Abstract
Familial hemophagocytic lymphohistiocytosis (FHL) is a life-threatening disorder of immune regulation caused by defects in lymphocyte cytotoxicity. Rapid differentiation of primary, genetic forms from secondary forms of hemophagocytic lymphohistiocytosis (HLH) is crucial for treatment decisions. We prospectively evaluated the performance of degranulation assays based on surface up-regulation of CD107a on natural killer (NK) cells and cytotoxic T lymphocytes in a cohort of 494 patients referred for evaluation for suspected HLH. Seventy-five of 77 patients (97%) with FHL3-5 and 11 of 13 patients (85%) with Griscelli syndrome type 2 or Chediak-Higashi syndrome had abnormal resting NK-cell degranulation. In contrast, NK-cell degranulation was normal in 14 of 16 patients (88%) with X-linked lymphoproliferative disease and in 8 of 14 patients (57%) with FHL2, who were identified by diminished intracellular SLAM-associated protein (SAP), X-linked inhibitor of apoptosis protein (XIAP), and perforin expression, respectively. Among 66 patients with a clinical diagnosis of secondary HLH, 13 of 59 (22%) had abnormal resting NK-cell degranulation, whereas 0 of 43 had abnormal degranulation using IL-2–activated NK cells. Active disease or immunosuppressive therapy did not impair the assay performance. Overall, resting NK-cell degranulation below 5% provided a 96% sensitivity for a genetic degranulation disorder and a specificity of 88%. Therefore, degranulation assays allow a rapid and reliable classification of patients, benefiting treatment decisions.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome characterized by excessive activation of macrophages and T cells.1-3 Familial hemophagocytic lymphohistiocytosis (FHL2-5) is caused by mutations in PRF1, UNC13D, STX11, and STXBP2, respectively.4-8 These genes all encode proteins required for lymphocyte cytotoxicity.9,10 In addition, Griscelli syndrome type 2 (GS2) and Chediak-Higashi syndrome (CHS), caused by mutations in RAB27A and LYST, respectively, are also associated with the development of HLH,11,12 display impaired lymphocyte cytotoxicity,11,13,14 and also manifest partial albinism. Furthermore, patients with X-linked lymphoproliferative syndrome (XLP) caused by mutations in SH2D1A and XIAP, respectively,15,16 may present with HLH,17 although an overt defect in lymphocyte cytotoxicity is not usually observed.16,18 Apart from these genetically defined familial or primary forms of the disease, patients without mutations in the known genes can also develop HLH. These may be secondary forms that frequently arise in the context of infections, severe autoimmune disease, or hematopoietic malignancies.2,19-21 It is difficult to distinguish between primary and secondary HLH, because the clinical features are identical and both forms are often triggered by an infection. Nevertheless, rapid identification of patients with primary disease is crucial because hematopoietic stem cell transplantation (HSCT) represents the only curative treatment.22
Currently, diagnosis of HLH is based on a set of clinical and laboratory criteria.23 Obtaining a molecular diagnosis for primary HLH is often time-consuming and costly. However, assays that assess cellular phenotype and function may rapidly differentiate between different inherited forms of HLH and guide sequencing efforts.24 Natural killer (NK)–cell cytotoxicity is the only functional immunologic parameter that has been evaluated systematically in regard to the clinical criteria; however, this assay has several limitations. First, the protocols used to study NK-cell cytotoxicity are labor intensive, usually involve radioactivity, and are not widely available. Second, many patients with HLH have few circulating NK cells. Defective NK-cell cytotoxicity can therefore reflect a reduced frequency rather than a reduced function of these cells. Third, although the NK-cell cytotoxicity assay has been used to subgroup HLH patients,25 it does not readily discriminate between primary and secondary HLH and therefore is not helpful for the differential diagnosis of the congenital forms of HLH.
In addition to cytotoxicity assays, flow cytometry–based assays that quantify perforin, SLAM-associated protein (SAP), and X-linked inhibitor of apoptosis (XIAP) expression can rapidly identify FHL2, XLP1, and XLP2 patients, respectively.26-28 The other genes associated with HLH all encode proteins implicated in the transport and exocytosis of lytic granules by cytotoxic lymphocytes. Therefore, assays quantifying lytic granule exocytosis are useful for the diagnosis of HLH patients.29-31 CD107a is a lysosomal protein that colocalizes with perforin in lytic granules of cytotoxic T lymphocytes (CTLs) and NK cells.32,33 Engagement of the TCR on CTLs and an array of activating receptors on NK cells can induce surface expression of CD107a, reflecting exocytosis of lytic granules.34-36 Degranulation assays quantifying CD107a surface expression have been applied to NK cells and CTLs for the diagnosis of the different forms of primary HLH.7,8,29-31 However, the performance of these assays in a large group of unselected patients referred for evaluation for HLH has not been tested. Moreover, the question of to what extent degranulation assays can help to discriminate between primary and secondary forms of HLH remains unanswered.
In the present study, we combined the experience of 4 European laboratories to define standardized user-friendly and robust protocols for the evaluation of NK-cell and CTL degranulation. These protocols were then evaluated prospectively in a large, unselected cohort of patients. We show that the CD107a based assays have a high sensitivity and specificity for the diagnosis of primary HLH associated with genetic disorders of granule exocytosis. The use of these functional assays cannot only significantly accelerate genetic diagnostics by facilitating more targeted sequencing, but can also guide therapy before a definite molecular diagnosis can be established.
Methods
Establishment of consensus protocols
In the European Union–funded Cure HLH project, 4 immunologic reference laboratories in Genoa, Italy; Stockholm, Sweden; London, United Kingdom; and Freiburg, Germany established standardized consensus protocols for the evaluation of patients with HLH. Previous experiences were discussed at a meeting in December 2008. Issues included technical details of the assays such as the Abs, reagents, and cell lines used, incubation times, and stimulation conditions, as well as reproducibility of the assays, normal values, display of results, utility of the assays in particular clinical settings, and confounding variables. We also considered practical issues in a diagnostic setting, such as sample transport and handling, person workload, and costs. Based on this discussion, consensus protocols were established, reevaluated, and implemented in a final version in January 2009. To assess inter-center variability, samples from 4 healthy blood donors were simultaneously analyzed in 3 of the participating laboratories. Institutional review board approval was obtained from all participating centers.
Patient samples
All patient samples that were referred to the participating laboratories in Stockholm (n = 159), Genoa (n = 92), and Freiburg (n = 220) for the evaluation of HLH between January 2009 and May 2011 were considered for analysis in this study. Patient samples in London were first analyzed with a screening test (n = 109),37 and only if abnormal or ambiguous were they analyzed with consensus protocol 2 (n = 23). Patient samples for which the same-day control sample obtained from a healthy donor showed abnormal results were excluded (n = 39 for resting and n = 7 for activated NK-cell degranulation).
The patient cohort included patients with the complete clinical picture of HLH,23 but also patients fulfilling only some clinical criteria, who were referred because HLH was considered a relevant differential diagnosis. Samples from patients with a proven genetic disease predisposing to HLH, but diagnosed in the absence of clinical symptoms of HLH because of an affected family member or because of other symptoms suggesting genetic disease (eg, partial albinism) were also included. The analysis also included a few samples from patients with a proven genetic diagnosis of FHL who had been analyzed before January 2009 with protocol 2 (see “Protocols for degranulation assays”). It was noted whether the samples were obtained from patients during acute disease or during remission and whether the patients received immunosuppressive therapy or treatment with the full HLH-2004 protocol23 at the time of analysis.
Patient classification
Molecular genetic analysis was performed for those patients in whom family history, consanguinity, or accompanying characteristics such as albinism, age at onset of HLH and its clinical course, or the results of the immunologic assays suggested genetic disease. Clinical follow-up information extending beyond 6 months after the immunologic tests was available for a significant number of the patients. On this basis, patients were retrospectively classified at the end of the study period into the following categories: (1) patients with proven genetic disease predisposing to HLH and affecting lymphocyte degranulation (ie, FHL3, FHL4, FHL5, CHS, and GS2); (2) patients with proven genetic disease predisposing to HLH not affecting lymphocyte degranulation (ie, FHL2, XLP1, and XLP2); (3) patients with secondary HLH (ie, patients who developed a single episode fulfilling the clinical criteria for HLH and sustained complete remission for at least 6 months after completion of HLH therapy); and (4) patients with complete or incomplete HLH and insufficient follow-up information to allow final classification. An overview of the center-specific distribution of the patients according to the different categories is provided in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Sample acquisition, handling, and PBMC isolation
Whole blood samples (5-10 mL) were collected by venous puncture in sodium heparin- or EDTA-containing vials and sent at room temperature with express mail delivery. All patient samples were sent with at least 1 healthy control sample. Within 24 hours of the venous puncture, PBMCs were isolated by Ficoll gradient centrifugation. For functional assays, cells were rested in complete medium (IMDM supplemented with 10% FBS and 1% penicillin/streptomycin/L-glutamine) for at least 2 hours. Cells were washed in FACS buffer (PBS supplemented with 2% FBS, 2mM EDTA, and 0.1% NaN3). Intracellular perforin, SAP, and XIAP stainings, in addition to cytotoxicity assays, were performed as detailed in supplemental Methods.
Cells and Abs
The target cell line K562 (ATCC) was maintained in complete medium. For flow cytometry, anti–CD3-PerCP (SK7, IgG1), anti-CD8–FITC (RPA-T8, IgG1), anti-CD16–FITC (3G8, IgG1), anti-CD56–APC (NCAM16.2, IgG2b), anti-CD107a–PE (H4A3, IgG1), anti-perforin–PE (δG9, IgG2b), and isotype mouse controls (MOPC-21, IgG1, and 27-35, IgG2b) mAbs were used (all BD Biosciences).
Protocols for degranulation assays
In Stockholm and Freiburg, sample delivery was possible before 10 am, allowing separation of PBMCs and functional analysis of resting NK cells on the same day (protocol 1). Phytohemagglutinin (PHA) blasts were generated to evaluate degranulation of both activated NK cells and CTLs. In London and Genoa, duration of shipment was more variable. Therefore, PBMCs were isolated and incubated overnight at 37°C in either medium alone or in medium supplemented with IL-2 to analyze at the same time resting or activated NK-cell degranulation, respectively (protocol 2). PHA blasts were used to evaluate CTL degranulation alone. Each laboratory generated its own reference values on at least 40 healthy donors. Step-by-step operating procedures are detailed in supplemental Methods. Data were acquired on a FACSCalibur (BD Biosciences) or a Navios (Beckman Coulter) flow cytometer and analyzed using FlowJo Version 7.6.5 or CellQuest Version 5.1 software (TreeStar and BD Biosciences, respectively). For analysis, CD3−CD56+ NK cells were gated and assessed for surface expression of CD107a. The term ΔCD107a is the difference between the percentage of NK cells expressing surface CD107a after K562 stimulation and the percentage of NK cells expressing surface CD107a after incubation with medium alone. For analysis of CTL degranulation, CD3+CD8+ T cells were gated and difference in the mean fluorescence intensity (MFI) of CD107a expression was compared with the unstimulated control sample.
Statistical analysis
Data were analyzed with the statistical programming environment R.38 Receiver operating characteristic (ROC) curves were used to graphically represent the relationship between sensitivity and specificity of a laboratory test over 2 diagnostic cutoff values. The graphics were generated using the R package pROC.39 Therefore, empirical ROC curves were built by moving over the range of all observed values, and corresponding confidence intervals were computed with bootstrap resampling. In addition, procedures were included to identify optimal thresholds or local maxima in sensitivity or specificity. Thresholds defined as optimal correspond to the best sum of sensitivity and specificity (ie, theYouden index).
Results
Evaluation of the consensus protocols for resting and activated NK-cell degranulation assays
NK-cell degranulation assays were performed on 494 patient samples in the 4 European laboratories. In 39 (8%) of the samples used for resting NK-cell analysis and in 7 (5%) of the samples used for activated NK-cell analysis, the day control was outside the normal range and the concomitantly analyzed patient samples were therefore not considered. This reflects the inherent nature of biologic assays that are sensitive to factors affecting the sample condition (ie, long delivery time or extreme heat or cold), as well as laboratory difficulties (ie, technical mistakes or reagent and instrument problems). Overall, reliable results were obtained when samples were delivered within 24 hours of venipuncture. Healthy controls showed a significant interindividual variability. The tenth percentile of normal controls was used as a center-specific lower limit of normal. This was 7% (Stockholm; n = 100) and 10% (Freiburg, n = 94) using fresh NK cells and 17% (Genoa, n = 54) and 15% (London, n = 37) using NK cells rested overnight in medium. Typically, CD107a staining was more than 10-fold increased on degranulating cells (Figure 1A). Prestimulation with IL-2 overnight or with PHA and IL-2 for 2-4 days consistently increased ΔCD107a levels with a 10th percentile above 30% using both protocols (n = 225, Figure 1C). No significant age-dependent variability was observed (including 6 samples analyzed in the first week of life) and the assay was informative using cord blood (n = 5; data not shown). However, because of the limited experience in this particular situation, it is suggested that, if abnormal, the assays should be repeated after the first 2 weeks of life. After HSCT, normal NK-cell degranulation was observed as early as 4 weeks after transplantation (n = 5). When a sample from the same healthy donor was simultaneously analyzed in 3 of the participating laboratories, some inter-laboratory variability was observed (Figure 1B,D), which may in part be explained by the fact that the K562 cells used in these assays were of different source and passage. Comparison of these different K562 cell lines in a single laboratory using 2 healthy donors revealed significant differences (supplemental Figure 1).
Evaluation of a consensus protocol for the analysis of NK-cell and CTL degranulation. (A-B) Degranulation of resting NK cells analyzed with protocol 1. (A) FACS plots illustrating the induction of CD107a expression in CD3−CD56+ NK cells using PBMCs from a healthy donor and a patient with FHL5 after incubation with medium or with NK-sensitive K562 target cells. (B) Results from the same sample analyzed in 3 different laboratories (▴, Genoa; □, Freiburg; and ●, Stockholm). (C-D) Degranulation of IL-2–stimulated NK cells. (C) NK-cell degranulation assay using PBMCs that had been stimulated for 48 hours with PHA and IL-2. (D) Results from the same sample analyzed in 3 different laboratories. (E-F) Degranulation of T-cell blasts. (E) FACS plots illustrating the induction of CD107a expression in 48h PHA/IL-2–stimulated CD8+ T cells from a healthy donor and from a patient with FHL5 after incubation with medium alone or medium plus anti-CD3/anti-CD28 beads. (F) Overlay of stimulated (white) and unstimulated (shaded gray) samples. Numbers inside quadrants represent ΔCD107a (A,C) and ΔMFI of CD107a (E-F). ΔCD107a indicates the difference in the percentage of cells expressing CD107a before stimulation subtracted from the percentage of cells expressing CD107a after stimulation. ΔMFI indicates the respective difference in MFI.
Evaluation of a consensus protocol for the analysis of NK-cell and CTL degranulation. (A-B) Degranulation of resting NK cells analyzed with protocol 1. (A) FACS plots illustrating the induction of CD107a expression in CD3−CD56+ NK cells using PBMCs from a healthy donor and a patient with FHL5 after incubation with medium or with NK-sensitive K562 target cells. (B) Results from the same sample analyzed in 3 different laboratories (▴, Genoa; □, Freiburg; and ●, Stockholm). (C-D) Degranulation of IL-2–stimulated NK cells. (C) NK-cell degranulation assay using PBMCs that had been stimulated for 48 hours with PHA and IL-2. (D) Results from the same sample analyzed in 3 different laboratories. (E-F) Degranulation of T-cell blasts. (E) FACS plots illustrating the induction of CD107a expression in 48h PHA/IL-2–stimulated CD8+ T cells from a healthy donor and from a patient with FHL5 after incubation with medium alone or medium plus anti-CD3/anti-CD28 beads. (F) Overlay of stimulated (white) and unstimulated (shaded gray) samples. Numbers inside quadrants represent ΔCD107a (A,C) and ΔMFI of CD107a (E-F). ΔCD107a indicates the difference in the percentage of cells expressing CD107a before stimulation subtracted from the percentage of cells expressing CD107a after stimulation. ΔMFI indicates the respective difference in MFI.
Evaluation of the consensus protocol for activated T-cell degranulation
The CTL degranulation assay was performed after 2-4 days of PHA/IL-2 activation (protocol 1) on 63 patient samples in Freiburg. This time point was chosen for an optimal parallel analysis of activated NK cells and CTLs, although the best results for CTL degranulation could be achieved at days 5-7. However, at days 5-7, there were too few NK cells remaining in the culture for the analysis of activated NK cells. To economize the workload, it was therefore decided to perform the activated CTL and NK-cell degranulation assays in parallel at a single time point after 2 days (up to 4 days, if sample delivery was close to a weekend) of culture. Different stimuli were compared for their ability to induce CD107a surface expression on activated NK cells and CTLs. Stimulation with anti-CD3/anti-CD28–coated beads induced higher CD107a expression than PHA, plate-bound anti-CD3, or Staphylococcal enterotoxin B (SEB) (not shown). Because CD107a expression was induced on the whole population of CD8+ T cells, and because the absolute (rather than the relative) increase of CD107a expression reflected the amount of granule exocytosis, we compared the MFI on unstimulated versus stimulated cells expressed as ΔMFI (Figure 1E-F). Healthy controls (n = 86) showed a ΔMFI in the reference range between 2.8 and 6.9 (10th to 90th percentile). Patient samples in which the day control was below 2.8 (n = 8) were not considered for analysis. Generation of PHA blasts was poor in some patients on immunosuppressive therapy. However, the test offered the possibility to evaluate degranulation in patients with too few NK cells for reliable analysis.
NK cell and CTL CD107a assays identify most patients with genetic disorders of degranulation
Among the patients for whom a genetic diagnosis of FHL3, FHL4, or FHL5 could be established, 75 of 77 patients (97%) displayed abnormal resting NK-cell degranulation (Figure 2A-C). Defective degranulation was arbitrarily defined as less than 5% degranulation, whereas abnormal degranulation was defined as being lower than 10% (corresponding to the 10th percentile of healthy controls, see “Statistical analysis” section below) but equal to or higher than 5%. Normal degranulation was observed in 1 patient with early-onset FHL4 and borderline degranulation in 1 patient with late-onset FHL5. Overall, 68 of 77 patients (88%) with FHL3-5 displayed defective NK-cell degranulation. This included a significant number of patients who manifested with HLH beyond the second year and as late as 35 years of life.40-42 Degranulation of activated NK cells was outside the normal range in 68 of 80 patients (85%) with FHL3-5 (Figure 2D-F), of which 64 of 80 patients (80%) displayed degranulation below 20%. Recovery of degranulation after IL-2 stimulation was observed in all 3 genetic forms of the disease and was more frequent in FHL4 or FHL5 patients (associated with late onset of disease). CTL degranulation was abnormal (ie, below the 10th percentile of healthy controls) in 20 of 23 patients (87%) with FHL3 or FHL5 (Figure 2G-H). We also analyzed 7 patients with a genetic diagnosis of CHS and 6 patients with GS2 (Figure 3A-C). Of these, 10 of 13 patients (77%) had defective and 1 of 13 patients (8%) had abnormal degranulation (Figure 3A). Degranulation of activated NK cells (Figure 3B) was abnormal in all but 1 patient and CTL degranulation (Figure 3C) in all investigated patients with GS2 or CHS.
NK-cell and CTL CD107a assays identify most patients with genetic disorders of degranulation. Results of CD107a degranulation assays using resting NK cells (A-C), IL-2–activated NK cells (D-F), or short-term CTL blasts (G-H) from patients with FHL3 (A,D,G), FHL4 (B,E), or FHL5 (C,F,H). ΔCD107a (%) indicates the difference in the percentage of cells expressing CD107a before stimulation subtracted from the percentage of cells expressing CD107a after stimulation; ΔCD107a (MFI), respective difference in MFI; Pr. 1, protocol 1; and Pr. 2, protocol 2. The gray shaded areas represent the range from the 10th to the 90th percentile of values obtained in healthy donors. Closed symbols represent patients manifesting with HLH before age 2; open symbols indicate manifestation of HLH after age 2.
NK-cell and CTL CD107a assays identify most patients with genetic disorders of degranulation. Results of CD107a degranulation assays using resting NK cells (A-C), IL-2–activated NK cells (D-F), or short-term CTL blasts (G-H) from patients with FHL3 (A,D,G), FHL4 (B,E), or FHL5 (C,F,H). ΔCD107a (%) indicates the difference in the percentage of cells expressing CD107a before stimulation subtracted from the percentage of cells expressing CD107a after stimulation; ΔCD107a (MFI), respective difference in MFI; Pr. 1, protocol 1; and Pr. 2, protocol 2. The gray shaded areas represent the range from the 10th to the 90th percentile of values obtained in healthy donors. Closed symbols represent patients manifesting with HLH before age 2; open symbols indicate manifestation of HLH after age 2.
Impaired NK-cell and CTL degranulation in patients with immunodeficiency and albinism. Results of CD107a degranulation assays using resting NK cells (A), IL-2–activated NK cells (B), or short-term CTL blasts (C) from patients with CHS (triangles) and GS2 (circles). Closed symbols represent patients manifesting with HLH before age 2; open symbols indicate manifestation of HLH after age 2. For additional explanations, see legend to Figure 2.
Impaired NK-cell and CTL degranulation in patients with immunodeficiency and albinism. Results of CD107a degranulation assays using resting NK cells (A), IL-2–activated NK cells (B), or short-term CTL blasts (C) from patients with CHS (triangles) and GS2 (circles). Closed symbols represent patients manifesting with HLH before age 2; open symbols indicate manifestation of HLH after age 2. For additional explanations, see legend to Figure 2.
NK-cell and CTL degranulation assays are normal in most patients with SAP, XIAP, and perforin deficiency
FHL2 should not affect lytic granule exocytosis, because perforin constitutes part of the lytic granule content, whereas XLP1 and XLP2 have not been associated with impaired NK-cell cytotoxicity.16,18 Therefore, to evaluate the specificity of the degranulation assays, we analyzed patients with FHL2 (n = 14), XLP1 (n = 8), and XLP2 (n = 8). Unexpectedly, abnormal degranulation was observed in 6 of 14 patients (57%) with FHL2 using resting NK cells (Figure 4A), but defective only in one. In 6 FHL2 patients, degranulation was also abnormal with activated NK cells (Figure 4B). Degranulation of activated CTLs was only investigated in 1 patient and was normal. Among 16 patients with XLP1 or XLP2 identified by flow cytometric intracellular staining of SAP or XIAP and confirmed by sequencing,27,28 3 had slightly abnormal degranulation of resting NK cells (Figure 4C) and in 1 of 5 patients (20%) degranulation of activated NK cells was abnormal (Figure 4D). Degranulation of CTLs was normal in 1 patient tested. Overall, NK-cell degranulation assays were moderately reduced in some patients with perforin deficiency, but normal in most patients with XLP.
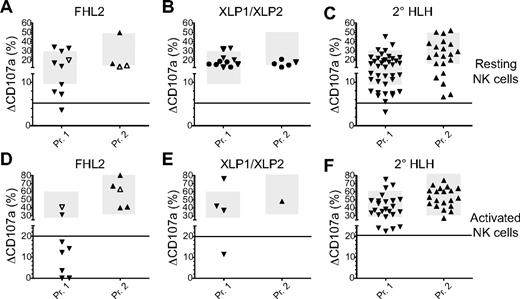
NK-cell degranulation assays are normal in most patients with FHL2, XLP, and secondary HLH. Results of CD107a degranulation assays using resting NK cells (A-C) and IL-2–activated NK cells (D-F) from patients with FHL2 (A,D), XLP1 and XLP2 (B,E), or secondary HLH (2° HLH; C,F). In panels A and C, closed symbols represent patients manifesting with HLH before age 2 and open symbols indicate manifestation of HLH after age 2. In panels B and E, triangles represent patients with XLP1 and circles represent patients with XLP2. For additional explanations, see legend to Figure 2.
NK-cell degranulation assays are normal in most patients with FHL2, XLP, and secondary HLH. Results of CD107a degranulation assays using resting NK cells (A-C) and IL-2–activated NK cells (D-F) from patients with FHL2 (A,D), XLP1 and XLP2 (B,E), or secondary HLH (2° HLH; C,F). In panels A and C, closed symbols represent patients manifesting with HLH before age 2 and open symbols indicate manifestation of HLH after age 2. In panels B and E, triangles represent patients with XLP1 and circles represent patients with XLP2. For additional explanations, see legend to Figure 2.
NK-cell and CTL degranulation assays in patients with secondary HLH
Patients who developed a single episode fulfilling the clinical criteria for HLH and sustained complete remission for at least 6 months after completion of HLH therapy were considered to have secondary HLH. This included patients with rheumatic disease, patients who were eventually diagnosed with malignancy, and patients with infectious diseases such as Leishmania and EBV. Thirteen of 59 patients (22%) with secondary HLH had defective or abnormal degranulation of resting NK cells (Figure 4E), whereas only 5 of 43 patients (12%) had degranulation of activated NK cells below the normal range (Figure 4F). Only 1 patient displayed defective NK-cell degranulation for resting cells and none had values below 20% for activated cells. No relevant difference in age, primary disease, or therapy could be discerned between secondary HLH patients with normal or abnormal degranulation (supplemental Table 2).
NK-cell and CTL degranulation assays are informative in patients undergoing immunosuppressive therapy
One important issue is whether the diagnostic assays are influenced by immunosuppressive therapy. To address this, we combined the results of all patients with FHL2, XLP1, XLP2, and secondary HLH (n = 59) for whom information on treatment was available and who were analyzed with 1 of the 2 protocols for resting NK-cell degranulation. We then compared patients who were investigated in the absence of immunosuppressive therapy (n = 25) with patients receiving immunosuppressive therapy at the time of blood sampling (n = 24) and with patients receiving the full HLH-2004 protocol (n = 10). There was no significant difference in the mean percentage of degranulating resting NK cells (Figure 5A). When paired samples for resting and activated NK-cell degranulation were analyzed, we found that immunosuppression did not have a relevant influence on the ability of PHA/IL-2 activation to increase degranulation (ie, a mean increase in ΔCD107a of 22% vs 22% in untreated patients). In contrast, treatment with the full HLH-2004 protocol had a significant impact (mean increase of 4%).
Immunosuppressive therapy does not significantly impair the performance of the degranulation assays. Results were pooled from those patients with FHL2, XLP, and secondary HLH for whom information on HLH-2004 or other immunosuppressive treatment (IS) at the time of analysis was available. (A) Analysis of resting NK cells. (B) Analysis of IL-2–activated NK cells.
Immunosuppressive therapy does not significantly impair the performance of the degranulation assays. Results were pooled from those patients with FHL2, XLP, and secondary HLH for whom information on HLH-2004 or other immunosuppressive treatment (IS) at the time of analysis was available. (A) Analysis of resting NK cells. (B) Analysis of IL-2–activated NK cells.
NK-cell and CTL degranulation in unclassified patients
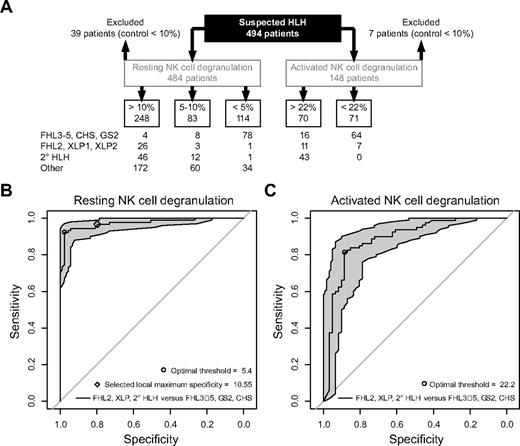
In a significant number of the patients, there was insufficient clinical follow-up information and incomplete genetic analysis to finally classify the case (Figure 6A). Most of these patients had infection-associated HLH or incomplete HLH and resting NK-cell degranulation in the normal range (n = 172) or between 5% and 10% (n = 60). However, there were 34 patients who displayed defective resting NK-cell degranulation (below 5%). Of these, 2 patients carried heterozygous UNC13D mutations, but protein expression had not been examined. Furthermore, 9 patients, 2 of whom displayed albinism, did not harbor mutations in the coding region of genes associated with HLH and defective degranulation. Three patients were diagnosed with Still disease and 1 with a fever syndrome, all of whom displayed normal function in all other assays and were not investigated further. One patient was diagnosed with Wolman disease. Finally, sequencing of genes is still ongoing in 10 patients, and in 8 patients, available clinical and genetic information was insufficient.
Summary of results obtained in this study and statistical evaluation. (A) Distribution of results in the different categories. “Other” indicates patients who either did not fulfill the criteria for HLH or for whom clinical and genetic information was insufficient for final classification. (B-C) Empirical ROC curves for resting (B) and activated (C) NK-cell degranulation assays based on a combined dataset generated with the 2 protocols.
Summary of results obtained in this study and statistical evaluation. (A) Distribution of results in the different categories. “Other” indicates patients who either did not fulfill the criteria for HLH or for whom clinical and genetic information was insufficient for final classification. (B-C) Empirical ROC curves for resting (B) and activated (C) NK-cell degranulation assays based on a combined dataset generated with the 2 protocols.
Statistical analysis
Altogether, 209 patients with suspected HLH were eligible to assess the diagnostic value of NK-cell degranulation. Empirical ROC curves were generated for both the resting and the activated NK-cell degranulation assays, including either separate datasets for protocol 1 (n = 141) and 2 (n = 68) or a combined dataset (Figure 6A). From these empirical curves, we have derived an optimal threshold: a cutoff point leading to the greatest sum of sensitivity and specificity. For the resting NK-cell assay, this optimal degranulation threshold was 5.4 for protocol 1, 5.8 for protocol 2, and 5.4 for the combined dataset (Figure 6B). For the activated NK-cell assay, the cutoff point was 21.9 for protocol 1, 34 for protocol 2, and 22.2 for the combined dataset (Figure 6C). These calculations are the basis for the 5% and 20% thresholds introduced in Figures 2 through 5. Overall, in testing secondary HLH, XLP, and FHL2 against FHL3-5, GS2, and CHS using a cutoff at 5% degranulation, the sensitivity was 96% and the specificity was 88%. Comparing patients manifesting with HLH at less than 2 years of age to those with later onset, sensitivity was comparable in the 2 age groups (94% and 97%, respectively), whereas specificity was higher in patients manifesting with HLH at less than 2 years of age (97% and 81%, respectively). Independent of age, the optimal specificity threshold for both protocols was identified at 10.55% (Figure 6B). These statistical findings provide a rational for defining resting NK degranulation below 5% as “defective” and values below 10% as “abnormal.”
Discussion
In the present study, we evaluated the performance of NK-cell and CTL degranulation assays in an unselected cohort of 468 patients being investigated for immunologic diagnostic evaluation of HLH and in 26 additional prescreened patients referred to 4 European reference laboratories. We established 2 different reference protocols. Protocol 1 foresees analysis of resting NK-cell degranulation on the day of sample processing and analysis of PHA/IL-2–stimulated NK cells and CTLs 2-4 days later. For protocol 2, only PBMC preparation was performed on the day of sample delivery, whereas analysis of resting and activated NK-cell degranulation was tested the following day after incubating PBMCs in either medium alone or in medium supplemented with IL-2, respectively. Moreover, in patients with few NK cells, PHA/IL-2 stimulation was performed to allow a functional assay on CTLs a few days later. Degranulation of resting NK cells from normal donors was higher after the overnight rest. Protocol 2 allowed later sample delivery. Overall, both protocols performed equally well, showing high sensitivity and specificity with regard to screening patients with genetic forms of HLH. When using the value of 5% degranulation as a cutoff, sensitivity was highest in patients below 2 years of age. Although the time required for genetic tests is decreasing, it cannot match functional tests that provide answers within 24 hours. Moreover, functional tests can direct genetic analyses and are particularly useful in situations in which conventional sequencing of exons only may miss splice-site mutations, deep intronic mutations, or other genetic aberrations that are frequent causes of FHL.43,44
Analysis of samples from the same donors in different laboratories (protocol 1) revealed some variations. For NK cells, this was at least in part because the K562 stimulator cells were of different source and passage. For CTLs, differences in the response to the PHA/IL-2 stimulation probably generated some variability. Nevertheless, the reference values obtained for healthy donors in the 4 laboratories, when related to those of patients with primary and secondary HLH, allowed a relatively simple classification of assay results. A cutoff at 10% degranulation for resting NK cells failed to identify only 2 of 77 patients (3%) with genetically determined degranulation deficiency, and this value represented the 10th percentile of the reference range in the laboratories using protocol 1 and the 5th percentile in the 2 laboratories using protocol 2. Patients with XLP1, XLP2, or “secondary” HLH showed normal values in most cases. Because most of these patients were analyzed at the time of active HLH, disease activity does not appear to be a major confounder for this assay. Moreover, most patients undergoing immunosuppressive treatment, including the full HLH-2004 protocol, had normal NK-cell degranulation, suggesting a limited impact of such therapy on the diagnostic value of this assay. Interestingly, 6 of 14 FHL2 patients (43%) displayed abnormal degranulation, which could reflect extrinsic factors such as anti-inflammatory cytokines subduing lymphocyte degranulation, intrinsic factors such as certain PRF1 mutations decreasing granule contents, or additional modifying mutations in genes affecting degranulation. Overall, although it remains essential to establish local reference values for this kind of functional assay, the threshold of 10% degranulation of resting NK cells for normal donors provides a reasonable estimation of what should be achieved.
Resting NK-cell degranulation between 5% and 10% was found in 9% of patients with genetic disease and in 22% of patients with secondary HLH (Figure 6). Although there was a clear trend toward a lower MFI of CD107a staining in patients with genetic degranulation disorders compared with those with secondary HLH, this was in several cases not sufficient to differentiate between the 2 diagnoses. The presence of monoallelic mutations causing a dose effect in degranulation needs to be addressed in future studies of patients with “secondary” HLH. For the individual patient, additional investigations have proven helpful. In most patients with genetic disease, assays with activated NK cells or CTLs also showed abnormal results. Nevertheless, we would recommend analyzing a repeat sample for patients with abnormal resting NK-cell degranulation (in the range of 5%-10%) before proceeding to genetic analysis unless the clinical situation demands prompt specific diagnosis. The interpretation can be difficult and should be done by an experienced reference laboratory.
Degranulation of resting NK cells was defective (below 5%) in 68 of 77 patients (88%) with an FHL variant of degranulation deficiency and in 10 of 13 patients (77%) with an albinism variant (Figure 6).45 In both cohorts, we observed previously that the nature of the mutation, disruptive or missense, could explain a certain heterogeneity of residual degranulation, as described for FHL3 patients.46 In contrast, resting NK-cell degranulation was above 5% in 29 of 30 patients (97%) with FHL2 or with 1 of the 2 XLP variants and in 58 of 59 patients (98%) with secondary HLH. Therefore, as suggested previously in smaller studies,29,30,37,42 the assay clearly discriminated between patients with genetic disorders predisposing to HLH that are associated with impaired degranulation and those that are not. Moreover, it also appeared useful in the discrimination of primary degranulation defects from secondary HLH. It should be noted, however, that there were also 34 unclassified patients with defective NK-cell degranulation. It is a relevant limitation of our study that detailed clinical follow-up and comprehensive genetic information were not available to allow definite classification of these patients. In this context, the classification of patients with secondary HLH has to be regarded with caution, because genetic analysis was not performed in all of them. Sequencing all 6 genes known to be associated with HLH and lymphocyte degranulation defects in all patients was beyond the scope of this study. Moreover, it is likely that additional, as-yet-unknown genetic defects associated with a defect in lytic granule exocytosis will be discovered in patients with HLH, adding an additional level of uncertainty. However, our study results show that the number of patients with degranulation defects not explained by the currently known genetic diseases is probably limited, at least in the analyzed European cohort. Among the unclassified patients, there were only 2 patients with an albinism phenotype and 7 patients with an FHL phenotype who had resting NK-cell degranulation below 5% and additional assays with activated NK cells or CTL or a clinical course highly suggestive of genetic disease, but no mutations in the known genes. Overall, based on the analysis of the 209 patients who could be clearly classified, resting NK-cell degranulation below the 5% threshold provided a sensitivity for a genetic degranulation disorder of 96% and a specificity of 88%. It therefore appears justified to initiate a donor search immediately in parallel with genetic analysis in patients with such results.
Twelve of 90 patients with genetically determined degranulation defects had resting NK-cell degranulation equal to or above 5% (4 were above 10%) and 7 additional patients had activated NK-cell degranulation above 20%. Fourteen of these 18 patients carried at least 1 likely hypomorphic missense or splice-site mutation, including 8 FHL5 patients with the exon 15 splice-site mutation known to be associated with a milder course of the disease. It has been reported previously in a small patient cohort that IL-2 can induce partial reconstitution of NK-cell degranulation in patients with nonsense mutations in STX11, but not in patients with nonsense mutations in UNC13D.30 Similar results were obtained for patients with nonsense mutations in STXBP2.7,8 Among our large cohort of patients, IL-2 reconstitution of NK-cell degranulation was more frequent in patients with FHL4 and FHL5 than in those with FHL3. However, a priori, it was not possible to predict the genetic defect based on the pattern of the degranulation results. As a note of caution, certain mutations in STX11 associated with HLH do not necessarily display strong defects in resting NK-cell degranulation.47,48 Summarizing the experience gained in the present study, we therefore suggest the following workflow for patients with HLH referred for diagnostic evaluation (Figure 7). All patients should be investigated for expression of perforin (and male patients also for expression of SAP and XIAP; see supplemental material for consensus protocols) and degranulation of resting and activated NK cells. CTL degranulation is optional, but helpful in patients with very few NK cells. Perforin expression is reduced or absent in the large majority of patients with FHL2, whereas evaluation of SAP and XIAP expression is not as reliable for identification of XLP patients (data not shown), and functional and genetic analyses are recommended in patients with a strong clinical suspicion of XLP despite normal protein expression. In patients with normal expression of the above-mentioned proteins, resting NK-cell degranulation above 10% and normal activated NK-cell degranulation, no immediate genetic investigation is indicated. In patients with resting NK-cell degranulation between 5% and 10%, the assay should be repeated, more urgently in those in whom activated NK cell or CTL assays are abnormal. In patients with resting NK-cell degranulation below 5%, genetic investigations should be initiated unless there is good evidence for a secondary HLH and activated NK-cell and CTL assays are normal. The blood film and hair microscopy will determine whether genes associated with albinism or those associated with FHL should be investigated. It should be noted that as-yet-undiscovered genetic defects associated with HLH may display different mechanisms and may not be detected by the current scheme of diagnostic assays.
Proposed laboratory diagnostic algorithm based on degranulation assays for patients presenting with HLH. Normal values have to be determined in the diagnostic laboratory for the evaluation of resting NK-cell degranulation. For the centers participating in this study, resting NK-cell degranulation was considered defective if < 5%, abnormal if 5%-10%, and normal if > 10%, cutoffs that have proven to be useful. Analyses of activated NK-cell degranulation is recommended for all patients. AICD indicates activation-induced cell death.
Proposed laboratory diagnostic algorithm based on degranulation assays for patients presenting with HLH. Normal values have to be determined in the diagnostic laboratory for the evaluation of resting NK-cell degranulation. For the centers participating in this study, resting NK-cell degranulation was considered defective if < 5%, abnormal if 5%-10%, and normal if > 10%, cutoffs that have proven to be useful. Analyses of activated NK-cell degranulation is recommended for all patients. AICD indicates activation-induced cell death.
What do our results imply for the role for NK-cell cytotoxicity assays, which have been most valuable in the past and provide one of the formal diagnostic criteria for HLH? Decreased NK-cell cytotoxicity remains an evaluated and valid criterion when establishing the diagnosis of HLH.23 However, once the clinical diagnosis is made, the main questions are whether the disease is primary or secondary and which genes should be sequenced to obtain a molecular diagnosis. In this context, NK-cell cytotoxicity assays alone are not informative. Moreover, the frequently low NK-cell numbers during active HLH make the results from these assays difficult to interpret. In addition to decreased NK-cell cytotoxicity being a diagnostic criterion for HLH, CTL cytotoxicity assays (see supplemental material for consensus protocols) can be used as confirmatory assays in some patients, and both types of assays are valuable for research purposes.
In summary, the results of our study demonstrate the high sensitivity and specificity of degranulation assays for the diagnosis of genetic disorders of cytotoxicity and provide a solid basis for an optimized laboratory diagnostic algorithm for patients being evaluated for HLH. This will allow more rapid identification of patients with genetic disease and thereby provide an early rationale for the initiation of donor search for HSCT, which is one of the key issues for improving the prognosis for this life-threatening disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the excellent technical assistance of the CCI Advanced Diagnostic Unit Freiburg and the Immunology Laboratory at Great Ormond Street Hospital, and thank E. Entesarian, M. Meeths, and S. Wood at Karolinska Institutet and E. Sieni and F. Brugnolo at Azienda Ospedaliera Universitaria Meyer for laboratory work.
This work was supported by the European Community's Seventh Framework Program (FP7/2007-2013 under grant agreement no. 201461), the Bundesministerium für Bildung und Forschung (BMBF-01-EO-0803 to S.E.), the Swedish Research Council, the Karolinska Institute Research Foundation, the Swedish Cancer Foundation, the Children's Cancer Foundation (to Y.T.B. and J.I.H.), the Åke Olsson Foundation, the Åke Wiberg Foundation, Jeansson's Foundation, the Histocytosis Association (to Y.T.B.), the National Institute for Health Research Biomedical Research Centers funding scheme (to K.C.G. and D.W.), the “Antonio Pinzino”–Associazione per la Ricerca sulle Sindromi Emofagocitiche, Italian Ministry of Health, Bando “Malattie Rare 2008,” and Progetti di Ricerca Finalizzta 2008 (Childhood Histiocytoses), Azienda Ospedaliera Universitaria Meyer (to M.A.), Associazione Italiana per la Ricerca sul Cancro (AIRC) Special Project 5×1000 n. 9962 (to L.M.), the Allergy Foundation of Sweden, and the Stockholm County Council (ALF project, to J.I.H.).
Authorship
Contribution: Y.T.B., D.P., A.M.-P., K.C.G., and S.E. designed the study; Y.T.B., D.P., A.M.-P., K.C.G., H.U., S.C.C., S.M., I.B., R.M., and D.W. performed the experiments; T.V., G.J., K.L., K.B., M.A., L.M., J.-I.H., and S.E. recruited the patients and provided clinical information; U.S., M.A., and J.-I.H. performed the genetic analysis; N.B. performed the statistical analysis; and Y.T.B., D.P., A.M.-P., K.C.G., and S.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephan Ehl, Centre of Chronic Immunodeficiency, Breisacher Str 117, 79106 Freiburg, Germany; e-mail: Stephan.Ehl@uniklinik-freiburg.de.
References
Author notes
Y.T.B., D.P., A.M.-P., and K.C.G. contributed equally to this work.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal