C-type lectin receptors on monocyte-derived DCs and myeloid DC subsets
| . | moDCs . | CD1c + mDCs . | CD16 + mDCs . | BDCA3 + mDCs . |
|---|---|---|---|---|
| CLEC9A | −* | − | − | + |
| DCIR | + | + | + | − |
| DC-SIGN | ++ | − | − | − |
| DEC205 | ++ | + | + | ++ |
| MR | ++ | − | − | − |
| . | moDCs . | CD1c + mDCs . | CD16 + mDCs . | BDCA3 + mDCs . |
|---|---|---|---|---|
| CLEC9A | −* | − | − | + |
| DCIR | + | + | + | − |
| DC-SIGN | ++ | − | − | − |
| DEC205 | ++ | + | + | ++ |
| MR | ++ | − | − | − |
Data represent 3 independent experiments performed with cells from different donors.
CLR indicates C-type lectin receptors; mDCs, myeloid dendritic cells; and moDCs, monocyte-derived dendritic cells.
CLR expression analyzed by flow cytometry; −, not expressed; +, < 1 log difference between staining and isotype control; and ++, > 1 log difference between staining and isotype control.
TLR-induced maturation reduces CLEC9A expression but not DEC205 expression on BDCA3+ mDCs
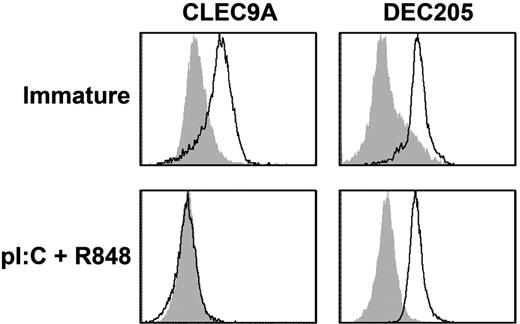
We previously showed that DEC205, an endocytic receptor that recognizes necrotic and apoptotic ligands,31 is expressed and functional on mature pDCs.32 Because CLEC9A is also a necrotic recognition receptor,20 we investigated whether CLEC9A expression is modulated on mDC maturation. BDCA3+ mDCs express both TLR3 and TLR8,5 which act synergistically to drive DC maturation.33 To induce full DC maturation, freshly isolated BDCA3+ mDCs were cultured overnight in the presence of the TLR3 ligand poly I:C and the TLR7/8 ligand R848, which induced high expression of the costimulatory molecules CD80 and CD86 and induced the production of proinflammatory cytokines (supplemental Figure 2). After overnight incubation with TLR ligands, CLEC9A completely disappeared from the cell surface of BDCA3+ mDCs, whereas DEC205 expression on mature BDCA3+ mDCs was comparable with that on freshly isolated BDCA3+ mDCs (Figure 2).
BDCA3+ mDCs lose CLEC9A expression on maturation, whereas DEC205 is still expressed. Expression of CLEC9A (left) and DEC205 (right) on freshly isolated BDCA3+ mDCs (top; immature) and BDCA3+ mDCs stimulated overnight with poly I:C and R848 (bottom; pl:C + R848). Flow cytometry plots are representative examples of 4 independent experiments performed with DCs from different donors. Filled gray histograms represent isotype controls, and open histograms represent cells stained with specific antibodies.
BDCA3+ mDCs lose CLEC9A expression on maturation, whereas DEC205 is still expressed. Expression of CLEC9A (left) and DEC205 (right) on freshly isolated BDCA3+ mDCs (top; immature) and BDCA3+ mDCs stimulated overnight with poly I:C and R848 (bottom; pl:C + R848). Flow cytometry plots are representative examples of 4 independent experiments performed with DCs from different donors. Filled gray histograms represent isotype controls, and open histograms represent cells stained with specific antibodies.
Cell-surface CLEC9A internalizes on receptor triggering
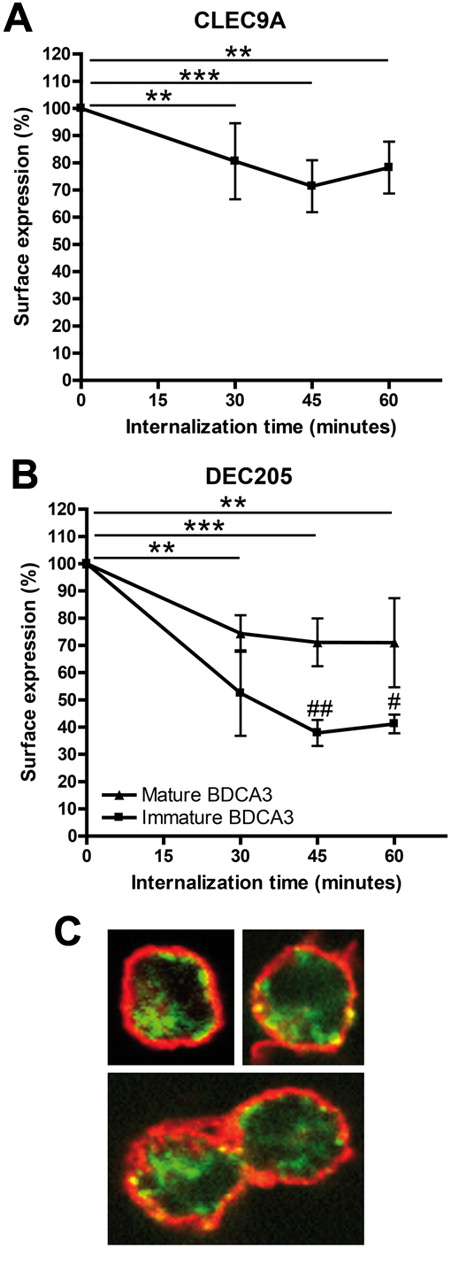
Many CLRs act as endocytic receptors on DCs and are therefore good targets for in vivo targeting in cancer immunotherapy. To study the potency of human CLEC9A as a targeting receptor, we investigated whether human CLEC9A is also internalized after receptor triggering in human immature BDCA3+ mDCs. Because natural ligands for CLEC9A have not yet been identified, we mimicked ligand binding by antibody ligation. CLEC9A internalization was already detectable 30 minutes after incubation at 37°C and surface levels of antibody-labeled CLEC9A reduced further after 45 minutes (Figure 3A). To exclude that reduced CLEC9A cell-surface expression was because of shedding after antibody binding, we investigated CLEC9A endocytosis by confocal microscopy. Although intracellular fluorescence after 45 minutes of internalization was very low, the presence of internalized CLEC9A antibodies in intracellular compartments could be confirmed (Figure 3C). Partial colocalization of CLEC9A with MHC class II on the plasma membrane indicates that not all CLEC9A has internalized, which supports the results depicted in Figure 3A. Together, our data clearly show that CLEC9A is expressed at the plasma membrane of immature human BDCA3+ mDCs and becomes rapidly internalized on receptor triggering.
Cell surface CLEC9A and DEC205 are internalized into BDCA3+ mDCs after receptor triggering. (A) CLEC9A internalization is induced in immature freshly isolated BDCA3+ mDCs by receptor triggering with αCLEC9A antibodies. (B) DEC205 internalization is induced by receptor triggering with αDEC205 antibodies on immature freshly isolated BDCA3+ mDCs (squares) and BDCA3+ mDCs matured overnight with 2 μg/mL poly I:C and 4 μg/mL R848 (triangles). BDCA3+ mDCs were labeled with αCLEC9A or αDEC205 antibodies on ice. Surface expression of antigen and antibody after incubation at 37°C was analyzed by labeling with Alexa 488–conjugated secondary antibodies. Data are expressed as percentage of surface expression at 4°C. Data shown are mean ± SD of 3 independent experiments. **P < .01; ***P < .001 compared with incubation at 4°C (t = 0). #P < .05; ##P < .01 immature vs mature BDCA3+ mDCs. (C) Confocal analysis of CLEC9A internalization in freshly isolated BDCA3+ mDCs. BDCA3+ mDCs were stained on ice with rabbit polyclonal αCLEC9A antibodies. Internalization at 37°C was allowed for 45 minutes, followed by staining with biotinylated goat anti–rabbit and Alexa 488–conjugated streptavidin (red). Extracellular MHC class II was stained with mouse anti–human HLA-DR/DP, followed by Alexa647-conjaged goat anti–mouse (green).
Cell surface CLEC9A and DEC205 are internalized into BDCA3+ mDCs after receptor triggering. (A) CLEC9A internalization is induced in immature freshly isolated BDCA3+ mDCs by receptor triggering with αCLEC9A antibodies. (B) DEC205 internalization is induced by receptor triggering with αDEC205 antibodies on immature freshly isolated BDCA3+ mDCs (squares) and BDCA3+ mDCs matured overnight with 2 μg/mL poly I:C and 4 μg/mL R848 (triangles). BDCA3+ mDCs were labeled with αCLEC9A or αDEC205 antibodies on ice. Surface expression of antigen and antibody after incubation at 37°C was analyzed by labeling with Alexa 488–conjugated secondary antibodies. Data are expressed as percentage of surface expression at 4°C. Data shown are mean ± SD of 3 independent experiments. **P < .01; ***P < .001 compared with incubation at 4°C (t = 0). #P < .05; ##P < .01 immature vs mature BDCA3+ mDCs. (C) Confocal analysis of CLEC9A internalization in freshly isolated BDCA3+ mDCs. BDCA3+ mDCs were stained on ice with rabbit polyclonal αCLEC9A antibodies. Internalization at 37°C was allowed for 45 minutes, followed by staining with biotinylated goat anti–rabbit and Alexa 488–conjugated streptavidin (red). Extracellular MHC class II was stained with mouse anti–human HLA-DR/DP, followed by Alexa647-conjaged goat anti–mouse (green).
DEC205, which is known to internalize on antibody binding,32,34 was included as a positive control. We previously showed that DEC205 functions as endocytic receptor on both resting and activated human pDCs.32 Because DEC205 is expressed on both immature and TLR-stimulated BDCA3+ mDCs, we studied DEC205 internalization after antibody binding on immature and mature BDCA3+ mDCs. Similar to CLEC9A, DEC205 surface expression of antibody-labeled DEC205 on immature BDCA3-DCs was reduced after 30 minutes and further reduced after 45 minutes at 37°C (Figure 3B). In addition, TLR-stimulated BDCA3+ mDCs internalized DEC205, although to a lesser extent than immature BDCA3+ mDCs (Figure 3B).
CLEC9A triggering does not induce DC maturation nor affect TLR-induced maturation
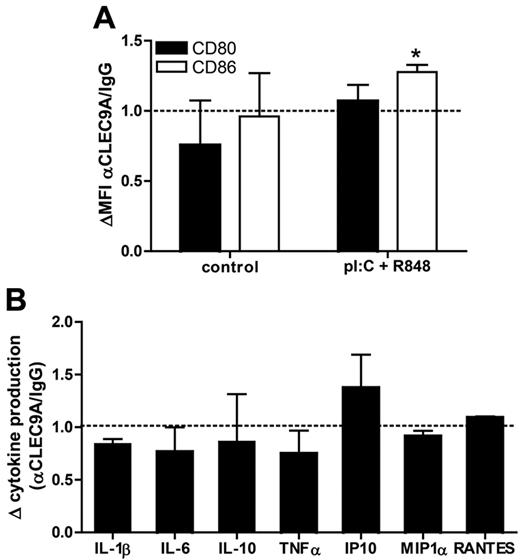
Because CLEC9A contains an ITAM-like motif in its cytoplasmic tail, it might function as an immune activating receptor. Using receptor chimeras in transduced macrophage cell lines, other investigators have demonstrated that activation of CLEC9A induces proinflammatory cytokine production.18 To determine whether CLEC9A triggering alone or in combination with TLR stimulation modulates human BDCA3-mDC activation, we analyzed both their phenotype and their cytokine production after overnight culture with anti-CLEC9A antibodies in the presence or absence of TLR ligands. In the absence of TLR ligands, CLEC9A triggering had no effect on expression of the costimulatory molecules CD80 and CD86 (Figure 4A), nor did it induce the secretion of the proinflammatory cytokines IL-1β, IL-6, IL-10, IL12p70, or TNFα and the chemokines IP-10, MIP1α, or RANTES by BDCA3+ mDCs (data not shown). In the presence of the TLR ligands poly I:C and R848 CLEC9A triggering slightly, but significantly, increased CD86 expression, whereas CD80 expression was not affected (Figure 4A). In addition, CLEC9A triggering did not enhance or reduce cytokine and chemokine secretion in activated DCs (Figure 4B). Overall, these results reveal that CLEC9A triggering with antibodies does not induce DC maturation, nor does it affect TLR-mediated DC maturation.
CLEC9A triggering does not affect DC phenotype or cytokine profile. Freshly isolated BDCA3+ mDCs were incubated overnight with mouse polyclonal anti–human CLEC9A or mouse IgG either in the presence or absence (control) of 2 μg/mL poly I:C and 4 μg/mL R848. (A) Expression of CD80 (black bars) and CD86 (white bars) was analyzed by flow cytometry after overnight incubation. Data are shown as mean fluorescence intensity of cells incubated with anti-CLEC9A relative to that of cells incubated with mouse IgG (MFI αCLEC9A/MFI IgG). (B) Cytokine and chemokine production after overnight incubation of BDCA3+ mDCs with anti–human CLEC9A or mouse IgG in the presence of 2 μg/mL poly I:C and 4 μg/mL R848. Data are shown as relative cytokine concentration (αCLEC9A/IgG). Graphs show the mean ± SEM of 3 independent experiments performed with BDCA3+ mDCs isolated from different donors. *P < .05.
CLEC9A triggering does not affect DC phenotype or cytokine profile. Freshly isolated BDCA3+ mDCs were incubated overnight with mouse polyclonal anti–human CLEC9A or mouse IgG either in the presence or absence (control) of 2 μg/mL poly I:C and 4 μg/mL R848. (A) Expression of CD80 (black bars) and CD86 (white bars) was analyzed by flow cytometry after overnight incubation. Data are shown as mean fluorescence intensity of cells incubated with anti-CLEC9A relative to that of cells incubated with mouse IgG (MFI αCLEC9A/MFI IgG). (B) Cytokine and chemokine production after overnight incubation of BDCA3+ mDCs with anti–human CLEC9A or mouse IgG in the presence of 2 μg/mL poly I:C and 4 μg/mL R848. Data are shown as relative cytokine concentration (αCLEC9A/IgG). Graphs show the mean ± SEM of 3 independent experiments performed with BDCA3+ mDCs isolated from different donors. *P < .05.
Antigens delivered to BDCA3+ DCs via CLEC9A are presented on MHC class II molecules and induce recall CD4 T-cell responses
Because CLEC9A is internalized on antibody binding, we investigated whether targeting to CLEC9A can induce antigen uptake and presentation to T lymphocytes. To study MHC class II–mediated antigen presentation, we used KLH as antigen. BDCA3+ mDCs and CD4+ T lymphocytes were isolated from the peripheral blood of patients with melanoma who were participating in ongoing vaccination trials with KLH-pulsed mature monocyte-derived DCs25 and who have KLH-responsive T lymphocytes. DCs cocultured with autologous CD4+ T cells in the continuous presence of soluble KLH induced strong T-cell proliferation, indicating that the isolated CD4+ T cells were responsive to KLH (Figure 5A). Similarly, KLH delivered via CLEC9A induced strong proliferative responses. Moreover, KLH-specific CD4+ T cells produced large amounts of IFN-γ on coculture with BDCA3+ mDCs loaded with KLH via CLEC9A in the presence of TLR ligands (Figure 5B). Th2 cytokines IL-4, IL-5, and IL-10 could not be detected (data not shown). Our data suggest that antibody-mediated delivery of antigens to CLEC9A results in efficient antigen uptake and subsequent antigen presentation to IFN-γ–producing CD4+ T cells.
Antigens delivered to BDCA3+ DCs via CLEC9A are presented to CD4+ T cells. Freshly isolated BDCA3+ mDCs were loaded with KLH via a 3-step labeling procedure, consisting of mouse polyclonal anti–human CLEC9A antibodies, followed by biotinylated goat anti–mouse IgG and subsequently KLH coupled to antibiotin molecules. As negative controls, mouse IgG or nonbiotinylated goat anti–mouse IgG were used. BDCA3+ mDCs cultured in the continuous presence of 10 μg/mL KLH served as a positive control. A total of 1 × 104 KLH-loaded BDCA3+ mDCs were cocultured with 1 × 105 autologous purified CD4+ T lymphocytes in the presence of 2 μg/mL poly I:C and 4 μg/mL R848. (A) After 4 days, proliferation of CD4 T lymphocytes was analyzed by 3H thymidine incorporation. (B) IFN-γ production in the supernatant was measured by ELISA. The graphs show mean ± SEM T-cell proliferation or IFN-γ production relative to the isotype control (mouse IgG) of 3 experiments with different donors performed in duplicate. *P < .05; **P < .01; ***P < .001.
Antigens delivered to BDCA3+ DCs via CLEC9A are presented to CD4+ T cells. Freshly isolated BDCA3+ mDCs were loaded with KLH via a 3-step labeling procedure, consisting of mouse polyclonal anti–human CLEC9A antibodies, followed by biotinylated goat anti–mouse IgG and subsequently KLH coupled to antibiotin molecules. As negative controls, mouse IgG or nonbiotinylated goat anti–mouse IgG were used. BDCA3+ mDCs cultured in the continuous presence of 10 μg/mL KLH served as a positive control. A total of 1 × 104 KLH-loaded BDCA3+ mDCs were cocultured with 1 × 105 autologous purified CD4+ T lymphocytes in the presence of 2 μg/mL poly I:C and 4 μg/mL R848. (A) After 4 days, proliferation of CD4 T lymphocytes was analyzed by 3H thymidine incorporation. (B) IFN-γ production in the supernatant was measured by ELISA. The graphs show mean ± SEM T-cell proliferation or IFN-γ production relative to the isotype control (mouse IgG) of 3 experiments with different donors performed in duplicate. *P < .05; **P < .01; ***P < .001.
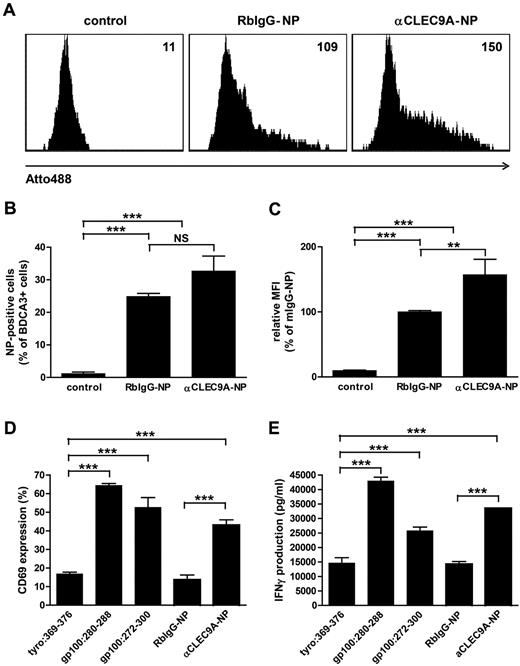
Tumor antigens delivered via CLEC9A to BDCA3+ mDCs are cross-presented to CD8+ T cells.
Cross-presentation of endocytosed antigens to cytotoxic CD8+ T cells is essential for the induction of antitumor immunity.35 To investigate whether antigens delivered to BDCA3+ mDCs via CLEC9A are cross-presented to CD8+ T cells, we used anti-CLEC9–coated PLGA nanoparticles with encapsulated gp100:272-300 peptide. PLGA is a biocompatible polymer that is widely used for (targeted) vaccine delivery,36,37 and gp100 is a melanoma-associated tumor antigen that is commonly used as target antigen immunotherapy of melanoma.38 The fluorescent dye Atto488 was incorporated in the PLGA nanoparticles to follow particle uptake. The uptake of anti-CLEC9A–coated nanoparticles by BDCA3+ mDCs was confirmed by flow cytometry after overnight incubation (Figure 6A-C). Although also isotype-coated nanoparticles were significantly taken up by BDCA3+ mDCs, increased fluorescence intensity of BDCA3+ mDCs after incubation with anti-CLEC9A–coated nanoparticles compared with isotype-coated nanoparticles suggests that uptake of anti-CLEC9A–coated nanoparticles was more efficient.
Tumor antigens delivered via CLEC9A to BDCA3+ mDCs are cross-presented to CD8+ T cells. Gp100 long peptide was targeted to CLEC9A via PLGA nanoparticles encapsulating gp100:272-300 and Atto488, coated with rabbit polyclonal αCLEC9A antibodies or rabbit IgG as control. (A-C) Uptake of nanoparticles by BDCA3+ mDCs after overnight incubation analyzed by flow cytometry. (A) Example of nanoparticle uptake by control BDCA3+ mDCs (no particles added; left), mDCs incubated with isotype antibody-coated particles (middle), or mDCs incubated with αCLEC9A-coated nanoparticles (right). Numbers indicate mean fluorescence intensity. (B) Percentage of Atto488-positive BDCA3+ mDCs. The graph shows mean ± SEM of 4 experiments with different donors performed in duplicate. (C) Relative fluorescence intensity of BDCA3+ mDCs. Data are presented as percentage of BDCA3+ mDCs incubated with RbIgG-coated nanoparticles. The graph shows mean ± SEM of 4 experiments performed in duplicate. (D-E) Freshly isolated BDCA3+ mDCs were incubated for 1-2 hours with 10μM irrelevant peptide (tyrosinase:369-376), 10μM gp100 short peptide (gp100:280-288), 10μM gp100 long peptide (gp100:272-300), or 50 μg/mL αCLEC9A- or RbIgG-coated PLGA nanoparticles encapsulating gp100:272-300 long peptide (corresponds to 0.5μM gp100:272-300) and Atto488. Next, DCs were cocultured overnight with allogeneic CD8+ T cells expressing gp100:280-288-specific TCR in the presence of 4 μg/mL R848 and 2 μg/mL poly I:C. T-cell activation was assessed by analysis of CD69 expression (D) and IFN-γ production (D). (D) Mean ± SD of percentage of CD8+ T cells expressing CD69 and is a representative example of 3 experiments with different donors performed in duplicate. (E) Mean ± SD IFN-γ production and is a representative example of 3 experiments with different donors performed in duplicate. **P < .01; ***P < .001; NS, not significant.
Tumor antigens delivered via CLEC9A to BDCA3+ mDCs are cross-presented to CD8+ T cells. Gp100 long peptide was targeted to CLEC9A via PLGA nanoparticles encapsulating gp100:272-300 and Atto488, coated with rabbit polyclonal αCLEC9A antibodies or rabbit IgG as control. (A-C) Uptake of nanoparticles by BDCA3+ mDCs after overnight incubation analyzed by flow cytometry. (A) Example of nanoparticle uptake by control BDCA3+ mDCs (no particles added; left), mDCs incubated with isotype antibody-coated particles (middle), or mDCs incubated with αCLEC9A-coated nanoparticles (right). Numbers indicate mean fluorescence intensity. (B) Percentage of Atto488-positive BDCA3+ mDCs. The graph shows mean ± SEM of 4 experiments with different donors performed in duplicate. (C) Relative fluorescence intensity of BDCA3+ mDCs. Data are presented as percentage of BDCA3+ mDCs incubated with RbIgG-coated nanoparticles. The graph shows mean ± SEM of 4 experiments performed in duplicate. (D-E) Freshly isolated BDCA3+ mDCs were incubated for 1-2 hours with 10μM irrelevant peptide (tyrosinase:369-376), 10μM gp100 short peptide (gp100:280-288), 10μM gp100 long peptide (gp100:272-300), or 50 μg/mL αCLEC9A- or RbIgG-coated PLGA nanoparticles encapsulating gp100:272-300 long peptide (corresponds to 0.5μM gp100:272-300) and Atto488. Next, DCs were cocultured overnight with allogeneic CD8+ T cells expressing gp100:280-288-specific TCR in the presence of 4 μg/mL R848 and 2 μg/mL poly I:C. T-cell activation was assessed by analysis of CD69 expression (D) and IFN-γ production (D). (D) Mean ± SD of percentage of CD8+ T cells expressing CD69 and is a representative example of 3 experiments with different donors performed in duplicate. (E) Mean ± SD IFN-γ production and is a representative example of 3 experiments with different donors performed in duplicate. **P < .01; ***P < .001; NS, not significant.
To study whether antigens delivered to BDCA3+ mDCs via CLEC9A are cross-presented to CD8+ T cells, BDCA3+ mDCs from an HLA-A2–positive donor were incubated with anti-CLEC9A–coated PLGA nanoparticles with encapsulated gp100:272-300 and cocultured with gp100:280-288–specific CD8+ T cells27 in the presence of the TLR ligands poly I:C and R848. After uptake, gp100:272-300 long peptide needs to be processed by DCs before it can be loaded onto MHC class I and presented to CD8+ T cells. DCs loaded with irrelevant peptide (tyrosinase:369-376) served as a negative control, whereas DCs loaded with gp100:280-288 short peptides, which directly bind extracellular HLA-A2 molecules, served as a positive control to ensure that the T cells were functional and specific. BDCA3-DCs loaded with soluble gp100 long peptide induced significant T-cell activation in terms of expression of the early activation marker CD69 and production of IFN-γ, indicating that BDCA3+ mDCs are able to cross-present gp100 peptides (Figure 6D-E). Interestingly, although isotype-coated nanoparticles were also taken up by BDCA3+ mDCs (Figure 6A-C), only anti-CLEC9A–coated nanoparticles induced CD69 expression and IFN-γ by gp100:280-288–specific CD8+ T cells (Figure 6D-E). Our results reveal that tumor antigens delivered via CLEC9A to BDCA3+ mDCs are cross-presented to CD8+ T cells.
Discussion
When considering in vivo targeting of DCs for immunotherapy, 2 key questions need to be addressed: (1) which DC subset, and (2) which surface receptor on that particular subset should be targeted? To the best of our knowledge, we here show for the first time that human BDCA3+ mDCs can (cross-)present antigens that are delivered via the endocytic CLR CLEC9A to CD4+ and CD8+ T cells.
Our data confirm and extend previous studies in which researchers demonstrated that CLR expression on mDC subsets is extremely heterogeneous.4,30 The selective expression of CLEC9A on BDCA3+ mDCs prompted us to study CLEC9A function on human BDCA3+ mDCs in more detail. CLRs are generally down-regulated after DC maturation. Accordingly, we found that CLEC9A is only expressed on immature BDCA3+ mDCs and that surface expression is rapidly lost after TLR-mediated maturation. In contrast, DEC205 expression remained high after maturation, which was also demonstrated previously for pDCs32 and moDCs.39,40 On binding of anti-CLEC9A antibodies, surface CLEC9A was internalized into the cell within 30 minutes, demonstrating that CLEC9A acts as endocytic receptor on human BDCA3+ mDCs. Unfortunately, we were unable to follow its intracellular path in more detail because of low cell numbers and low fluorescence intensity. In murine DCs, CLEC9A does not colocalize with lysosomal compartments after internalization. Sancho et al suggested that this nonlysosomal localization may favor cross-presentation of transported antigens.20 Because antigens delivered to DCs via human CLEC9A are cross-presented as well, human CLEC9a may follow a similar intracellular path as murine CLEC9A. At the same time, there are some fundamental differences between human and murine CLEC9A with respect to glycosylation and receptor dimerization.18 This might directly affect antigen routing, and therefore the endocytic route of CLEC9A in human DCs deserves further investigation.
Ligation of CLEC9A with anti-CLEC9A antibodies did not induce phenotypical maturation or cytokine production by BDCA3+ mDCs. Likewise, CLEC9A triggering in the presence of TLR3 and TLR7/8 ligands did not affect TLR-ligand induced expression of maturation markers or secretion of cytokines and chemokines. Several other CLRs, like BDCA2, DCIR, and DEC205, influence the maturation state of DCs by modulating TLR-induced gene expression but do not induce DC maturation in the absence of TLR ligands.32,41-44 CLRs are able to shape immune responses via the signaling motifs in their cytoplasmic tails or via the association with adaptor molecules that induce downstream signals. CLEC9A contains an ITAM-like motif in its cytoplasmic tail.18,20 ITAM-like and ITAM motifs signal via receptor tyrosine phosphorylation by Src kinases and subsequently docking and activation of Syk kinase.45 Also in murine CD8+ DCs, anti-CLEC9A antibodies did not influence in the expression of costimulatory molecules in the absence or presence of TLR-ligation.11,19,22 In contrast, in RAW macrophages, CLEC9A/dectin1 receptor chimeras signaled through their ITAM-like motifs via Syk kinase to induce the production pro-inflammatory cytokines on ligand binding.18 Because natural ligands of CLEC9A have not been identified, it remains to be seen whether ligand binding to CLEC9A modulates DC maturation, as it should be considered that antibody binding may not completely reflect natural ligand binding and that receptor signaling depends both on the receptor and the nature of the ligand.46,47
In mice, CLEC9A proved to be a potent targeting receptor for tumor immunotherapy.19 For induction of effective antitumor responses activation of both CD8+ cytotoxic T lymphocytes and CD4+ T helper 1 cells is required.35 We here show that KLH delivered to BDCA3+ mDCs via anti-CLEC9A antibodies induces efficient recall CD4+ T-cell responses. In the presence of TLR-stimulated BDCA3+ mDCs, KLH-specific T cells produced large amounts of the Th1 cytokine IFN-γ, whereas Th2 cytokines IL-4, IL-5, and IL-10 were absent. DCs polarize immune responses via secretion of cytokines and IL-12p70 favors the differentiation of IFN-γ–producing T helper 1 cells. On activation with TLR3 and TLR7/8 ligands, BDCA3+ mDCs produced high levels of the proinflammatory cytokines IL-1β, IL-6, and TNFα.
However, in line with previous studies,9,10 production of IL-12p70 by BDCA3+ mDCs was rather low. This could point to induction of Th1 responses independent from IL-12.48 On the other hand, Poulin et al10 demonstrated that TLR-stimulated BDCA3+ mDCs produce high levels of IL-12p70 only after CD40 ligation by T cells, a finding that is consistent with several studies in which investigators demonstrated that BDCA3 mDCs and mouse CD8+ DCs only produce IL-12 in concert with additional activation signals.9,10,49 Also in the absence of TLR stimulation, targeted delivery of KLH induced proliferation of KLH-specific CD4+ T cells without inducing IFN-γ production (data not shown). For proliferation, memory CD4+ T-cell responses do not require strong costimulation.50 However, during in vivo targeting, adjuvants such as TLR ligands or CD40 ligand will be necessary to induce efficient naive T-cell responses.19,22
For the first time, we demonstrate that human BDCA3+ mDCs can cross-present antigens internalized through CLEC9A. The long peptide derived from gp100 delivered via anti-CLEC9A–coated nanoparticles was efficiently processed by BDCA3+ mDCs and cross-presented to gp100:280-288 CD8+ T cells. Although isotype control antibody-coated nanoparticles were also taken up or bound by BDCA3+ mDCs, this did not lead to cross-presentation of the antigen. This finding suggests that uptake or binding of the nanoparticles alone is not sufficient to induce degradation of the nanoparticles and processing and cross-presentation of the antigen and that uptake via CLEC9A results in transport to intracellular compartments involved in antigen processing for cross-presentation. CLEC9A targeting with gp100-containing nanoparticles was performed in the presence of soluble TLR ligands poly I:C and R848, to induce expression of costimulatory molecules and cytokine production by DCs. Jongbloed et al even described that poly I:C stimulation of BDCA3+ mDCs is required for cross-presentation of soluble antigens.9
Because we used antibody-coated nanoparticles encapsulating the antigen to target CLEC9A, it is possible to encapsulate TLR ligands within the same particle for in vivo targeting of DCs. Thus, the (tumor) antigen and adjuvant will be delivered simultaneously to the DC and only mature DCs will present antigens to T cells. Because CLEC9A triggering alone did not induce expression of costimulatory molecules or cytokine production, it might even be required to deliver the CLEC9a antibodies and TLR ligands in the same particle to prevent the induction of unwanted immune tolerizing responses, as occurs in mice when antigens are delivered to CLEC9A in the absence of adjuvants.19,22 We recently showed that targeted delivery to DC of antigens and TLR ligands coencapsulated in PLGA nanoparticles greatly enhances immune responses, both in vitro in human monocyte-derived DCs and in vivo in mice.51
In human DCs, CLEC9A is exclusively expressed by BDCA3+ mDCs, which represent only a small percentage of total DCs in peripheral blood and secondary lymph node organs. It is currently unknown whether for immunotherapy it is better to target receptors with restricted expression on limited cell subsets or to target multiple APCs via more widely expressed receptors, such as DEC205 or DCIR. However, in a recent study in mice, investigators showed that targeting CLEC9A induced more potent CD4+ T-cell and humoral responses compared with DEC205.23 The authors suggested this finding may be attributable to prolonged circulation of CLEC9A-specific antibodies in the circulation, because anti-CLEC9A antibodies are not sequestered by other cells, as occurs with for instance anti-DEC205. Thus, the high specificity of anti-CLEC9A antibodies for BDCA3+ mDCs and the low frequencies of BDCA3+ mDCs might also lead to prolonged antigen presentation in humans because of potential longer persistence of anti-CLEC9A antibodies in the circulation.
In conclusion, we show that in human BDCA3+ mDCs antigens delivered via anti-CLEC9A antibodies are efficiently endocytosed, processed, and (cross-)presented via MHC class I and MHC class II to both CD4+ and CD8+ T cells. Therefore, CLEC9A provides a suitable target for delivery of antigens to increase the efficiency of vaccines against infectious or malignant diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ben Joosten, Tjitske Duiveman-de Boer, and Michel Olde Nordkamp for technical assistance.
This work was supported by grants from the Dutch Cancer Society (KUN2006-3699, KUN2009-4402), the European Union (ENCITE HEALTH-F5-2008-201842, Imunanomap MRTN-CT-2006-035946 ERC Advanced grant nr.269019), and The Netherlands Organization for Scientific Research (NWO-Vidi-917.76.363). C.G.F. received the NWO Spinoza award.
Authorship
Contribution: G.S. and L.J.J.K. designed and performed research, analyzed data, and wrote the paper; L.J.C. and M.K. performed research; P.J.T., J.T., G.J.A., and C.G.F. contributed to experimental design and the writing of the paper; G.D.B. contributed vital reagents; and I.J.M.d.V. supervised the study and contributed to experimental design and the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: I. Jolanda M. de Vries, Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: j.devries@ncmls.ru.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal