In this issue of Blood, Kanaji et al propose that balanced expression levels of filamin A and GPIbα in megakaryocytes are a major determinant of platelet size.1
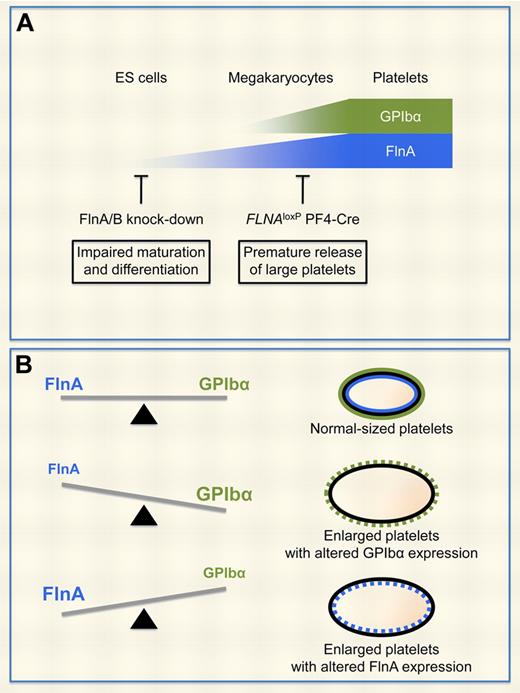
FlnA and GPIbα regulate platelet size. (A) Increased surface expression of GPIbα is a marker of megakaryocyte maturation and differentiation, whereas FlnA expression levels increase as early as in ES cells. Ablation of FlnA expression in FLNAloxP PF4-Cre megakaryocytes results in the premature production of enlarged platelets with reduced GPIbα expression.4 Kanaji et al show that FlnA and FlnB regulate ES cell maturation and differentiation.1 (B) Balanced expression levels of FlnA and GPIbα in megakaryocytes regulate the production of normal-sized platelets. FlnA-null and GPIbα-null platelets, as well as platelets over-expressing GPIbα, are enlarged. Conversely, FlnA subcellular localization is altered in GPIbα-null platelets and in HEK293 cells overexpressing GPIbα.
FlnA and GPIbα regulate platelet size. (A) Increased surface expression of GPIbα is a marker of megakaryocyte maturation and differentiation, whereas FlnA expression levels increase as early as in ES cells. Ablation of FlnA expression in FLNAloxP PF4-Cre megakaryocytes results in the premature production of enlarged platelets with reduced GPIbα expression.4 Kanaji et al show that FlnA and FlnB regulate ES cell maturation and differentiation.1 (B) Balanced expression levels of FlnA and GPIbα in megakaryocytes regulate the production of normal-sized platelets. FlnA-null and GPIbα-null platelets, as well as platelets over-expressing GPIbα, are enlarged. Conversely, FlnA subcellular localization is altered in GPIbα-null platelets and in HEK293 cells overexpressing GPIbα.
Filamins are cytoskeletal proteins that play a critical role in cell motility and signaling, as they cross-link actin filaments, tether membrane glycoproteins, and serve as scaffolds for signaling intermediates.2 The mammalian filamin family consists of 3 homologous members: filamin A (FlnA), FlnB, and FlnC, of which FlnA is the most abundant and widely expressed. Mutations of the X-linked FLNA gene, which encodes FlnA, lead to brain malformations, skeletal and cardiovascular defects, hemorrhage, and premature death.
The role of FlnA in platelets and megakaryocytes has been the focus of several papers in the past 2 years, thanks in part to the generation of mouse models3-5 and studies of patients with FLNA mutations.6,7 In platelets, FlnA anchors the von Willebrand factor receptor GPIb-IX-V complex and β1 and β3 integrins to the underlying actin cytoskeleton. Mice specifically lacking FlnA in the megakaryocyte lineage, such as FLNAloxP GATA1-Cre and FLNAloxP PF4-Cre mice, have a severe thrombocytopenia, enlarged platelets, and increased tail bleeding time,3,4 similar to GPIbα-null mice.8,9 In FLNA-null platelets, GPIbα is not linked to the actin cytoskeleton, and its surface expression is reduced and altered. A time course analysis indicates that the association of GPIbα with the actin cytoskeleton occurs in mature megakaryocytes and depends on expression of FlnA. Consistently, female patients with heterozygous FLNA mutations exhibit a bleeding tendency and giant platelets, reminiscent of Bernard-Soulier syndrome.7
Here, Kanaji et al propose that expression levels of both FlnA and FlnB in embryonic stem (ES) cells modulate maturation and differentiation toward platelet-producing megakaryocytes (see figure panel A).1 The authors use an elegant system in which expression levels of FlnA and FlnB are down-regulated as early as in ES cells using a short hairpin RNA and are monitored using GFP as a tracer. Most ES cells express GFP when first generated, but lose both GFP and the targeting short hairpin RNA as they proliferate and differentiate into megakaryocytes, indicating that expression of FlnA and FlnB confers a selective advantage. The results differ from the previously described FLNAloxP PF4-Cre mouse model, as comparable numbers of CD61+ cells can be obtained from FLNAloxP PF4-Cre and control FLNAloxP fetal liver cells.4 The discrepancy may likely be attributed to late FlnA ablation in the mouse model, as expression of the Cre recombinase is under the control of the megakaryocyte-specific PF4 promoter, or to normal FlnB expression.
In contrast to control cells, GFP+ megakaryocytes expressing low levels of FlnA and FlnB exhibit abnormal proplatelet projections, characterized by enlarged swelling and thick shafts.1 Further, these proplatelets produce platelets that are 2 to 3 times larger than those derived from control cells, and have decreased GPIbβ expression, indicating that FlnA and FlnB are required for the production of normal-sized platelets and efficient trafficking of the GPIb-IX-V complex to the platelet surface. Similar results are obtained, although to a lesser extent, when only expression of FlnA is knocked down, consistent with previous studies.3,4,10
Past studies have focused on GPIbα expression and function in the absence of the FlnA linkage. The novelty of the study by Kanaji et al is that the authors further examine whether balanced expression of FlnA and GPIbα affects efficient trafficking out of the endoplasmic reticulum (ER), particularly whether GPIbα conversely affects FlnA distribution in megakaryocytes and platelets.1 Interestingly, overexpression of FlnA, like lack of FlnA expression,10 results in retention of GPIbα within the ER in transfected HEK293 cells. Conversely, excessive expression of enhanced GFP-fused GPIbα, without GPIbβ and GPIX, traps FlnA in the ER in HEK293 cells, and ES cell–derived megakaryocytes expressing excessive enhanced GFP-fused GPIbα produce platelets that are 2 to 3 times larger than those derived from control cells. Together, the data indicate that the right balance between the expression levels of FlnA and GPIbα is required for optimal trafficking to the plasma mem-rane and normal platelet size (see figure panel B).
Consistently, platelets isolated from transgenic mice expressing excessive levels of a chimeric protein composed of the transmembrane and cytoplasmic domains of GPIbα fused to the extracellular domain of the IL4 receptor (IL4R-GPIbα)9 are larger and contain more immature IL4R-GPIbα than control platelets, confirming in vivo that high expression levels of GPIbα, not only its reduced expression, results in the formation of enlarged platelets.
In conclusion, the study by Kanaji et al introduces (1) the role of FlnA and FlnB in ES cell maturation and differentiation, and (2) the concept of balanced expression levels of FlnA and GPIbα in megakaryocytes in the regulation of platelet size. Their paper advances our understanding of the role of these proteins in hematopoietic cells, but also points to exciting new directions for research. It would be particularly interesting to investigate whether balanced expression of FlnA and β1 and β3 integrins similarly regulates megakaryocyte function to produce “fully balanced” platelets.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal