Peripheral blood monocytes originate from a common myeloid precursor in the bone marrow and migrate into tissues to differentiate into macrophages and dendritic cells on interaction with their microenvironment. In this issue of Blood, Zhang et al report that autophagy is mandatory for the differentiation of monocytes into macrophages on stimulation with GM-CSF or M-CSF.1
The authors contend that autophagy prevents the calpain-mediated activation of a caspase-dependent apoptotic cell death pathway, which is in accordance with the observation that, in most circumstances, autophagy is an adaptation pathway that promotes survival of the cell in stressful conditions. However, in some settings autophagy leads to a nonapoptotic programmed cell death called autophagic or type II cell death.2 Because a limited activation of several caspases, which are key actors of apoptotic cell death, is also required for M-CSF–driven differentiation of monocytes into macrophages,3 tight control of the intricate interplay between death and differentiation is required to generate mature macrophages. The identification of such a subtle regulation opens potential therapeutic avenues in cancer and inflammatory diseases.
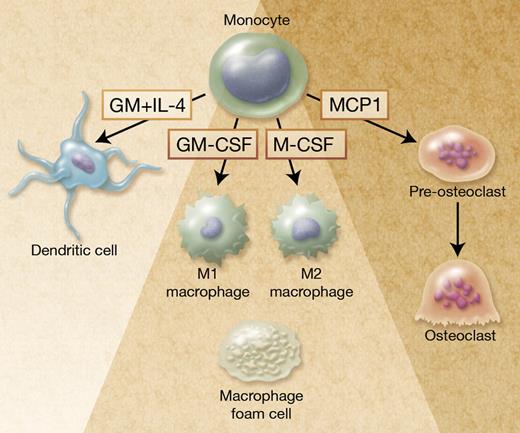
Autophagy in monocyte-derived cells. Monocytes are plastic cells that differentiate into M1-polarized macrophages on exposure to GM-CSF and M2-polarized macrophages on M-CSF exposure. Zhang et al report that autophagy is required for monocyte differentiation into macrophages and prevents cell death. Autophagy is also activated in monocytes exposed to GM-CSF and IL-4 to generate dendritic cells,1 and to MCP1 to generate pre-osteoclasts.9 In addition, autophagy is activated in lipid-loaded macrophages (foam cells) found in atherosclerotic lesions10 and in some infected macrophages to favor pathogen elimination and antigen presentation (not shown). Professional illustration by Alice Y. Chen.
Autophagy in monocyte-derived cells. Monocytes are plastic cells that differentiate into M1-polarized macrophages on exposure to GM-CSF and M2-polarized macrophages on M-CSF exposure. Zhang et al report that autophagy is required for monocyte differentiation into macrophages and prevents cell death. Autophagy is also activated in monocytes exposed to GM-CSF and IL-4 to generate dendritic cells,1 and to MCP1 to generate pre-osteoclasts.9 In addition, autophagy is activated in lipid-loaded macrophages (foam cells) found in atherosclerotic lesions10 and in some infected macrophages to favor pathogen elimination and antigen presentation (not shown). Professional illustration by Alice Y. Chen.
Autophagy is a major intracellular degradation system that clears out superfluous or degraded cell materials in the lysosome, and serves as a dynamic recycling process. There are 3 discrete types of autophagy. Microautophagy refers to the engulfment of cytoplasm portions through invagination of the lysosome membrane. Chaperone-mediated autophagy is the selective degradation of cytosolic proteins targeted to the lysosomes by exposure of a KFERQ-like motif and association to the Hsc70 complex. Macroautophagy consists in de novo synthesis of isolation membranes or phagophores. These membranes form specific vacuoles called autophagosomes that fuse with lysosomes to become autolysosomes and degrade the material contained within it, including organelles and proteins. The molecular cascade that executes authophagy has been identified in recent years.2 A low level of autophagy occurs in virtually all cells to renew altered proteins and organelles. A strong amplification of macroautophagy was detected in monocytes undergoing macrophage differentiation, in both human monocytes cultured in vitro and mouse cells examined in vivo.1
A role for autophagy has been identified in some other hematopoietic lineages. Conditional deletion of the essential autophagy gene Atg7 in the mouse hematopoietic system demonstrated that autophagy was required for hematopoietic stem cell maintenance. In the absence of Atg7, the hematopoietic stem and progenitor cells accumulate mitochondria, reactive oxygen species, and DNA damage, and a severe myeloproliferation appears, leading to premature death of the animal.4 During the maturation of reticulocytes, the clearance of mitochondria, which involves an atypical protein of the Bcl-2 family called Nix, is at least in part an autophagy-dependent mechanism (in this case called mitophagy),5 and autophagy was shown to shape the T-lymphocyte repertoire.6
Autophagy may contribute to the macrophage differentiation of monocytes by selectively clearing some cellular components to generate amino acids, free fatty acids, glucose, and ATP that are required for differentiation-associated structural remodeling. Monocytes are heterogeneous cells with several phenotypically and functionally distinct subsets that differentially respond to cytokines.7 In addition, the macrophages generated on stimulation with different cytokines have distinct functions, for example, GM-CSF–induced macrophages (M1 macrophages) respond to interferon γ by releasing pro-inflammatory cytokines whereas M-CSF–induced macrophages (M2 macrophages) are involved in tissue repair (see figure).8 Increasing evidence indicates that autophagy can be selective, that is, degrade selective proteins and organelles.2 Further studies will indicate whether the capability of a monocyte subset to activate autophagy, and the selectivity of the activated catabolism, participates in the functional specificity of this subset and the generation of polarized macrophages.
Monocytes can also differentiate into osteoclasts in the bone, which can be reproduced by exposure of the cells to the combination of M-CSF and receptor activator of NF-kB ligand (RANKL; see figure) or to monocyte chemotactic protein-1 (MCP-1). Autophagy was recently observed during osteoclastic differentiation of monocytes exposed to MCP-1.9 Germ line and somatic mutations in SQSTM1 gene, that encodes the ubiquitin-binding adaptor protein P62/SQSTM1 or sequestosome 1, have been identified in Paget disease of bone. P62/SQSTM1 is a scaffold with multiple protein-protein interaction motifs that plays a role in the signaling pathways activated by RANKL. Because the protein is also involved in autophagy, the mutated protein could contribute to the disease pathogenesis by affecting this degradation system, leading to altered osteoclastogenesis.2
Pathogen-associated molecular patterns, recognized by Toll-like and NOD-like receptors at the surface of macrophages, stimulate autophagy. In infected macrophages, autophagy enhances the delivery of ubiquitin-coated pathogens to the lysosome and contributes to the presentation of antigenic peptides at the cell surface. Macrophage autophagy is also observed in atherogenic pathophysiologic conditions, for example, autophagy, and contributes to intracellular lipid breakdown in lipid-loaded macrophages and generates free cholesterol for ABCA1-mediated efflux.10 Enhancement of this first step in the macrophage reverse cholesterol transport to the liver is considered a good antiatherogenic strategy, indicating that controlled stimulation of autophagy in macrophage foam cells is a potential therapeutic approach in atheroscleroris.
If macrophages are essential for normal development, innate and acquired immunity, and tissue repair, they also contribute to almost every disease through their immunologic and wound-healing functions. All established solid tumors recruit macrophages that, in 80% of cases, promote progression and metastasis. The recruitment of macrophages to adipose tissue contributes to the low-level inflammatory state identified in obesity.
Macrophages also contribute to the propagation of many autoimmune and inflammatory diseases such as rheumatoid arthritis. Current attempts at blocking M-CSF action with kinase inhibitors or agents that prevent the cytokine binding to its receptor suggest that the strategy is more efficient in cancer than in inflammatory diseases.8 If confirmed in genetically modified models, the role of autophagy in monocyte differentiation suggests another potential approach to prevent macrophage recruitment into tissues, that is, inhibition of autophagy may induce the death of differentiating monocytes. Therapeutic manipulation of autophagy in the monocyte/macrophage lineage will require in-depth analysis of the specificities of autophagic pathways in the diverse cellular subsets on stimulation with different cytokines.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■