In this issue of Blood, Biyashev and colleagues report their findings about the critical role of miR-27b in controlling endothelial tip cell fate, branching and venous specification through down-regulation of the newly described miR-27b targets, Spry2 and Dll4.1
Angiogenesis is a fundamental and dynamic process in vertebrates for generation of new blood vessels and capillaries from pre-existing blood vessels. This process initially involves proliferation, sprouting, and migration of endothelial cells. The newly generated blood vessel sprout is guided by migrating tip cells, in response to growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Endothelial cells are extremely sensitive to signals emanating from the extracellular microenvironment and a substantial number of studies implicate endothelium-associated small noncoding RNAs, known as microRNAs (miRNAs), in fine-tuning this process.
The first evidence implicating miRNAs in the regulation of angiogenesis was provided by a study involving mice homozygous for a hypomorphic allele of DICER1, an endoribonuclease in the RNase III family that is essential for processing of functional miRNAs.2 These hypomorphs lacked angiogenesis and died between embryonic days 12.5 and 14.5. This and other studies have clearly implicated a pivotal role for endothelial miRNAs in the regulation of angiogenesis. miRNAs that promote angiogenesis include miR-126, miR-130a, miR-210, and miR-292. The most studied is miR-126, an endothelial cell–specifc miRNA that promotes angiogenesis in response to proangiogenic stimuli, and represses negative regulators of angiogenic signaling pathway.3,4
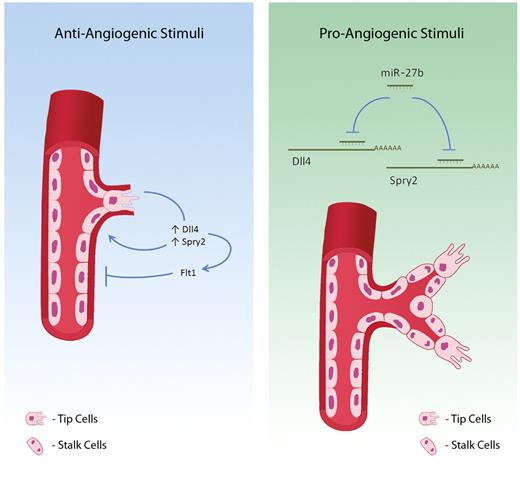
In this report Biyashev and colleagues show that miR-27b has a crucial role in determining tip cell sprouting and is also involved in venous specification. They demonstrate that silencing of miR-27b in zefrafish and mouse tissue impairs vessel sprouting and filipodia formation, in agreement with previous findings from Kuehbacher and colleagues where they showed that inhibition of miR-27b significantly reduces endothelial cell sprouting in an in vitro setting.5 Urbich and colleagues showed that miR-27a/b promotes angiogenesis by targeting endogenous angiogenesis inhibitor SEMA6A and controlling endothelial sprouting.6 Now Biyashev et al demonstrate that Dll4 and Spry2 are targets of miR-27b and therefore the effectors of miR-27b action on the angiogenic switch. Dll4 and Spry2 have been previously implicated in vascular guidance and branching of the tubular structures and further supports this study.7,8 miR-27b acts posttranscriptionally by silencing Dll4 and Spry2 expression that contributes to vessel sprouting and venous specification. This report also shows that miR-27b controls arterial-venous specification via silencing of Dll4, thus impairing Notch and Ephrin B2 (EfnB2) signaling. Spry2 silencing further enhances Flt4, another determinant of venous specification (see figure). Importantly, knockdown of Spry2 or Dll4 activity rescues the phenotype resulting from miR-27b silencing in zebrafish and mice.
The structural differences between arteries and veins were largely attributed to distinct blood flow dynamics and structural differences. But lately, distinct genetic footprints associated specifically with either arterial or venous endothelial cells have been identified.9 This reports by Biyashev et al adds to such previous studies and identifies miR-27b as a molecule that participates in differentiating between arterial and venous endothelial cells via controlled expression of EfnB2, EfnB4, Flt1 and Flt4. Biyashev and colleagues further show that pigment epithelial-derived factor (PEDF), an endogenous angiogenesis inhibitor, can down-regulate miR-27b in the activated endothelial cells, thus leading to impaired angiogenesis via up-regulation of Dll4 and Spry2 proteins and affecting venous specification.
Recently the miR-23b cluster, of which miR-27b is part, as been demonstrated to have proangiogenic properties and targets Spry2 and Semaphorin.10 While miR-23b regulates Spry2, whether this represents a direct targeting of by the miR-23b cluster remains unknown. Collectively, Biyashev and colleagues demonstrate the involvement of miR-27b in the control of Notch signaling and regulating of tip cell fate, sprouting, and venous specification. This study places miR-27b at the core of mechanisms leading to angiogenic balance, serving as a systems integrator of these opposing angiogenic signals to regulate context-dependent angiogenic response. miR-27b is the second miRNA described as critical for angiogenesis, in addition to miR-126. In this regard, miR-126 is necessary for the development and maintenance of the embryonic vasculature.3,4
Mir-27b is a systems integrator of pro- and antiangiogenic signals at the posttranscriptional level via repression of Dll4 and Spry2 expression. Antiangiogenic stimuli result in down-regulation of miR-27b expression that allows for Spry2 and Dll4 expression, which blocks capillary branching and tip endothelial cell fate, respectively. Furthermore, miR-27b down-regulates Flt1 through Dll4/Notch signaling, further repressing vascular sprouting. Proangiogenic stimuli result in miR-27b expression, enabling posttranscriptional silencing of Dll4 and Spry2 and suppression of Flt1, which enhances tip cell numbers and promotes vascular sprouting. Such context-dependent bimodal action of mir-27b shapes appropriate angiogenesis response in health and disease.
Mir-27b is a systems integrator of pro- and antiangiogenic signals at the posttranscriptional level via repression of Dll4 and Spry2 expression. Antiangiogenic stimuli result in down-regulation of miR-27b expression that allows for Spry2 and Dll4 expression, which blocks capillary branching and tip endothelial cell fate, respectively. Furthermore, miR-27b down-regulates Flt1 through Dll4/Notch signaling, further repressing vascular sprouting. Proangiogenic stimuli result in miR-27b expression, enabling posttranscriptional silencing of Dll4 and Spry2 and suppression of Flt1, which enhances tip cell numbers and promotes vascular sprouting. Such context-dependent bimodal action of mir-27b shapes appropriate angiogenesis response in health and disease.
It is now clear that miR-27b plays a crucial role in regulating the process of angiogenesis by achieving a fine balance between stimulators and suppressors of endothelial proliferation, migration, and differentiation. Future studies will most likely further identify new roles for miR-27b in regulating the function of other cell types associated with blood vessels such as pericytes. Tumor-associated endothelial cells reveal suppression of miR-27b associated with increased expression of Dll4 and Spry2. Therefore, silencing miR-27b using antagomirs to control tumor angiogenesis and growth or enhancing miR-27b activity in the setting of ischemic damage associated with myocardial infarction and stroke to favor angiogenesis could be explored as potential therapies.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal