Abstract
Monocytes are programmed to undergo apoptosis in the absence of stimulation. Stimuli that promote monocyte-macrophage differentiation not only cause cellular changes, but also prevent the default apoptosis of monocytes. In the present study, we demonstrate that autophagy is induced when monocytes are triggered to differentiate and that the induction of autophagy is pivotal for the survival and differentiation of monocytes. We also show that inhibition of autophagy results in apoptosis of cells that are engaged in differentiation. We found that the differentiation signal releases Beclin1 from Bcl-2 by activating JNK and blocks Atg5 cleavage, both of which are critical for the induction of autophagy. Preventing autophagy induction hampers differentiation and cytokine production; therefore, autophagy is an important transition from monocyte apoptosis to differentiation.
Introduction
Macrophages regulate the innate immune responses to acute and chronic inflammation. In the absence of differentiation, circulating monocytes are believed to undergo apoptotic cell death. Therefore, the half-life of monocytes in blood is suggested to be relatively short: approximately 1 day in mice and 3 days in humans.1,2 Peripheral blood monocytes also die by an apoptotic process when cultured in the absence of growth or activation stimuli.3 Once monocytes are stimulated by an inflammatory response, they activate prosurvival pathways, migrate to tissues, and differentiate into macrophages. Two of the most important growth factors for this process, GM-CSF and M-CSF, induce monocyte-macrophage lineage differentiation and prosurvival pathways both in vivo and in vitro. Previous studies have determined many of the signaling pathways activated by GM-CSF or M-CSF,3-5 but the molecular mechanisms by which they induce survival and differentiation of monocytes are still unclear.
Autophagy is a ubiquitous process believed to occur in all eukaryotic cells in which cytosol and organelles are sequestered within double-membrane vesicles that deliver their contents to lysosomes for degradation and/or recycling of the resulting macromolecules.6,7 Among the many functions of autophagy are cellular homeostasis,8 anti-aging,9,10 and development.11 In host-pathogen interactions, autophagy functions as a fundamental cellular homeostasis pathway and is designed to effectively counter intracellular pathogens and microbial insult to the cellular environment.12,13 In addition, autophagy has been proposed to protect from various diseases, including cancer,14 cardiomyopathy,15 and neurodegenerative disorders.16 Because autophagy plays a role in ubiquitous areas, developing an understanding of its physiologic and pathologic functions has grown increasingly important because their manipulation may affect the treatment of diseases.
Autophagy regulates both cell survival and cell death. Originally, it was found to protect against cell death during nutrient starvation7 ; however, later studies showed that excessive autophagy might also induce cell death.17 Several studies have shown that there is cross-talk between autophagic and apoptotic pathways. For example, Bcl-2, the well-characterized anti-apoptotic protein, can inhibit Beclin1/Atg6-mediated autophagy by binding to Beclin1.18 Apoptotic induction of caspase cleavage of several key autophagic proteins has also been described previously.19 Atg5, which was previously considered to be an autophagy-specific protein,20 can be cleaved after death stimuli, and the cleavage product can promote mitochondrial-mediated apoptosis.21 All of these data suggest that a switch between autophagy and apoptosis may exist in many physiologic settings.20
Although the role of autophagy in cell differentiation remains elusive, there is some evidence that it is an important event for differentiation in many different kinds of cells,22 including erythrocytes,23 lymphocytes,24-26 and adipocytes.27,28 Autophagy appears to be primarily involved in the differentiation of cell types that require marked architectural remodeling, such as erythroid cells, in which the presumable function is in mitochondrial clearance.23 Whereas differentiating agents such as vitamin D3 induce autophagy in the monocyte-like leukemic cell line HL-60,29 vitamin D3 was shown separately to induce autophagy in human monocytes/macrophages via cathelicidin.30 These reports suggest that autophagy could be involved in monocyte differentiation; however, although autophagy has been shown to be important for the maintenance and differentiation of the hematopoietic stem cells from which they are derived, there have been no reports on the role of autophagy during monocyte-macrophage differentiation.31
In the present study, we sought to determine whether autophagy plays a role in protection from cell death during monocyte-macrophage differentiation. We found that differentiation of human primary monocytes with GM-CSF or M-CSF triggered autophagy in these cells. Autophagy prevents monocytes from apoptosis and also induces monocyte-macrophage differentiation, and this is attributable to JNK-mediated Bcl-2 phosphorylation and subsequent Beclin1–Bcl-2 disassociation. GM-CSF also blocks the generation of pro-apoptotic truncated Atg5 by inhibiting calpain activity. Inhibiting autophagy leads to the blockage of monocyte-macrophage differentiation; therefore, our findings demonstrate that induction of autophagy is essential for monocyte-macrophage differentiation.
Methods
Cell culture
Monocytes elutriated from human peripheral blood were obtained from the National Institutes of Health Blood bank (according to protocol 99CC0168). Monocytes were cultured in RPMI 1640 medium supplemented with 10% (vol/vol) FBS, 2mM glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL). For differentiation, cells were cultured for 7 days in the presence of recombinant human GM-CSF (10 ng/mL).
Reagents and Abs
Lipopolysaccharide (LPS), 3-methyl adenine (3-MA), and chloroquine (CQ) were from Sigma-Aldrich; DQ Red BSA was from Invitrogen (D-12051); anti–Bcl-2 (610539), anti-Beclin1 (612112), anti-p62 (610832), anti-JNK (554286), CD11b-PE (553311), CD86-PE (555658), Ly6C-FITC (553104), and FITC–annexin V (556419) were from BD Biosciences; anti–p-Bcl-2 (2827), anti–Bcl-2 (4223), anti–Caspase-3 (9662), anti-Beclin1 (3738), and anti-VDAC (4866) were from Cell Signaling Technology; anti-LC3 (L7543) and anti-actin (A3853) were from Sigma-Aldrich; anti-Atg5 (NB110-53818) was from Novus Biologicals; anti-LC3 (AP1800a), anti-PI3KC3 (AP8014a), and anti-Atg5 (C-term; AP1812b) were from Abgent; anti-phospho JNK (44682) was from Invitrogen; and CD14-FITC (11-0149-73) and F4/80-APC (17-4801-80) were from eBiosciences. The siRNAs targeting human Beclin1 (pool L-010552-00-00-0005, single siRNA J-010552-05 and J-010552-06), human Atg5 (pool L-004374-00-0005), pool siRNA control (D001206-13-20), and single siRNA control (D-001810-01-05) were from Thermo-Dharmacon. JNK1/2 siRNAs (5′-AAA GAA UGU CCU ACC UUC UTT-3′ and 5′-AGA AGG UAG GAC AUU CUU UTT-3′) targeting 2 different identical regions of human JNK1/2 mRNAs were synthesized by Thermo-Dharmacon. Recombinant human GM-CSF (215-GM), M-CSF (216-MC), IL-4 (204-IL), IFN-γ (285-IF), and recombinant mouse M-CSF (416-ML) were from R&D Systems.
Immunoblotting and co-immunoprecipitation
Cells were lysed in lysis buffer (20mM Tris, pH 7, 0.5% NP-40, 250mM NaCl, 3mM EDTA, 3mM EGTA, 2mM DTT, 0.5mM PMSF, 20mM β-glycerol phosphate, 1mM sodium vanadate, and 1 mg/mL of leupeptin) and were analyzed by immunoblotting after SDS-PAGE. Proteins were visualized by ECL according to the manufacturer's instructions (Thermo). For immunoprecipitation assays, proteins were precipitated with different Abs and protein G-agarose beads and analyzed by Western blotting.
Confocal microscopy
Confocal microscopy was performed with cells stained with DQ-BSA, anti-LC3, or anti-p62 according to the manufacturer's protocol. Stained cells were visualized on a Zeiss Observer Z1 microscope equipped with an Apo63 × 1.4 oil DIC II objective. Confocal images were generated on a Zeiss LSM510 META laser scanning microscope.
Transmission electron microscopy
Monocytes treated or not with GM-CSF for 14 hours were fixed with 1% glutaraldehyde in 0.1M cacodylate buffer (pH 7.0) and examined by National Institutes of Health electron microscopy core facility using an H7600 microscope (Hitachi) with a digital camera (Advanced Microscopy Techniques).
RNA isolation
Total RNA was extracted with TRIzol reagent according to the manufacturer's guidelines (Invitrogen). Any remaining DNA was removed with the DNA-free kit (Ambion) and was repurified with the RNeasy kit (QIAGEN).
Real-time PCR
TaqMan real-time gene expression assays were run on an ABI StepOnePlus system according to manufacturer's protocol (Applied Biosystems). Gene expression was normalized to that of GAPDH. The following minor groove binder assays from Applied Biosystems were used for gene-expression analysis: GAPDH, Hs02786624_g1; human TNF-α, Hs01113624_g1; and human IL12p40, Hs00233688_m1.
Flow cytometry
Flow cytometry was performed using a 2-laser (488 and 585 nm) FACSCalibur flow cytometer (BD Biosciences) according to the manufacturer's protocol.
Recruitment and sorting of peritoneal monocytes/macrophages
Thioglycolate-activated monocytes/macrophages were isolated from C57BL/6 mice by peritoneal lavage 1-4 days after IP injection of 1 mL of 3% sterile thioglycolate medium. The cells from thioglycolate-induced peritonitis were costained with anti-Ly6C and anti-F4/80, and F4/80+ populations were sorted.
Mouse primary monocytes isolation from BM
BM cells were isolated by removing leg bones from muscles and cultured for 4 hours. After removing the floating cells, the remaining attached cells were the enriched monocytes. The purity of these cells was detected by flow analysis. In addition, pure mouse primary monocytes were sorted by costaining with anti-Ly6C and anti-F4/80.
Annexin V/PI apoptosis assays
Monocytes were costained with FITC–annexin V and propidium iodide (PI) according to the manufacturer's protocol and analyzed with a flow analyzer.
Calpain activity assay
Calpain activity was detected with the Calpain-Glo Protease Assay Kit (Promega).
Electroporation
Monocytes was transfected with Amaxa Nucleofector II and the kits supplied (VPA-1008) according to the manufacturer's instructions (Lonza). After electroporation, monocytes were cultured in the monocyte-growing medium supplied with the kit before collection and different treatments.
Statistics
Two group comparisons were performed using the Student t test. Multiple group comparisons were performed using 1-way ANOVA and the Fisher least significant difference test. P < .05 was considered statistically significant.
Results
Autophagy is induced during monocyte differentiation
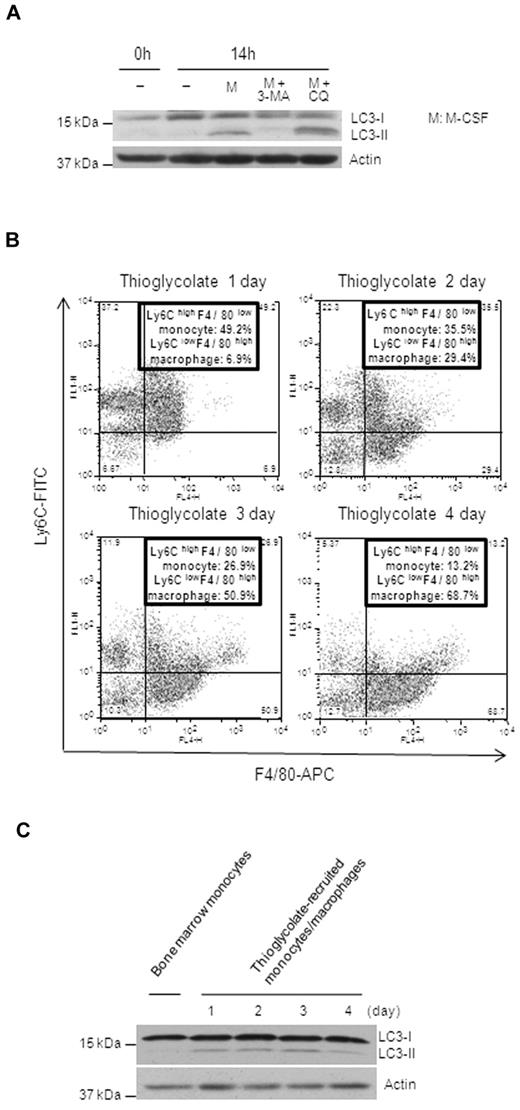
To explore the role of autophagy in monocyte differentiation, we examined whether autophagy is activated in response to differentiation stimuli. We obtained elutriated human monocytes from National Institutes of Health blood bank. All elutriated cell samples used in our study contained more than 85% CD11b+CD14+ monocytes by flow analysis, and more than 95% cells were alive, as shown by PI staining (data not shown). Cells were treated with GM-CSF to induce monocyte to macrophage differentiation. Microtubule-associated protein light chain 3 (LC3) was used as a marker for the induction of autophagy because, during autophagy, cytosolic LC3-I is processed to its lipidated LC3-II form.32 LC3-II then locates to newly forming autophagophores and subsequently participates in membrane elongation and cargo recognition while continuing to be present in mature autophagosomes as well.32 Therefore, processing of LC3 and a punctate LC3 pattern represent the formation of autophagosomes and autophagic responses. In the present study, LC3-II was detected in substantial amounts only in GM-CSF–treated cells beginning at approximately 3 hours, and the maximum amount of LC3-II occurred at 12 hours after treatment (Figure 1A). LC3-II processing was blocked by the autophagy inhibitor 3-MA, which inhibits the activity of PI3KC3 and the formation of autophagosomes (Figure 1B). Addition of the lysosome-autophagosome fusion inhibitor CQ, which inhibits autophagy at a later step in the pathway, caused an additional accumulation of LC3-II, indicating increased autophagic flux in GM-CSF–treated cells (Figure 1B). Fluorescence microscopy revealed a punctate LC3 pattern after GM-CSF treatment (Figure 1C). To confirm that GM-CSF caused autophagic vacuoles, monocytes treated or not with GM-CSF for 14 hours were analyzed by electron microscopy. Images showed that only GM-CSF–treated monocytes had the characteristic autophagic vacuoles surrounded by double membranes with partially degraded intracellular material inside (Figure 1D and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Quantification of cells with punctate LC3 showed an approximate 6-fold increase of cells with autophagosomes during differentiation, whereas 3-MA attenuated this increase (Figure 1E). Because monocytes can also differentiate to macrophages on M-CSF or differentiate to dendritic cells when exposed to the combination of GM-CSF and IL-4, we then determined autophagy induction by these stimuli. Induction of autophagy was observed when monocytes were differentiated with M-CSF or the combination of GM-CSF and IL-4 (supplemental Figure 2).
Autophagy is activated during human monocyte differentiation. (A-B) Immunoblot analysis of lysates from human primary elutriated monocytes incubated with or without GM-CSF (10nM) for the indicated times. (B) Where indicated, cells were pretreated with 3-MA at 10mM or CQ at 50μM. Representative data from at least 3 independent experiments for panels A and B are shown. The ratio of LC3II/LC3I was quantified from 3 experiments and is presented in bar graphs (A-B). (C) Human monocytes treated or not with GM-CSF for 14 hours, stained with anti-LC3 Ab and then Alexa Fluor 568 goat anti–mouse IgG (red), and processed for confocal microscopy. Scale bars indicate 5 μm. (D) Human monocytes were incubated with or without GM-CSF for 14 hours and processed for electron microscopy. Black arrows indicate autophagosomes with double membranes and partially degraded material. Scale bars indicate 500 nm. (E) Human monocytes were incubated with or without GM-CSF (10nM) in the absence or presence of 3-MA (10mM) for 14 hours, fixed, and stained with 4′,6-diamidino-2-phenylindole to visualize the nuclei (blue), and immunolabeled with an anti-LC3 Ab followed by 568 goat anti–mouse IgG (red). Scale bars indicate 20 μm. Representative images from 4 independent experiments are shown in panels B through D and quantitation of the percentage of cells with LC3+ punctate from the 4 experiments is shown in panel E (*P < .002 for comparisons between GM-CSF alone and untreated or cotreatment).
Autophagy is activated during human monocyte differentiation. (A-B) Immunoblot analysis of lysates from human primary elutriated monocytes incubated with or without GM-CSF (10nM) for the indicated times. (B) Where indicated, cells were pretreated with 3-MA at 10mM or CQ at 50μM. Representative data from at least 3 independent experiments for panels A and B are shown. The ratio of LC3II/LC3I was quantified from 3 experiments and is presented in bar graphs (A-B). (C) Human monocytes treated or not with GM-CSF for 14 hours, stained with anti-LC3 Ab and then Alexa Fluor 568 goat anti–mouse IgG (red), and processed for confocal microscopy. Scale bars indicate 5 μm. (D) Human monocytes were incubated with or without GM-CSF for 14 hours and processed for electron microscopy. Black arrows indicate autophagosomes with double membranes and partially degraded material. Scale bars indicate 500 nm. (E) Human monocytes were incubated with or without GM-CSF (10nM) in the absence or presence of 3-MA (10mM) for 14 hours, fixed, and stained with 4′,6-diamidino-2-phenylindole to visualize the nuclei (blue), and immunolabeled with an anti-LC3 Ab followed by 568 goat anti–mouse IgG (red). Scale bars indicate 20 μm. Representative images from 4 independent experiments are shown in panels B through D and quantitation of the percentage of cells with LC3+ punctate from the 4 experiments is shown in panel E (*P < .002 for comparisons between GM-CSF alone and untreated or cotreatment).
To confirm this observation in cells from a different source, we examined the differentiation of mouse BM monocytes. Cells were isolated from mouse BM and, after enrichment, 90% of the enriched cells were Ly6ChighF4/80low monocytes (supplemental Figure 3A). M-CSF–induced differentiation of mouse monocytes also caused LC3 processing, which was blocked by 3-MA, and accumulation of LC3-II was potentiated by CQ, indicating autophagic flux (Figure 2A). Similar results were observed in sorted pure Ly6ChighF4/80low monocytes from BM (supplemental Figure 3B). To confirm the occurrence of autophagy during monocyte-macrophage differentiation in vivo, we chose an in vivo differentiation model by treating mice with thioglycolate. Monocytes were recruited to the peritoneum by thioglycolate and differentiated into mature macrophages after 3 days. Flow analysis of thioglycolate-elicited peritoneal cells showed that the percentage of Ly6ClowF4/80high macrophages gradually increased, whereas the percentage of Ly6ChighF4/80low monocytes reduced from day 1 to day 4, which reflected the monocyte-macrophage differentiation in vivo (Figure 2B). Almost 70% of total peritoneal cells were fully differentiated macrophages by day 4 (Figure 2B). We also sorted these peritoneal F4/80+ cells to check for the induction of autophagy. Compared with the sorted Ly6ChighF4/80low BM monocytes, these peritoneal F4/80+ cells had significantly increased levels of LC3-II, indicating the induction of autophagy (Figure 2C). The longer kinetics of LC3-II presence in these cells compared with that seen in the in vitro GM-CSF–induced macrophages was most likely due to the gradual, nonsynchronized recruitment of monocytes to the peritoneum by thioglycolate. These data suggest that autophagy occurs during monocyte-macrophage differentiation.
The induction of autophagy is observed during monocyte differentiation in vivo. (A) Representative immunoblot analysis of lysates from mouse BM monocytes incubated with or without murine M-CSF at a concentration of 20nM for 14 hours. Where indicated, cells were treated in the presence or absence of 3-MA (10mM) or CQ (50μM). (B) Representative flow analysis of cells from thioglycolate-induced peritonitis costained with F4/80-APC and Ly6C-FITC for the indicated times. (C) Ly6ChighF4/80low BM monocytes and the F4/80+ population from panel B were sorted and their lysates compared by Western blot analysis. Representative immunoblot analyses are shown.
The induction of autophagy is observed during monocyte differentiation in vivo. (A) Representative immunoblot analysis of lysates from mouse BM monocytes incubated with or without murine M-CSF at a concentration of 20nM for 14 hours. Where indicated, cells were treated in the presence or absence of 3-MA (10mM) or CQ (50μM). (B) Representative flow analysis of cells from thioglycolate-induced peritonitis costained with F4/80-APC and Ly6C-FITC for the indicated times. (C) Ly6ChighF4/80low BM monocytes and the F4/80+ population from panel B were sorted and their lysates compared by Western blot analysis. Representative immunoblot analyses are shown.
p62 is colocalized with LC3 in GM-CSF–treated monocytes
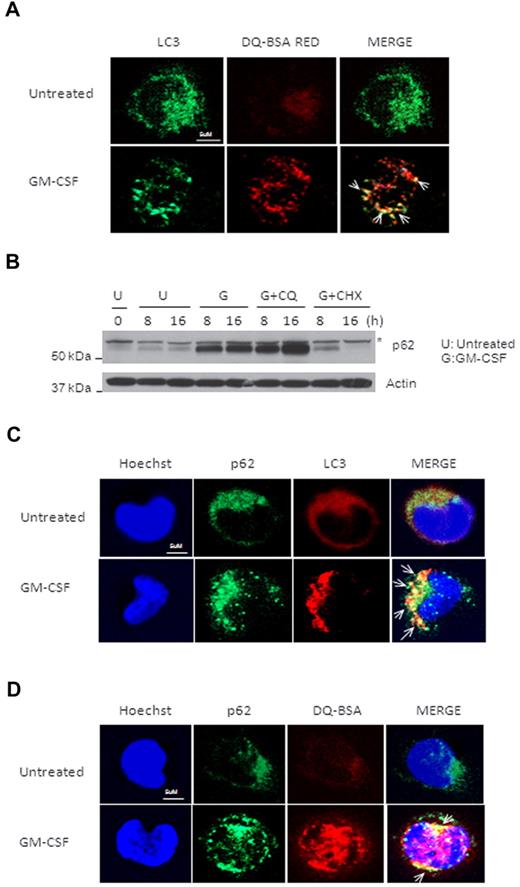
To examine the convergence of autophagosomes and lysosomes in GM-CSF–induced autophagy, we tested intracellular proteolytic activity with DQ Red BSA (DQ-BSA) and determined p62 protein degradation. DQ-BSA is a derivative of BSA that is labeled with a self-quenched red fluorescent dye and can be dequenched by lysosomal proteases. Whereas no dequenching of DQ-BSA occurred in control cells, DQ-BSA Red was observed in GM-CSF–treated monocytes and, more importantly, the dequenched, DQ-BSA–containing vesicles were colocalized with LC3-positive puncta (Figure 3A). We then investigated autophagic degradation of p62 in GM-CSF–treated monocytes. Strikingly, the p62 protein level was markedly increased after treatment due to GM-CSF–induced up-regulation of p62 expression (Figure 3B and data not shown). Blocking lysosome-autophagosome fusion by CQ induced the additional accumulation of p62, and blocking new protein synthesis by CHX led to p62 degradation (Figure 3B). In addition, p62 was found to be colocalized with LC3 and DQ-BSA Red in GM-CSF–stimulated monocytes (Figure 3C-D). These results suggest that both p62 and DQ-BSA are present in the same compartment of autophagosomes and are degraded by autophagy in activated monocytes.
p62 is colocalized with GM-CSF–induced LC3+ puncta and degraded by autophagy in activated monocytes. (A) Human primary monocytes were incubated with or without GM-CSF for 14 hours, and then treated with DQ-BSA for another 2 hours. Cells were fixed and stained with anti-LC3 Ab, followed by an FITC goat anti–mouse IgG (green). Representative confocal images of colocalization of LC3 and red fluorescence of DQ-BSA are shown. (B) Human primary monocytes were pretreated with cycloheximide (CHX) at 1μM or CQ at 50mM, and then incubated with GM-CSF (10nM) for the indicated times and analyzed by immunoblotting against p62. One representative experiment from at least 3 independent experiments is shown. Asterisk indicates one special band of truncated p62 Ab. (C) Human primary monocytes were incubated with or without GM-CSF for 14 hours, and then fixed and costained with anti-p62 Ab followed by a FITC goat anti–mouse IgG (green) and anti-LC3 Ab and then rhodamine goat anti–rabbit IgG (red). Representative confocal images of colocalization of p62 and LC3 are shown. (D) Human primary monocytes were incubated with or without GM-CSF for 14 hours and DQ-BSA for another 2 hours, then fixed and stained with anti-p62 Ab, followed by an FITC goat anti–mouse IgG (green). Representative confocal images of colocalization of p62 and red fluorescence of DQ-BSA are shown. Scale bars indicate 5 μm. Arrows indicate colocalization of green and red fluorescence (A,C,D).
p62 is colocalized with GM-CSF–induced LC3+ puncta and degraded by autophagy in activated monocytes. (A) Human primary monocytes were incubated with or without GM-CSF for 14 hours, and then treated with DQ-BSA for another 2 hours. Cells were fixed and stained with anti-LC3 Ab, followed by an FITC goat anti–mouse IgG (green). Representative confocal images of colocalization of LC3 and red fluorescence of DQ-BSA are shown. (B) Human primary monocytes were pretreated with cycloheximide (CHX) at 1μM or CQ at 50mM, and then incubated with GM-CSF (10nM) for the indicated times and analyzed by immunoblotting against p62. One representative experiment from at least 3 independent experiments is shown. Asterisk indicates one special band of truncated p62 Ab. (C) Human primary monocytes were incubated with or without GM-CSF for 14 hours, and then fixed and costained with anti-p62 Ab followed by a FITC goat anti–mouse IgG (green) and anti-LC3 Ab and then rhodamine goat anti–rabbit IgG (red). Representative confocal images of colocalization of p62 and LC3 are shown. (D) Human primary monocytes were incubated with or without GM-CSF for 14 hours and DQ-BSA for another 2 hours, then fixed and stained with anti-p62 Ab, followed by an FITC goat anti–mouse IgG (green). Representative confocal images of colocalization of p62 and red fluorescence of DQ-BSA are shown. Scale bars indicate 5 μm. Arrows indicate colocalization of green and red fluorescence (A,C,D).
Inhibition of autophagy suppresses GM-CSF–induced monocyte survival
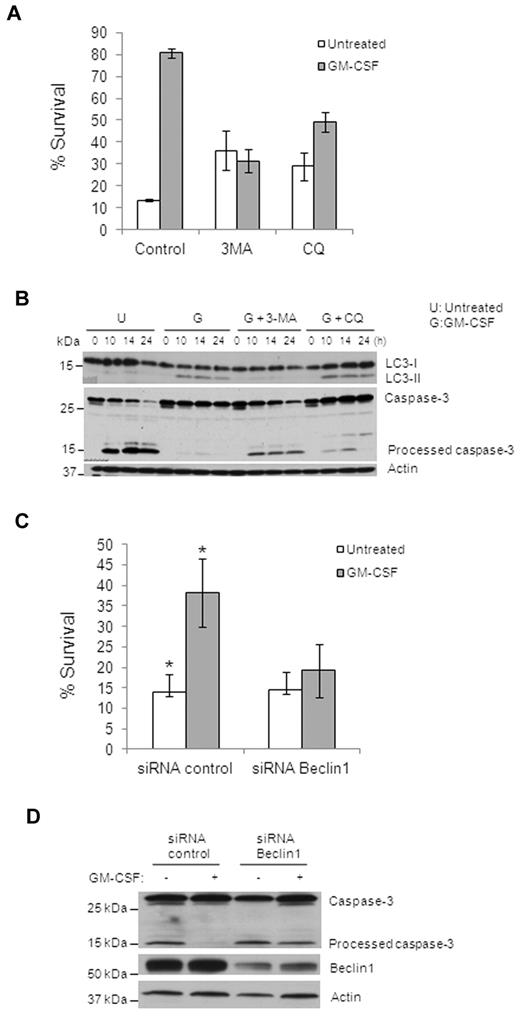
Previous studies demonstrated that autophagy regulates both cell survival and cell death and that there is cross-talk between autophagy and apoptosis.17 To assess the importance of autophagy in monocyte differentiation, we examined the effect of autophagy on monocyte cell death. GM-CSF increased survival of monocytes from approximately 10%-15% to 80% after 48 hours in culture (Figure 4A). This increase in viability was prevented efficiently by 3-MA or CQ, although CQ was slightly less effective and 3-MA or CQ alone without GM-CSF promoted survival to some extent (Figure 4A and supplemental Figure 4). Examining caspase activation in these cells confirmed these observations, because Caspase-3 was processed in control monocytes, little or no Caspase-3 cleavage was found in GM-CSF–treated cells, and Caspase-3 cleavage was recovered in GM-CSF–treated cells by 3-MA or CQ pretreatment (Figure 4B). To further prove that autophagy is necessary for monocyte survival, we suppressed autophagy by knocking down Beclin1, which is required for the initiation of autophagy. Monocytes were transfected with either control or Beclin1 siRNAs for 36 hours and then treated with GM-CSF for another 24 hours. Whereas transfection was toxic to monocytes, as we have reported previously,33 there were sufficient remaining viable cells for these experiments. Both Beclin1 pool siRNAs and 2 Beclin1 single siRNAs efficiently reduced Beclin1 expression compared with nontargeting control siRNAs (Figure 4D and supplemental Figure 6B). Knockdown of Beclin1 inhibited GM-CSF–induced LC3-II production in the remaining monocytes (supplemental Figure 5). Whereas GM-CSF was able to induce a 2- to 4-fold increase in the survival of the control siRNA–transfected cells, no significant increase in viability was seen in the Beclin1-knockdown cells after GM-CSF treatment (Figure 4C and supplemental Figure 6A,C), indicating that GM-CSF–induced survival was dependent on Beclin1-mediated autophagy. Consistently, GM-CSF treatment prevented the generation of fully processed Caspase-3 in control siRNA–transfected cells, but was not able to do so in Beclin1-knockdown cells (Figure 4D). In addition, knockdown of Atg5 in GM-CSF–treated monocytes had a similar effect on cell survival (supplemental Figure 7). Finally, inhibition of caspase activity with z-VAD increased monocyte viability and the total number of differentiated macrophages without GM-CSF (supplemental Figure 8). These data indicate that GM-CSF–induced autophagy is critical for monocyte survival.
Inhibition of autophagy suppresses GM-CSF–induced human primary monocyte survival. (A) Cell death of human monocytes with different treatments (GM-CSF, 10nM; 3-MA, 10mM; or CQ, 50 mM) for 48 hours was assessed using annexin V/PI staining and flow cytometry. Percentage of annexin V/PI double-negative cells (percentage survival) are shown. Dead cells indicate all cells positive for annexin V, including annexin V+/PI− early apoptosis and annexin V+/PI+ later apoptosis (A,C). Error bars indicate ± SEM. Data are representative of at least 3 independent experiments. (B) Immunoblot analysis with Abs to LC3, Caspase-3, and actin for GM-CSF–treated primary human monocytes. Representative data are shown for GM-CSF alone and pretreatment with 3-MA (10mM) or CQ (50μM). (C) Cell death assessed by flow cytometry of annexin V/PI–stained cells with Beclin1 siRNA or control siRNA in treated or not with GM-CSF. The percentage of annexin V/PI double-negative cells (percentage survival) is shown. Error bare indicate ± SEM. Data are representative of at least 3 independent experiments. *P < .03. (D) Immunoblot analysis of lysates of monocytes transfected with human Beclin1 siRNA or control siRNA probed with anti–Caspase-3, anti-Beclin1, and anti-actin Abs. Data are representative of at least 3 experiments.
Inhibition of autophagy suppresses GM-CSF–induced human primary monocyte survival. (A) Cell death of human monocytes with different treatments (GM-CSF, 10nM; 3-MA, 10mM; or CQ, 50 mM) for 48 hours was assessed using annexin V/PI staining and flow cytometry. Percentage of annexin V/PI double-negative cells (percentage survival) are shown. Dead cells indicate all cells positive for annexin V, including annexin V+/PI− early apoptosis and annexin V+/PI+ later apoptosis (A,C). Error bars indicate ± SEM. Data are representative of at least 3 independent experiments. (B) Immunoblot analysis with Abs to LC3, Caspase-3, and actin for GM-CSF–treated primary human monocytes. Representative data are shown for GM-CSF alone and pretreatment with 3-MA (10mM) or CQ (50μM). (C) Cell death assessed by flow cytometry of annexin V/PI–stained cells with Beclin1 siRNA or control siRNA in treated or not with GM-CSF. The percentage of annexin V/PI double-negative cells (percentage survival) is shown. Error bare indicate ± SEM. Data are representative of at least 3 independent experiments. *P < .03. (D) Immunoblot analysis of lysates of monocytes transfected with human Beclin1 siRNA or control siRNA probed with anti–Caspase-3, anti-Beclin1, and anti-actin Abs. Data are representative of at least 3 experiments.
Autophagy is required for monocyte differentiation
We next investigated the effect of autophagy on the differentiation of monocytes. Morphologically, undifferentiated monocytes were loosely adherent round cells, whereas monocytes cultured with GM-CSF differentiated into clusters of macrophages. Untreated monocytes died gradually (supplemental Figure 9). After GM-CSF stimulation, monocytes remained as round, floating cells on the first day; started to become bigger, adherent cells by day 3; and finally differentiated into tightly adherent, egg-like macrophages by day 6 (Figure 5A left panels). The presence of 3-MA resulted in the loss of these macrophage clusters, and many cells died by day 6 (Figure 5A middle panels). CQ also had an inhibitory effect on GM-CSF–induced differentiation (Figure 5A right panels). Although some living cells remained in the CQ-treated group at later time points, most of the cells did not fully differentiate into macrophages. FACS analysis showed that both 3-MA and CQ blocked the expression of the characteristic macrophage markers CD14 and CD11b in the GM-CSF–treated cells (Figure 5B). Functionally, GM-CSF–induced macrophages can further differentiate into M1-type activated macrophages after LPS and IFN-γ treatment, which induced an Ag-presenting function.34 We detected the dramatic increase in the Ag-presenting maker CD86 after 24 hours of LPS and IFN-γ treatment in GM-CSF–derived macrophages (Figure 5C top panels). However, in the cells treated with 3-MA or CQ, CD86 expression was substantially lower (Figure 5C middle and bottom panels). In addition, both 3-MA and CQ blocked the LPS/IFN-γ–mediated up-regulation of the inflammatory cytokines TNFα and IL-12 (Figure 5D). Blockage of macrophage marker CD11b expression (supplemental Figure 10A), LPS/IFN-γ–activated CD86 (supplemental Figure 10B), and up-regulation of inflammatory cytokines (supplemental Figure 10C) were observed when Beclin1 was knocked down in GM-CSF–treated cells. Tthese data suggest that monocytes in which autophagy was inhibited failed to differentiate into functional macrophages.
Autophagy is required for human primary monocyte differentiation. (A) Morphological changes associated with GM-CSF–induced monocyte/macrophage differentiation from days 1, 3, and 6. Representative phase-contrast images are shown for GM-CSF treatment alone (left panel) and for pretreatment with 3-MA (middle panel) or CQ (right panel). Scale bar indicates 100 μm. (B) Representative FACS plots showing CD14 and CD11b expression in GM-CSF–treated monocytes/macrophages treated or not with 3-MA or CQ. Live Cells were gated and stained with FITC–anti-CD14 Ab and PE–anti-CD11b Ab. (C) Representative FACS plots showing LPS/IFN-γ–stimulated CD86 expression in GM-CSF macrophages pretreated or not with 3-MA or CQ. Gated live cells stained with PE–anti-CD86 Ab are shown. (D) Quantitative real-time PCR analysis of human TNF-α and IL-12p40 mRNA in macrophages pretreated with 3-MA or CQ, presented relative to GAPDH mRNA. P < .005 for comparisons between GM-CSF alone and cotreatment with 3MA or CQ for both graphs. Data are representative of 5 (A) or 3 (B-D) experiments. Histogram shows mean ± SD in panel D.
Autophagy is required for human primary monocyte differentiation. (A) Morphological changes associated with GM-CSF–induced monocyte/macrophage differentiation from days 1, 3, and 6. Representative phase-contrast images are shown for GM-CSF treatment alone (left panel) and for pretreatment with 3-MA (middle panel) or CQ (right panel). Scale bar indicates 100 μm. (B) Representative FACS plots showing CD14 and CD11b expression in GM-CSF–treated monocytes/macrophages treated or not with 3-MA or CQ. Live Cells were gated and stained with FITC–anti-CD14 Ab and PE–anti-CD11b Ab. (C) Representative FACS plots showing LPS/IFN-γ–stimulated CD86 expression in GM-CSF macrophages pretreated or not with 3-MA or CQ. Gated live cells stained with PE–anti-CD86 Ab are shown. (D) Quantitative real-time PCR analysis of human TNF-α and IL-12p40 mRNA in macrophages pretreated with 3-MA or CQ, presented relative to GAPDH mRNA. P < .005 for comparisons between GM-CSF alone and cotreatment with 3MA or CQ for both graphs. Data are representative of 5 (A) or 3 (B-D) experiments. Histogram shows mean ± SD in panel D.
GM-CSF induces JNK activation to mediate the disassociation of Beclin1 and Bcl-2, the induction of autophagy, and monocyte survival
All 3 MAPKs are known to be activated during monocyte-macrophage differentiation.35-37 To investigate the potential mechanism of GM-CSF–induced autophagy in monocytes, we investigated whether any of these 3 MAPKs are involved in this process using the specific inhibitor of each MAPK pathway. Whereas GM-CSF–stimulated LC3-II generation was blocked by the JNK inhibitor SP600125 (SP), it was not prevented by U0126 or SB203580 (SB), inhibitors of the ERK and p38 MAPK, respectively (Figure 6A). M-CSF–induced LC3 processing was also blocked by SP (supplemental Figure 11A). These data suggest that JNK activation might be required for autophagy induction. To explore the role of JNK in the induction of autophagy, we first confirmed JNK activation by GM-CSF. JNK activation in human primary monocytes could be detected as early as 5 minutes and peaked 2 hours after GM-CSF treatment, as determined with an anti–phospho-JNK Ab (Figure 6B). The activation of JNK was blocked by the JNK inhibitor SP (supplemental Figure 12A). Because Beclin1 function in autophagy is inhibited by Bcl-2 through its interaction with Beclin1,18 and because the association of Beclin1 and Bcl-2 is modulated by JNK-mediated phosphorylation of Bcl-2,38 we then investigated the phosphorylation of Bcl-2 and the interaction of Beclin1 and Bcl-2 in GM-CSF–treated monocytes. With a specific anti–phospho-Bcl-2 Ab (P-S70), Bcl-2 phosphorylation was detected in GM-CSF–treated monocytes, but not in the untreated cells (Figure 6C). We next examined the interaction of Beclin1 and Bcl-2 in cells treated or not with GM-CSF for 6 hours because Bcl-2 phosphorylation peaked at this time point (Figure 6C). Co-immunoprecipitation experiments with an anti–Bcl-2 Ab revealed that Bcl-2 bound to Beclin1 in nonstimulated monocytes, and that this interaction was diminished after GM-CSF treatment (Figure 6D). Co-immunoprecipitation with an anti-Beclin1 Ab confirmed that GM-CSF triggered the Beclin1-PI3KC3 interaction but disrupted the Bcl-2–Beclin1 interaction (supplemental Figure 13A). The Bcl-2–Beclin1 disassociation was maintained until at least 14 hours after treatment (supplemental Figure 13B). More importantly, inhibition of JNK activation by the JNK inhibitor SP significantly reduced the disassociation of Beclin1 and Bcl-2 (Figure 6D). These results indicate that GM-CSF likely induces autophagy by activating JNK and, subsequently, the release of Beclin1 from Bcl-2 during monocyte differentiation.
GM-CSF induces JNK activation to mediate the disassociation of Beclin1 and Bcl-2, the induction of autophagy, and monocyte survival. (A) Immunoblot analysis with anti-LC3 and actin for monocytes pretreated with JNK inhibitor SP (10μM), MEK inhibitor U0126 (10μM), or p38 inhibitor SB (5μM), and then treated with GM-CSF for 14 hours. (B) Immunoblot analysis with Abs to phosphorylated JNK or JNK of monocytes treated with GM-CSF for the indicated times. (C) Human monocytes were treated with or without GM-CSF for the indicated times and lysates were subjected to immunoblot analysis with Abs to phosphorylated Bcl-2 and actin. (D) Western blots of endogenous Beclin1 co-immunoprecipitated by anti–Bcl-2 Ab. (E) Human monocytes pretreated with the JNK inhibitor SP (10μM) in the presence or absence of GM-CSF for the indicated times and lysates were subjected to immunoblot analysis with Abs to phosphorylated Bcl-2, LC3, and Actin. (F) Cell death assessed by annexin V/PI staining showing apoptosis in monocytes untreated or pretreated with JNK inhibitor SP in the presence or absence of GM-CSF for 48 hours. Percentage of annexin V/PI double-negative cells (percentage survival) are shown. Dead cells indicate all cells positive for annexin V, including annexin V+/PI− early apoptosis and annexin V+/PI+ later apoptosis. Error bars indicate± SEM. Data are representative of 3 independent experiments. (G) Lysates were subjected to immunoblotting with Caspase-3 and actin Abs. Western blot data are representative of at least 3 experiments.
GM-CSF induces JNK activation to mediate the disassociation of Beclin1 and Bcl-2, the induction of autophagy, and monocyte survival. (A) Immunoblot analysis with anti-LC3 and actin for monocytes pretreated with JNK inhibitor SP (10μM), MEK inhibitor U0126 (10μM), or p38 inhibitor SB (5μM), and then treated with GM-CSF for 14 hours. (B) Immunoblot analysis with Abs to phosphorylated JNK or JNK of monocytes treated with GM-CSF for the indicated times. (C) Human monocytes were treated with or without GM-CSF for the indicated times and lysates were subjected to immunoblot analysis with Abs to phosphorylated Bcl-2 and actin. (D) Western blots of endogenous Beclin1 co-immunoprecipitated by anti–Bcl-2 Ab. (E) Human monocytes pretreated with the JNK inhibitor SP (10μM) in the presence or absence of GM-CSF for the indicated times and lysates were subjected to immunoblot analysis with Abs to phosphorylated Bcl-2, LC3, and Actin. (F) Cell death assessed by annexin V/PI staining showing apoptosis in monocytes untreated or pretreated with JNK inhibitor SP in the presence or absence of GM-CSF for 48 hours. Percentage of annexin V/PI double-negative cells (percentage survival) are shown. Dead cells indicate all cells positive for annexin V, including annexin V+/PI− early apoptosis and annexin V+/PI+ later apoptosis. Error bars indicate± SEM. Data are representative of 3 independent experiments. (G) Lysates were subjected to immunoblotting with Caspase-3 and actin Abs. Western blot data are representative of at least 3 experiments.
Treatment of cells with the JNK inhibitor SP greatly reduced GM-CSF–stimulated LC3-II production (Figure 6A,E) and Bcl-2 phosphorylation (Figure 6E). Inhibition of JNK activation markedly blocked GM-CSF–induced cell survival (Figure 6F and supplemental Figure 12B) and abolished the GM-CSF–induced inhibition of Caspase-3 cleavage (Figure 6G). The effect of the JNK inhibitor SP was confirmed by knocking down JNK1 and JNK2 with JNK1/2 siRNAs (supplemental Figure 12C). JNK1/2 siRNAs also reduced GM-CSF–induced LC3-II processing and monocyte survival (supplemental Figure 12C-D). The JNK inhibitor SP also dramatically blocked the GM-CSF–induced p62 induction (supplemental Figure 11B). These results suggest that JNK activation is critical for the induction of autophagy and protection from cell death.
GM-CSF blocks cleavage of the autophagy protein Atg5
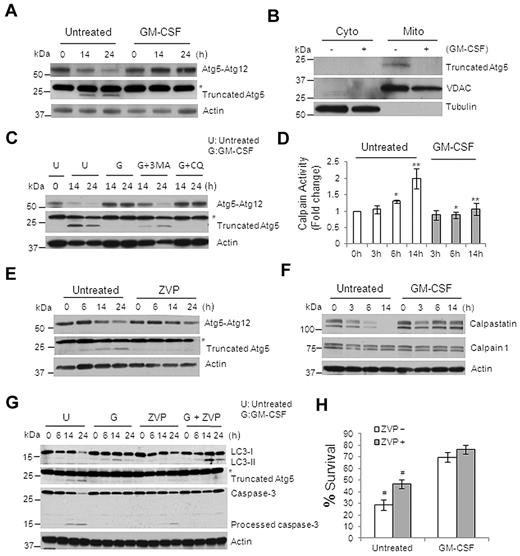
Because Atg5 is a protein that plays an important role in autophagy, and because the cleavage product of Atg5 promotes apoptosis,20,21 we next examined the expression of Atg5 protein in monocytes treated or not with GM-CSF. A truncated form of Atg5 matching the size of the reported apoptosis-promoting cleaved Atg5 was produced, and the level of Atg5-Atg12 conjugate required for autophagy occurrence was decreased in untreated monocytes (Figure 7A). However, GM-CSF treatment stabilized the level of the Atg5-Atg12 conjugate and blocked the generation of the truncated Atg5 (Figure 7A). Similar results were obtained in M-CSF–treated monocytes (supplemental Figure 14). It has been reported previously that the truncated Atg5 localizes to the mitochondria and promotes mitochondrial-mediated apoptosis.21 To determine whether the cleaved product of Atg5 in untreated monocytes was in the mitochondria, we performed cell fractionation experiments and only detected the truncated-Atg5 protein in the mitochondrial fraction of untreated monocytes, not in the cytosolic fraction or in the treated cells (Figure 7B). The addition of 3-MA, but not CQ, prevented GM-CSF from blocking the generation of the truncated Atg5 (Figure 7C). This result indicates that preventing the generation of the proapoptotic Atg5 fragment by GM-CSF happens at the early phase of autophagy induction because CQ inhibits autophagy at later stage of the process.
GM-CSF blocks the cleavage of apoptosis-related truncated Atg5 by inhibiting calpain activity. (A) Immunoblot analysis with Abs to Atg5 or truncated Atg5 in lysates of human primary monocytes treated or not with GM-CSF. (B) Immunoblot analysis of subcellular fractionations (cytoplasm and mitochondria) of human monocytes that were or were not treated with GM-CSF for 14 hours, probed with anti-truncated Atg5, anti-VADC, and anti–β-Tubulin. (C) Immunoblot analysis with Abs to truncated Atg5 in human primary monocytes treated or not treated with GM-CSF. Where indicated, cells were pretreated with 3-MA (10μM) or CQ (50μM) for the indicated times. (D) Calpain activity assay of lysates from monocytes treated or not with GM-CSF. Error bars indicate ± SEM. Data are representative of 5 independent experiments, *P < .02; **P < .002. (E) Immunoblot analysis with Abs to Atg5 or truncated Atg5 in lysates from human primary monocytes treated or untreated with the calpain inhibitor ZVP (10μM). (F) Immunoblot analysis with Abs to Calpain1, calpastatin, and Actin in lysates of GM-CSF–incubated monocytes. (G) Immunoblot analysis with Abs to LC3, truncated Atg5, Caspase-3, and actin in human primary monocytes lysates treated with GM-CSF and ZVP (10μM). (H) Histogram of cell death assessed by flow cytometry of annexin V/PI–stained cells treated or not with GM-CSF and ZVP for 24 hours. The percentages of annexin V/PI double-negative cells (percentage survival) are shown. Dead cells indicate all cells positive for annexin V, including annexin V+/PI− early apoptosis and annexin V+/PI+ later apoptosis. Error bars indicate ± SEM. Data are representative of at least 3 independent experiments. #P < .02. Western blot data are representative of at least 3 (A-C,E) or 5 (F-G) experiments. Asterisk indicates a nonspecial band of truncated Atg5 Ab (A,C,E,G).
GM-CSF blocks the cleavage of apoptosis-related truncated Atg5 by inhibiting calpain activity. (A) Immunoblot analysis with Abs to Atg5 or truncated Atg5 in lysates of human primary monocytes treated or not with GM-CSF. (B) Immunoblot analysis of subcellular fractionations (cytoplasm and mitochondria) of human monocytes that were or were not treated with GM-CSF for 14 hours, probed with anti-truncated Atg5, anti-VADC, and anti–β-Tubulin. (C) Immunoblot analysis with Abs to truncated Atg5 in human primary monocytes treated or not treated with GM-CSF. Where indicated, cells were pretreated with 3-MA (10μM) or CQ (50μM) for the indicated times. (D) Calpain activity assay of lysates from monocytes treated or not with GM-CSF. Error bars indicate ± SEM. Data are representative of 5 independent experiments, *P < .02; **P < .002. (E) Immunoblot analysis with Abs to Atg5 or truncated Atg5 in lysates from human primary monocytes treated or untreated with the calpain inhibitor ZVP (10μM). (F) Immunoblot analysis with Abs to Calpain1, calpastatin, and Actin in lysates of GM-CSF–incubated monocytes. (G) Immunoblot analysis with Abs to LC3, truncated Atg5, Caspase-3, and actin in human primary monocytes lysates treated with GM-CSF and ZVP (10μM). (H) Histogram of cell death assessed by flow cytometry of annexin V/PI–stained cells treated or not with GM-CSF and ZVP for 24 hours. The percentages of annexin V/PI double-negative cells (percentage survival) are shown. Dead cells indicate all cells positive for annexin V, including annexin V+/PI− early apoptosis and annexin V+/PI+ later apoptosis. Error bars indicate ± SEM. Data are representative of at least 3 independent experiments. #P < .02. Western blot data are representative of at least 3 (A-C,E) or 5 (F-G) experiments. Asterisk indicates a nonspecial band of truncated Atg5 Ab (A,C,E,G).
Because calpain is known to cleave Atg5,21 we sought to determine the activity of calpains in treated and untreated monocytes. Untreated monocytes showed an increase in calpain activity, whereas GM-CSF–treated cells did not (Figure 7D). This kinetic profile matched the generation of the Atg5 truncation (Figure 7A,C) and the time course of Caspase-3 cleavage (Figures 4B and 6G). Treatment of monocytes with the calpain inhibitor Z-Val-Phe methyl ester (ZVP) largely prevented the generation of truncated Atg5 and stabilized the Atg5-Atg12 conjugate (Figure 7E). To determine how calpain activity was regulated, we examined the expression of calpains, along with calpastatin, an endogenous inhibitor of calpains. Whereas the Calpain1 level remained unchanged, calpastatin expression gradually decreased in untreated cells (Figure 7F); however, in GM-CSF–treated cells, the calpastatin protein level was stable (Figure 7F). 3-MA, but not CQ, substantially blocked GM-CSF–induced calpastatin stabilization (supplemental Figure 15A) and increased Calpain activity (supplemental Figure 15B). The JNK inhibitor SP slightly reduced the calpastatin level (supplemental Figure 15C). Because the proteosome inhibitor MG132 blocked calpastatin decrease in the untreated monocytes, this decrease was probably mediated by changes in protein stability (supplemental Figure 15D). In addition to reducing the generation of the truncated Atg5, ZVP enhanced the production of LC3-II in untreated monocytes and partially prevented the generation of the cleaved Caspase-3 (Figure 7G). Moreover, ZVP increased cell survival by almost 2-fold in untreated monocytes, but had no effect on survival in GM-CSF–treated cells (Figure 7H and supplemental Figure 16). Treating monocytes with both GM-CSF and ZVP further enhanced LC3-II accumulation compared with GM-CSF alone (Figure 7G). These data suggest that GM-CSF prevents the generation of the proapoptotic Atg5 fragment through modulation of calpain activity, and that calpain activity may likewise exert an inhibitory effect on autophagy by cleavage of Atg5.
Discussion
Macrophages are chief participants in host inflammatory responses. Therefore, deregulation of macrophage differentiation and function may lead to disease, including autoimmune disease and cancer.39,40 The molecular processes that occur during monocyte-macrophage differentiation, particularly in the human system, are not completely understood. Primary monocytes have characteristics that are different from monocyte-related cell lines and are programmed to undergo apoptosis in the absence of stimulation both in vitro and in vivo.2-5 Therefore, it was important to carry out our studies in primary monocytes, because these cells have to conquer spontaneous apoptosis during the differentiation process. The results of the present study show that autophagy is induced during monocyte differentiation, and that the prevention of default apoptosis by autophagy in monocytes is pivotal for the differentiation process. Inhibition of differentiation-induced autophagy leads to apoptosis. We found that autophagy and its protection from cell death occurred irrespective of whether monocytes were differentiated with GM-CSF, M-CSF, or the combination of GM-CSF and IL-4. We made similar findings in both human elutriated monocytes and mouse BM monocytes, suggesting that our discovery is a general phenomenon during monocyte-macrophage differentiation.
Studies investigating the molecular mechanisms of cross-talk between apoptosis and autophagy are still in their infancy. However, recent data have revealed possible molecular mechanisms that link these processes. Bcl-2, the well-characterized apoptosis guardian, appears to be important in autophagy, because it binds to Beclin1/Atg6 and inhibits Beclin1-mediated autophagy.18,41,42 We observed a strong interaction between Beclin1 and Bcl-2 in unstimulated monocytes, and when monocytes were incubated with GM-CSF, the Beclin1 and Bcl-2 interaction was disrupted, leading to the release of Beclin1 and the formation of the kinase complex Beclin1-PI3KC3 and autophagy. The modification of LC3 and the disassociation of Beclin1 from Bcl-2 were first detected after 3 hours of GM-CSF treatment, indicating the onset of autophagy. Starvation-induced disassociation of Beclin1 from Bcl-2 was shown previously to involve the phosphorylation of Bcl-2 by JNK1 on multiple residues of the protein (T69, S70, and S87).38,41 We have now shown that the induction of autophagy during monocyte differentiation is also dependent on JNK1 activation and is associated with S70 phosphorylation of Bcl-2, because JNK inhibition blocked the phosphorylation of Bcl-2, the disassociation of Beclin1 and Bcl-2, and the production of LC3-II. Like the direct autophagic inhibitors, 3-MA and CQ, prevention of autophagy by JNK inhibition led to Caspase-3 cleavage and cell death in GM-CSF–treated monocytes, indicating that this was a crucial pathway for survival. Whereas the MEK/ERK and p38 signaling pathways have been previously linked to autophagy,43-46 ERK and p38 inhibitors had no effect on the GM-CSF–induced production of LC3-II.
Another molecule that may link apoptosis and autophagy during monocyte-macrophage differentiation is Atg5. Atg5 conjugates with Atg12 and localizes to autophagophores dissociating just before or after completion of autophagosome formation, and is required for targeting of LC3 to the isolation membranes.47 It has also been proposed to have a role in apoptosis,12,20,48 because a 24-kDa truncation (comprising residues 1-193) mediated by calpain promotes apoptosis through an interaction with mitochondrial proteins.21 In agreement with the results of the present study, a recent study showed that Calpain1 plays an important role in controlling autophagy by regulating the levels of full-length Atg5 and the Atg5-Atg12 conjugate.49 Silencing of Calpain1 or the use of a calpain inhibitor prevents the cleavage of Atg5 and increases the Atg5-Atg12 conjugate and autophagy.49 We found that GM-CSF treatment prevented the cleavage of Atg5 in part by blocking the degradation of endogenous calpain inhibitor calpastatin, which in turn inhibited calpain activity and stabilized the level of conjugated Atg5-Atg12 and thereby promoted autophagy. Therefore, in monocytes lacking differentiation stimuli, truncated Atg5 was produced and the Atg5-Atg12 conjugate was decreased. Subcellular fractionation of monocytes showed that truncated Atg5 was located in monocyte mitochondria, suggesting that it enables mitochondrial-mediated apoptosis.
According to our present findings, most monocytes do not survive when autophagy is blocked during differentiation, especially when blocked in its early stages by 3-MA. However, a small number of cells survived after 7 days of GM-CSF treatment in both 3-MA– and CQ-treated cells. Compared with the 3-MA group, more cells survived in the CQ group, possibly because it was less robust at preventing the decrease of truncated Atg5 by GM-CSF. Although some cells survived, the surviving cells that were treated with the autophagy inhibitors lacked functional differentiation compared with cells treated with GM-CSF alone. In 3-MA– or CQ-treated cells, the macrophage differentiation marker CD14 was undetectable and CD11b expression was lower than fully differentiated macrophages. In addition, these cells did not respond well to LPS/IFN-γ, suggesting that autophagy may be involved not only in the prevention of cell death, but also more directly in the differentiation process. In addition, we found that GM-CSF–induced autophagy is fully functional and can mediate protein degradation, because both p62 and DQ-BSA were degraded by autophagolysosome in activated monocytes. We are currently investigating how autophagy regulates monocyte/macrophage differentiation, particularly, what proteins are the targets of autophagy that engage monocytes to differentiate.
It has been known for some time within the field of immunology that short-term serum starvation of primary monocytes facilitates their differentiation into macrophages. Indeed, many laboratories incorporate serum starvation in their standard in vitro differentiation protocols. Although we did not serum starve the monocytes in the present study, we have used this technique previously, and would agree that short-term serum starvation does in fact render monocytes more likely to undergo macrophage differentiation, as measured by the various standard methods. In agreement with our present results, we found that 2-hour serum starvation of monocytes was sufficient to induce autophagy, as measured by LC3-II formation (data not shown). Therefore, given our current data, we propose that one of the ways in which differentiation is potentiated by serum starvation is through the induction of autophagy.
The autophagic inhibitor CQ has long been used in the treatment of rheumatoid arthritis. The mechanisms that render this treatment is effective have not been completely elucidated, but are believed to work in part by preventing the release of TNFα and other proinflammatory cytokines from monocytes and macrophages.50 Because macrophages produce larger amounts of proinflammatory cytokines than do monocytes, our present data also suggest that an additional reason that CQ treatment is effective in the treatment of this disease is because of its ability to inhibit macrophage differentiation and maturation through blocking autophagy.
In conclusion, in the present study, we have shown that autophagy is important during monocyte-macrophage differentiation. Our findings support a novel function of autophagy in monocyte-macrophage differentiation, which will be helpful in understanding the role of autophagy in infectious, autoimmune, and inflammatory diseases.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor Hans-Uwe Simon (University of Bern, Bern, Switzerland) for help with the Atg5 study; the National Institutes of Health (NIH) Blood Bank for elutriated monocytes; Barbara J. Taylor and Subhadra Banerjee (National Cancer Institute [NCI], NIH, Bethesda, MD) for help with cell sorting; Yvona Ward and Ross Lake (NCI) for help with confocal images; and Kunio Nagashima (SAIC-Frederick, NCI) for help with electron micrographs.
This study was supported by the Intramural Research Program of The Center for Cancer Research (NCI).
National Institutes of Health
Authorship
Contribution: Y.Z. designed and performed the research, analyzed the data, and wrote the manuscript; M.J.M. designed part of the research and revised the manuscript; K.C. and S.C. performed part of the research; and Z.-g.L. (adviser for Y.Z.) designed the research; contributed vital reagents, instruments, and analytical tools; analyzed data; and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zheng-gang Liu, Cell and Cancer Biology Branch, NCI, NIH, Bldg 37, Rm 1130, 37 Convent Dr, Bethesda, MD 20892; e-mail: zgliu@helix.nih.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal