Abstract
On November 16, 2011, the Food and Drug Administration approved ruxolitinib (a JAK1 and JAK2 inhibitor) for use in the treatment of high and intermediate risk myelofibrosis. This is welcome news for those patients in whom such therapy is indicated and treatment benefit outweighs attendant risk. The question is who are these patients, what should they expect in terms of both short-term effects and long-term impact, and why would they choose ruxolitinib over other JAK inhibitors that are freely available for use in a research setting. Ruxolitinib and most other JAK inhibitors exert a salutary effect on constitutional symptoms and splenomegaly but have yet to produce histopathologic or cytogenetic remissions, reverse bone marrow fibrosis, or improve survival over best supportive care. Furthermore, the palliative value of JAK inhibitors is diminished by notable side effects, including anemia, thrombocytopenia, gastrointestinal disturbances, metabolic abnormalities, peripheral neuropathy, and hyperacute relapse of symptoms during treatment discontinuation. Therefore, risk-benefit balance favors use of currently available JAK inhibitors in only a select group of patients with myelofibrosis, and their potential value in polycythemia vera, outside of special circumstances (eg, intractable pruritus), is undermined by the absence of evidence for a disease-modifying effect and presence of arguably superior alternatives.
Introduction
When the late Murray Silverstein (1928-1998), a Mayo Clinic physician-scientist par excellence,1 introduced me to the subspecialty practice of myeloproliferative neoplasms (MPNs) in the early 1990s, hydroxyurea had already been established as the antithrombotic treatment of choice for polycythemia vera (PV).2 Subsequent controlled studies demonstrated a similar therapeutic value for hydroxyurea in high-risk essential thrombocythemia (ET)3,4 and low-dose aspirin in PV.5 Accordingly, the combination of hydroxyurea and aspirin has become the current “standard of care” in the first-line treatment of high risk PV or ET.6 The safety and nonleukemogenicity of hydroxyurea, in this context, have repeatedly been highlighted by large retrospective studies,7-9 and there is no controlled evidence to the contrary.10
Second-line cytoreductive drugs in PV and ET, for patients who are either resistant or intolerant to hydroxyurea, include interferon IFN-α, busulfan, and anagrelide.11 There is no hard evidence to implicate any one of these 3 drugs as being leukemogenic,12-14 whereas the data in this regard are significant enough to discourage the use of chlorambucil,15 P32,16 or pipobroman.17 The latter drug was randomly compared with hydroxyurea in patients with PV and found to be significantly more leukemogenic.17 However, the particular study17 did not include a control arm that would have allowed accurate assessment of leukemia risk associated with hydroxyurea therapy. On the other hand, a retrospective international study of more than 1500 patients with WHO-defined PV found no excess leukemia risk associated with treatment exposure to either hydroxyurea or busulfan.9
Although not necessarily considered “standard of care,” hydroxyurea is also widely used in myelofibrosis (MF), including primary (PMF) and post-PV/ET MF, for controlling splenomegaly, leukocytosis, and thrombocytosis.18 Hydroxyurea has limited efficacy in the management of MF-associated anemia or constitutional symptoms, which are both relevant to quality of life and prognosis.19,20 Other drugs that are variably effective in MF-associated anemia include erythropoiesis-stimulating agents, androgens, corticosteroids, danazol, thalidomide, and lenalidomide.21 Other treatment modalities in MF include allogenic stem cell transplantation, splenectomy, and involved-field radiotherapy.21 Unlike drug therapy, allogenic stem cell transplantation is potentially curative in MF, but its overall impact on risk-adjusted survival remains controversial.22
“Unmet need”: or dearth of Food and Drug Administration (FDA)–approved drugs?
Based on the above, it is evident that there is a dire need for additional therapeutic choices in MF, but it is more difficult to argue for the same in either ET or PV. Two recent international studies involving more than 2000 patients with ET or PV demonstrated a near-normal life expectancy in ET23 and a median survival of approximately 19 years in PV24 ; the corresponding 10-year leukemic transformation rates were approximately 0.7% and 2.5%. In PMF, both median survival (< 6 years)20 and leukemic transformation rate (5-year risk, ∼ 9%)25 are markedly worse and quality of life is severely compromised. Therefore, there is indeed an unmet need for both disease-modifying and palliative drugs in MF. In PV and ET, clinical trial endpoints must capture meaningful health outcome to justify the preference of new drugs (eg, IFN-α, JAK inhibitors) over the status quo (eg, hydroxyurea, busulfan).
Targeting JAK-STAT: how robust is the scientific rationale?
Two distinct but inter-related pathogenetic processes are implicated in causing disease symptoms in MF: clonal myeloproliferation26 and an accompanying, putatively reactive inflammatory state.27 Stem cell–derived clonal myeloproliferation is the primary driver of disease in MPN and, based on the experience with chronic myelogenous leukemia, its selective suppression appears to be essential for disease modification. Unlike chronic myelogenous leukemia, the molecular underpinnings of BCR-ABL1–negative MPN are poorly understood and the disease-initiating events remain elusive. Therefore, it is unlikely that we will run into an imatinib-like drug, for BCR-ABL1–negative MPN, any time soon.
Beginning in 2005, a number of MPN-associated mutations have been described: JAK2, MPL, LNK, CBL, IDH1, IDH2, TET2, EZH2, DNMT3A, ASXL1, SF3B1, IKZF1, TP53, CUX1, and others.28,29 With the exception of JAK2V617F, mutational frequency for each one of these mutations is less than 20% and often less than 10%.29 Furthermore, these mutations are neither specific to MPN nor mutually exclusive, and none of them can always be traced back to the ancestral clone.28 Whether or not some of these “late” genetic events instead contribute to leukemic transformation has been suggested30,31 but needs experimental validation. On the other hand, a close genotype-phenotype association exists between JAK2 mutations and erythrocytosis32 and SF3B1 mutations and ring sideroblasts,33 suggesting the limit of “targeted therapy,” in this context, to phenotype modification.
The above not withstanding, JAK-STAT is still an attractive drug target in BCR-ABL1-negative MPN because (1) JAK2V617F occurs in the majority of patients29 ; (2) JAK2 unmutated patients might harbor other JAK-STAT activating mutations (eg, MPL or LNK mutations)34,35 ; (3) JAK2, MPL, and LNK mutations induce MPN-like disease in mice and might promote disease-associated stromal changes34-38 ; and (4) JAK-STAT is an integral component of dysregulated cytokine expression39 and immune response,40 which often accompany MF.
Preclinical data: mouse models and more
Table 1 summarizes preclinical information on ATP mimetic JAK inhibitors that have been introduced into clinical trials: ruxolitinib, SAR302503, CYT387, lestaurtinib, SB1518, AZD1480, BMS911543, LY2784544, NS-018, and XL019.41-54 The table includes inhibitory concentrations in the setting of in vitro kinase assays and proliferation of JAK2V617F-bearing cell lines or primary cells. The latter involved both endogenous and cytokine-supported colony formation assays. Relevant cell lines include Ba/F3 murine pro-B cell lines transformed to express human JAK2V617F, HEL human erythroleukemia cells that are homozygous for JAK2V617F, and SET-2 human essential thrombocythemia cell lines expressing heterozygous JAK2V617F. Some of the drugs have also been evaluated in JAK2V617F transplant or transgenic mouse models or Ba/F3-JAK2V617F (or HEL/SET-2) inoculated nude mice.
JAK inhibitors for MPNs: preclinical data
| Drug . | JAK2V617F IC50, nM . | JAK2-wt IC50, nM . | JAK1 IC50, nM . | JAK3 IC50, nM . | TYK2 IC50, nM . | Other targets with < 100nM IC50 . | Ba/F3 cells JAK2V617F proliferation IC50, nM . | Endogenous PV erythroid colony growth IC50, nM . | Erythroid colony growth (PV patients) IC50, nM . | Erythroid colony growth (healthy controls) IC50, nM . | Mouse models where efficacy was shown . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ruxolitinib44 | NA | 2.8 | 3.3 | 428 | 19 | None reported | 126 | 67 | 223 | 407 | Ba/F3-JAK2V617F |
| SAR30250345-47 | 3 | 3 | 105 | 1040 | 405 | FLT3, RET | 300-580 | < 300-600 | 600-1200 | > 1200 | JAK2V617F transplant |
| Lestaurtinib48 | NA | < 1 | NA | 3 | NA | FLT3, TRKA VEGFR2, RET | NA | NA | NA | NA | HEL xenografts |
| CYT38783 | NA | 18 | 11 | 155 | 17 | PKD3, PKCμ CDK2, ROCK2 JNK1, TBK1 | 1500 | 500-1000 | 2000-4000 | 2000-4000 | JAK2V617F transplant |
| SB151841 | 19 | 23 | 1280 | 520 | 50 | FLT3 | 160 | 63 | 260 | 230 | Ba/F3-JAK2V617F SET-2-JAK2V617F |
| AZD148049 | NA | < 0.5 | 1.3 | 3.9 | NA | Aurora-A, TRKA FGFR1, FLT4 | NA | NA | NA | NA | NA |
| BMS91154353 | NA | 1.1 | 356 | 73 | 66 | None reported | 70 | 75 | 300 | 1500 | NA |
| LY278454454 | NA | NA | NA | NA | NA | NA | 68 | NA | NA | NA | Ba/F3-JAK2V617F |
| NS-01852 | NA | < 1 | 33 | 39 | 22 | SRC, FYN ABL, FLT3 | 60 | 224 | 529 | 952 | Ba/F3-JAK2V617F JAK2V617F-transgenic |
| XL01950,57 | NA | 2 | 134 | 195 | 344 | NA | NA | NA | NA | NA | HEL xenografts |
| 2* | 23* | 102* | 33* | None reported* | NA | NA | NA | NA | MPL W515 transplant* |
| Drug . | JAK2V617F IC50, nM . | JAK2-wt IC50, nM . | JAK1 IC50, nM . | JAK3 IC50, nM . | TYK2 IC50, nM . | Other targets with < 100nM IC50 . | Ba/F3 cells JAK2V617F proliferation IC50, nM . | Endogenous PV erythroid colony growth IC50, nM . | Erythroid colony growth (PV patients) IC50, nM . | Erythroid colony growth (healthy controls) IC50, nM . | Mouse models where efficacy was shown . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ruxolitinib44 | NA | 2.8 | 3.3 | 428 | 19 | None reported | 126 | 67 | 223 | 407 | Ba/F3-JAK2V617F |
| SAR30250345-47 | 3 | 3 | 105 | 1040 | 405 | FLT3, RET | 300-580 | < 300-600 | 600-1200 | > 1200 | JAK2V617F transplant |
| Lestaurtinib48 | NA | < 1 | NA | 3 | NA | FLT3, TRKA VEGFR2, RET | NA | NA | NA | NA | HEL xenografts |
| CYT38783 | NA | 18 | 11 | 155 | 17 | PKD3, PKCμ CDK2, ROCK2 JNK1, TBK1 | 1500 | 500-1000 | 2000-4000 | 2000-4000 | JAK2V617F transplant |
| SB151841 | 19 | 23 | 1280 | 520 | 50 | FLT3 | 160 | 63 | 260 | 230 | Ba/F3-JAK2V617F SET-2-JAK2V617F |
| AZD148049 | NA | < 0.5 | 1.3 | 3.9 | NA | Aurora-A, TRKA FGFR1, FLT4 | NA | NA | NA | NA | NA |
| BMS91154353 | NA | 1.1 | 356 | 73 | 66 | None reported | 70 | 75 | 300 | 1500 | NA |
| LY278454454 | NA | NA | NA | NA | NA | NA | 68 | NA | NA | NA | Ba/F3-JAK2V617F |
| NS-01852 | NA | < 1 | 33 | 39 | 22 | SRC, FYN ABL, FLT3 | 60 | 224 | 529 | 952 | Ba/F3-JAK2V617F JAK2V617F-transgenic |
| XL01950,57 | NA | 2 | 134 | 195 | 344 | NA | NA | NA | NA | NA | HEL xenografts |
| 2* | 23* | 102* | 33* | None reported* | NA | NA | NA | NA | MPL W515 transplant* |
NA indicates not available.
Tested on the structurally similar compound EXEL-8232.57
All 10 drugs listed in Table 1 are potent inhibitors of JAK2-wt (enzymatic IC50 < 1-23nM or Ba/F3-JAK2V617F cellular IC50 = 60-1500nM), and some have been shown to display similar inhibitory activity against JAK2V617F,45 JAK2 exon 12,46 or MPL mutations46 ; however, none of them is specific to mutant as opposed to wild-type JAK2. Some of these drugs appear to be highly selective to JAK2 (eg, BMS911543), whereas others were equally potent against JAK1 (eg, ruxolitinib, CYT387), TYK2 (eg, CYT387), FLT3 (eg, lestaurtinib, SAR302503, SB1518, NS-018), or other kinases (Table 1). Interestingly, there is already information on JAK2V617F, JAK2 wild-type, and JAK1 point mutations that confer resistance to ruxolitinib and other JAK inhibitors.55,56 In general, JAK2 inhibition is associated with abrogation of STAT3/5, Akt, and ERK phosphorylation and induction of apoptotic cell death. Some JAK inhibitors are thought to be more selective to clonal as opposed to normal erythropoiesis (eg, ruxolitinib, SAR302503, BMS911543, LY2784544, and NS-018)44,46,52-54 but the lack of standardized assays in this regard makes such observations suspect.
Very few of the drugs listed in Table 1 have undergone evaluation in mouse MPN models. In a mouse transplant PV model, SAR302503 treatment improved survival and decreased hematocrit, JAK2V617F allele burden, endogenous colony formation, STAT5 phosphorylation, splenomegaly, hepatic extramedullary hematopoiesis (EMH), and reticulin fibrosis.45 In a similar murine model, CYT387 treatment lowered both hematocrit and leukocyte levels without affecting platelet count.43 CYT387 reduced spleen size, reticulin fibrosis, inflammatory cytokine levels, and the burden of JAK2V617F-expressing cells.43 In a JAK2V617F transgenic mice with MF phenotype, treatment with NS-018 improved survival, prevented progression of anemia, alleviated cachexia, and controlled leukocytosis, organomegaly and EMH but did not reverse fibrosis.52 EXEL-8232, a structurally similar compound to XL-019, was evaluated in a mouse MPLW515L transplant ET model where it led to resolution of thrombocytosis, leukocytosis, hepatosplenic EMH, and bone marrow fibrosis, without causing anemia.57
Mechanism of action: do we know?
What is becoming increasingly evident is the limited concordance between preclinical and clinical data regarding JAK inhibitor therapy in MPN. For example, unlike the experience in mouse MPN models, JAK inhibitor therapy in humans has had little effect on bone marrow histopathology or JAK2V617F allele burden.58-60 Retrovirus-based transplantation models do not necessarily recapitulate mutant protein expression levels or the phenotypic diversity seen in human disease.61,62 Although the particular issue has been addressed by transgenic models,63 in whom mutant gene expression could be manipulated, and by gene knock-in models,64 where endogenous promoters control gene expression, none of them accounts for the underlying genetic complexity seen in human MPN.29 In other words, in human MPN, especially in MF, targeting a signaling network or multiple oncogenic pathways, rather than an isolated JAK-STAT pathway, might be necessary to secure histopathologic remissions and eradication of the ancestral clone.65-67
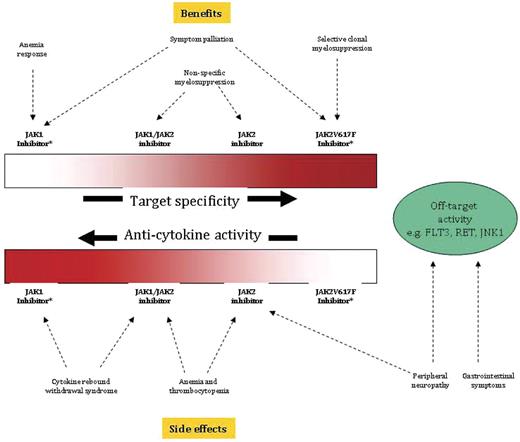
The absence of drug-induced selective clonal suppression is not a trivial shortcoming because of the implications regarding the prospect of JAK inhibitor therapy to modify disease rather than simply palliate symptoms. Current information from ruxolitinib clinical trials suggests a mechanism of action that includes a nonspecific myelosuppressive effect (possibly responsible for its salutary effect on leukocytosis, thrombocytosis, and splenomegaly and its side effects of anemia and thrombocytopenia) and an anti–JAK-STAT–mediated down-regulation of inflammatory cytokine activity (possibly responsible for its palliative effect on constitutional symptoms and cachexia; Figure 1).39,58 The latter contention is supported by the drug's potent suppression of proinflammatory cytokines in patients with MF,58 an observation that was also seen in JAK2V617F-transduced mice receiving another JAK1/JAK2 inhibitor (CYT387).43 More selective JAK2 inhibitors appear to have a less pronounced anticytokine effect (possibly because of less anti-JAK1 activity) and more pronounced anti-erythropoiesis effect (expected on-target activity; Figure 1).59 On the other hand, the remarkable effect of JAK1 and JAK1/JAK2 inhibitors on splenomegaly, which was seen in both animal models and human disease,58,59 suggests a particular dependence of splenic EMH on JAK-STAT–driven myeloproliferation or JAK-STAT–dependent cytokines.
Mutant protein target specificity and anticytokine activity of JAK inhibitors used for the treatment of myeloproliferative neoplasms and their corresponding benefits and side effects. *Pure JAK1 or JAK2V617F inhibitors are currently not available.
Mutant protein target specificity and anticytokine activity of JAK inhibitors used for the treatment of myeloproliferative neoplasms and their corresponding benefits and side effects. *Pure JAK1 or JAK2V617F inhibitors are currently not available.
Specific targeting of JAK2V617F, if it were possible, might spare patients the untoward effects of wild-type JAK1 (cytokine rebound syndrome) or JAK2 (myelosuppression) inhibition, but therapeutic outcome might be limited to suppression of a subclone with a subordinate role in disease phenotype or clonal evolution (Figure 1),68 Current “JAK2-selective” drugs do not meaningfully distinguish between wild-type JAK2 and JAK2V617F,69 and are therefore subjected to consequences of wild-type JAK2 inhibition, which includes induction or worsening of anemia.59 Off-target activity outside the JAK family of kinases might explain the occurrence of certain side effects; for example, gastrointestinal disturbances are commonly seen with drugs that concomitantly inhibit FLT3 (eg, lestaurtinib, SAR302503, or SB1518). However, differences in molecular mechanisms of action, including drug pharmacokinetics, probably contribute to the overt expression of both on-target and off-target drug effects and might underlie the association of neuropathy, for example, with both JAK2-selective (XL019) and JAK1/JAK2 inhibitors (eg, CYT387; Table 2). Finally, the antianemia effect of CYT387,70 which is also occasionally seen with ruxolitinib,58 might represent alleviation of cytokine-mediated ineffective erythropoiesis, improved red cell survival stemming from drug-induced reduction in spleen size, or engagement of yet to be identified erythropoietic target.
Clinical trials results for JAK inhibitors in MF
| Drug . | Current development stage . | Constitutional symptoms response, % . | Palpable/MRI spleen response, % . | Anemia response, % . | Leukocytosis response, % . | Thrombocytosis response, % . | JAK2V617F allele burden response, % . | Side effects . | Annual cost, $ (US) . |
|---|---|---|---|---|---|---|---|---|---|
| Ruxolitinib58,71,73 | FDA-approved; phase 3 (n = 528); phase 1/2 (n = 153) | > 50 | 29-42 (by MRI) | 14 | * | 44 | Unremarkable | Anemia; thrombocytopenia; “ruxolitinib withdrawal syndrome”† | > 80 000 |
| SAR30250359 | Phase 3; phase 1/2 (n = 59) | > 50 | 39 | 0 | 72 | 90 | 45 | Nausea/diarrhea; anemia; thrombocytopenia; transaminasemia; hyperlipase/amylasemia | NA |
| Lestaurtinib60 | Phase 2 (n = 22) | NR | > 18 | 25‡ | NR | NR | Unremarkable | Nausea/diarrhea; anemia; thrombocytopenia | NA |
| CYT38782,83 | Phase 2 (n = 163) | > 50 | 45 | 50 | NR | NR | Unremarkable | Thrombocytopenia; headaches, first dose effect§; peripheral neuropathy; transaminasemia; hyperlipase/amylasemia | NA |
| SB151887 | Phase 2 (n = 34) | > 50 | 32 (by MRI) | ‖ | NR | NR | NR | Nausea/diarrhea | NA |
| LY278454488 | Phase 1/2 (n = 19) | NR | > 22 | NR | NR | NR | Unremarkable | Nausea/diarrhea; anemia electrolyte abnormalities/TLS?; increases in serum creatinine | NA |
| XL01989 | Halted (n = 21) | > 50 | 33 | NR | ¶ | NR | NR | Peripheral neuropathy | NA |
| AZD1480 | Phase 1/2 | No information available | NA | NA | NA | NA | NA | NA | NA |
| BMS911543 | Phase 1/2 | No information available | NA | NA | NA | NA | NA | NA | NA |
| NS-018 | Phase 1/2 | No information available | NA | NA | NA | NA | NA | NA | NA |
| Drug . | Current development stage . | Constitutional symptoms response, % . | Palpable/MRI spleen response, % . | Anemia response, % . | Leukocytosis response, % . | Thrombocytosis response, % . | JAK2V617F allele burden response, % . | Side effects . | Annual cost, $ (US) . |
|---|---|---|---|---|---|---|---|---|---|
| Ruxolitinib58,71,73 | FDA-approved; phase 3 (n = 528); phase 1/2 (n = 153) | > 50 | 29-42 (by MRI) | 14 | * | 44 | Unremarkable | Anemia; thrombocytopenia; “ruxolitinib withdrawal syndrome”† | > 80 000 |
| SAR30250359 | Phase 3; phase 1/2 (n = 59) | > 50 | 39 | 0 | 72 | 90 | 45 | Nausea/diarrhea; anemia; thrombocytopenia; transaminasemia; hyperlipase/amylasemia | NA |
| Lestaurtinib60 | Phase 2 (n = 22) | NR | > 18 | 25‡ | NR | NR | Unremarkable | Nausea/diarrhea; anemia; thrombocytopenia | NA |
| CYT38782,83 | Phase 2 (n = 163) | > 50 | 45 | 50 | NR | NR | Unremarkable | Thrombocytopenia; headaches, first dose effect§; peripheral neuropathy; transaminasemia; hyperlipase/amylasemia | NA |
| SB151887 | Phase 2 (n = 34) | > 50 | 32 (by MRI) | ‖ | NR | NR | NR | Nausea/diarrhea | NA |
| LY278454488 | Phase 1/2 (n = 19) | NR | > 22 | NR | NR | NR | Unremarkable | Nausea/diarrhea; anemia electrolyte abnormalities/TLS?; increases in serum creatinine | NA |
| XL01989 | Halted (n = 21) | > 50 | 33 | NR | ¶ | NR | NR | Peripheral neuropathy | NA |
| AZD1480 | Phase 1/2 | No information available | NA | NA | NA | NA | NA | NA | NA |
| BMS911543 | Phase 1/2 | No information available | NA | NA | NA | NA | NA | NA | NA |
| NS-018 | Phase 1/2 | No information available | NA | NA | NA | NA | NA | NA | NA |
NA indicates not available; NR, not clearly reported; and TLS, tumor lysis syndrome.
Mean leukocyte count after 3 months of treatment decreased from 29.8 × 109/L to 16.0 × 109/L.
Characterized by hyperacute relapse of symptoms, painful enlargement of spleen, and cardiopulmonary distress.
Two of 8 transfusion-dependent patients became transfusion-independent.
Characterized by transient lightheadedness and hypotension occurring only during the first dose.
Two patients were reported to obtain anemia response but no denominator given.
Responses in leukocyte counts mentioned but accurate figures not given.
Ruxolitinib: from phase 1 to drug approval in approximately 4 years
Ruxolitinib (INCB018424) is the first JAK inhibitor evaluated in a clinical trial for MPN (phase 1 study in patients with MF was initiated in 2007) and is also the first one to be FDA-approved for use in high and intermediate risk MF (November 16, 2011).
Phase 3 studies
Ruxolitinib has undergone 2 separate randomized studies in MF, referred to as the COntrolled MyeloFibrosis Study with ORal JAK inhibitor Treatment (COMFORT)-1 and COMFORT-2, whose results were the basis for the drug's FDA approval. COMFORT-1 was a double-blind, placebo-controlled study that included 309 patients with International Prognostic Scoring System (IPSS)20 high or intermediate-2 MF (50% PMF, 31% post-PV MF, and 18% post-ET MF). Additional eligibility criteria included more than or equal to 5-cm palpable spleen and more than or equal to 100 × 109/L platelet count. Patients were randomized to either placebo (n = 154) or ruxolitinib (n = 155). Ruxolitinib was administered at a dose of either 15 mg twice daily (platelet count, 100-200 × 109/L) or 20 mg twice daily (platelet count > 200 × 109/L). The primary endpoint of the study was achievement of more than or equal to 35% spleen volume reduction by MRI or CT at 24 weeks.
The results of the COMFORT-1 trial were first reported early in 2011.71 After a median follow-up of 32.2 weeks, 41.9% of patients on ruxolitinib (vs 0.7% on placebo) achieved spleen response. Median duration of spleen response was not reached at that point. In addition, 45.9% of patients on ruxolitinib (vs 5.3% on placebo) experienced a more than or equal to 50% improvement in constitutional symptoms. This palliative benefit was apparent among all risk groups and was not affected by JAK2 mutational status.72 According to the FDA document available in public domain (http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf), grade 3 or grade 4 anemia (45.2% vs 19.2%) and thrombocytopenia (12.9% vs 1.3%) were reported significantly more with ruxolitinib than they were with placebo. Similarly, red blood cell transfusions were documented in 60% of patients receiving ruxolitinib versus 38% receiving placebo. There was no significant difference between the 2 arms in the number of deaths (10 vs 14 deaths on placebo; P = .33). However, after a median follow-up of approximately one year, the number of deaths had increased to 13 in the ruxolitinib and 24 in the placebo arms (P = .04).72
COMFORT-2 was an open-label study and included 219 patients with IPSS high (49%) or intermediate-2 (51%) risk MF (53% PMF, 31% post-PV MF, and 16% post-ET MF) that were randomized to either best available therapy (BAT; n = 73) or ruxolitinib (n = 146).73 Eligibility criteria and drug administration schedule were the same as in COMFORT-1. The primary endpoint of the study was achievement of more than or equal to 35% spleen volume reduction by MRI or CT at 48 weeks; response rates were 28.5% for ruxolitinib and 0% for BAT. The corresponding values at 24 weeks were 31.9% and 0% and median duration of response was 48 weeks. Thrombocytopenia (44.5% vs 9.6%) and anemia (40.4% vs 12.3%) were again the most frequent side effects of ruxolitinib; the corresponding frequency comparisons for grade 3 or grade 4 thrombocytopenia and anemia were 7.5% vs 4.1% and 11% vs 4.1%, respectively.73 There was no difference in survival between the 2 arms (2.7% death rate for ruxolitinib and 4.1% for BAT). A third ruxolitinib phase 3 study is currently ongoing in patients with hydroxyurea-intolerant/resistant PV (ruxolitinib vs best available therapy; www.clinicaltrials.gov).
Phase 1/2 studies in MF
Short-term results.
In the first phase 1/2 study using ruxolitinib in MF,58 eligibility criteria were largely similar to those of the aforementioned COMFORT trials. Dose limiting toxicity (DLT) was thrombocytopenia, and the maximum tolerated dose (MTD) was declared at 25 mg twice daily or 100 mg once daily. A total of 153 ruxolitinib-treated patients were included in the study; and after a median follow-up of approximately 15 months, the anemia, spleen, and constitutional symptom response rates were 14%, 44%, and more than 50%, respectively.58 The latter was accompanied by a rapid and significant reduction in proinflammatory cytokines. Mean leukocyte count after 3 months of treatment (15 or 25 mg twice daily) decreased from 29.8 × 109/L to 16.0 × 109/L, and 7 (44%) of 16 patients with baseline thrombocytosis normalized their platelet count. Ruxolitinib had little effect on JAK2V617F allele burden or bone marrow fibrosis. Side effects included thrombocytopenia and anemia at rates similar to those reported here for the COMFORT trials.
Long-term results.
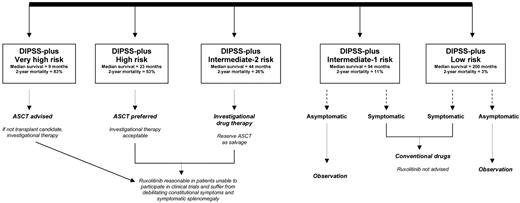
Longer-term follow-up of the phase 1/2 ruxolitinib study in MF were recently reported by both the Mayo Clinic and MD Anderson Cancer Center (MDACC), the only 2 study centers who participated in this first study. The Mayo study included 51 patients (66% high or intermediate-2 risk per Dynamic International Prognostic Scoring System-plus; Figure 2)74 who were enrolled between October 2007 and February 2009, and follow-up was updated in July 2011.75 Response rates were 29% for spleen, 21% for anemia, and 63% for constitutional symptoms.76 Grade more than or equal to 2 thrombocytopenia (26%) and anemia (33%) were the most frequent side effects. Treatment discontinuation rates at 1, 2, and 3 years were 51%, 72%, and 89%, respectively, and were mostly secondary to loss of treatment response or toxicity. Drug discontinuation was almost always associated with rapid relapse of symptoms, sometimes leading to hospitalization, splenic infarct, and cardiopulmonary distress.77 As of July 2011, 18 deaths (36%) and 5 (10%) leukemic transformations were documented, and a retrospective comparison of risk-adjusted survival showed no significant difference between ruxolitinib-treated and untreated (n = 410) patients (P = .58).75
Current treatment algorithm for myelofibrosis. DIPSS-plus indicates Dynamic International Prognostic Scoring System-plus74,96 ; and ASCT, allogenic stem cell transplantation.
The MDACC experience with ruxolitinib in the phase 1/2 setting involved 107 patients (91% high or intermediate-2 per IPSS).78,79 After a median follow-up of 32 months, 54% of the patients were still taking the drug. Death and leukemic transformation rates were 31% and 8.4%, respectively. The authors compared the survival of their ruxolitinib-treated patients with a historical control of “protocol-candidate” patients recruited from their center and 2 other Italian centers and suggested improved survival for the former (P = .02).78 The death (36% vs 31%) and leukemia (10% vs 8.4%) transformation rates in the Mayo versus MDACC reports were similar; and yet, retrospective risk-adjusted survival analysis suggested the absence or presence of survival advantage in the Mayo versus MDACC reports, respectively.75,78 The reasons for this discrepancy are currently not clear. The results of the aforementioned COMFORT-2 randomized study that compared ruxolitinib against BAT were similar to those of the Mayo Clinic report in showing no survival difference between the 2 arms. The results from COMFORT-1 (placebo-controlled) showed no survival difference at an early time point but favored ruxolitinib in the most recent analysis.
Phase 1/2 studies in PV and ET
Ruxolitinib has also been evaluated in hydroxyurea-refractory or intolerant PV (n = 34) or ET (n = 39).80 The drug induced phlebotomy independence in almost all patients and more than 50% spleen reduction in 59% of the patients. Response rates for leukocytosis (63%) or thrombocytosis (69%) appeared to be inferior to those expected from currently used conventional drugs, such as IFN-α and busulfan.11 The same can be said about the reported complete response rate (49%) for platelet count in ET.11 Treatment discontinuation rate was particularly high (23%) in ET, and grade 3 adverse events included leukopenia and peripheral neuropathy. As expected, ruxolitinib was effective in alleviating pruritus and other constitutional symptoms but had little to no effect on JAK2V617F allele burden.
Other JAK inhibitors: we need more
Nine other JAK inhibitor ATP mimetics have undergone clinical trials in humans (Table 1). Among them, only 1 (SAR302503) has reached phase 3 stage of development. Phase 2 studies involving CYT387, lestaurtinib, and SB1518 are currently ongoing. Phase 1 studies involving AZD1480, BMS911543, LY2784544, and NS-018 have been initiated. Clinical trial development with XL019 has been halted because of significant drug-induced neuropathy.
SAR302503 (TG101348)
Aside from ruxolitinib, SAR302503 is the only other JAK inhibitor that has currently entered phase 3 stage of drug development. The phase 1/2 study accrued 59 MF patients with high- or intermediate-risk disease, and its eligibility criteria were different from the aforementioned ruxolitinib trials in terms of allowing patients with a platelet count of moer than or equal to 50 to less than 100 × 109/L.59 DLT included hyperamylasemia, and MTD was declared at 680 mg/day. The median treatment dose during the extension phase of the study was 440 mg/day (range, 120-680 mg/day). After 6 cycles of treatment with SAR302503, 39% of patients experienced more than or equal to 50% palpable spleen response and more than or equal to 50% constitutional symptoms response. In addition, 72% and 90% of patients with baseline leukocytosis and thrombocytosis, respectively, normalized their counts. Responses to SAR302503 were not affected by JAK2 mutational status, although 45% of 20 patients with baseline allele burden greater than 20% had a more than or equal to 50% decrease in JAK2V617F allele burden.59
Frequent side effects included nausea and diarrhea (majority of patients), induction of red cell transfusion dependency (35%), grade 3 or 4 thrombocytopenia (24%), and asymptomatic increases in serum lipase (27%) and transaminases (27%). Fifteen patients (25%) discontinued treatment during the first 6 cycles of therapy. A recent update disclosed that 37% of SAR302503-treated patients remain on therapy and have received a median of 29 treatment cycles (range, 24-41 cycles; one treatment cycle = 28 days).81 Furthermore, more than half of the patients treated at dose levels more than or equal to 240 mg/day experienced a durable more than or equal to 50% decrease in palpable spleen size. The report also confirmed the durability of JAK2V617F allele burden reduction after 24 cycles of treatment: median 21% (range, 6%-100%) versus 60% (range, 23%-100%) at baseline (P = .03). An international phase 3 study in high/intermediate-2 risk MF has just been initiated (SAR302503 vs placebo).
CYT387
In a phase 1/2 multicenter study, 163 patients with high or intermediate-2 risk MF were enrolled at the time of the most recent study report (median duration of treatment, 6.6 months; range, 0.25-20.4 months).82 DLT included grade 3 hyperlipasemia and grade 3 headaches, and the MTD was declared at 300 mg/day; current starting doses are 150 mg/day, 300 mg/day, or 150 mg twice a day. In the first 60 patients completing at least 3 cycles of treatment with CYT387, spleen, anemia, and constitutional symptom response rates, per conventional response criteria,76 were 45%, 50%, and more than or equal to 50%, respectively.83 Responses were also seen in patients who had previously failed treatment with ruxolitinib, SAR302503, or pomalidomide. Unexpectedly, 58% of transfusion-dependent patients became transfusion-independent, defined as being transfusion-free for more than or equal to 12 weeks, while on protocol drug therapy, and capped by a hemoglobin level of more than or equal to 8 g/dL.83 These responses were durable (median, 20 weeks; range, 12-54 weeks) and not affected by JAK2V617F mutational status.
Most common side effects included transient lightheadedness and hypotension seen only with the first dose and occurring in the majority of patients, grade 3 or 4 thrombocytopenia (16%), hyperlipasemia (3%), and mostly grade 1 sensory peripheral neuropathy (28%).83 Unlike the case with ruxolitinib and SAR302503, treatment-related grade 3 or 4 anemia was infrequent (< 1%). At the time of the most recent report, 25 (15%) of the 163 study subjects had discontinued treatment.82
Lestaurtinib
Lestaurtinib (CEP-701) at 80 mg twice-daily dose was given to 22 JAK2-mutated MF patients in a phase 2 study.60 Overall response per conventional criteria76 was 27% (6 patients). Among 8 transfusion-dependent patients, 2 (25%) became transfusion-independent. Four patients were reported to achieve more than 50% reduction in spleen size, but the number of patients who were evaluable for spleen response was not provided by the report. Median duration of response was more than 14 months. Treatment did not affect bone marrow histopathology, JAK2V617F allele burden, or inflammatory cytokine levels. Side effects included diarrhea (73%), nausea (50%), and grade 3 or 4 anemia (14%) and thrombocytopenia (23%). After a median follow-up of less than 1.5 years, 21 patients (91%) discontinued therapy mostly because of lack of response and 6 (27%) deaths were reported. A phase 1 study with a new capsule formulation (as opposed to liquid formulation), to overcome excess plasma protein binding, is currently ongoing in patients with JAK2V617F-positive MF84 ; at the time of the most recent communication, 19 patients were enrolled and MTD had not been reached, whereas reductions in spleen size and JAK2V617F allele burden were already being noted.
In another phase 2 study, 39 high-risk patients with JAK2V617F-positive PV (n = 27) or ET (n = 12) received lestaurtinib.85 The results were disappointing, with the drug causing worsening of leukocytosis and thrombocytosis and side effects including thrombosis in 5 patients. Fifteen patients experienced a reduction in spleen size and 5 resolution of pruritus. Reduction in phlebotomy requirement was delayed, and the drug had minimal effect on JAK2V617F allele burden.
SB1518 (pacritinib)
DLT during a phase 1 study of SB1518 was diarrhea and occurred at 600 mg/day.86 This phase 1 study included 21 MF patients in whom the spleen response rate was 24%. MTD was declared at 400 mg/day. A subsequent phase 2 study in MF included 33 patients and reported a 39% spleen response rate and a 40% to 65% decrease in the “intensity” of constitutional symptoms. A 36% treatment discontinuation rate was reported, and side effects included diarrhea (81%), nausea (41%), vomiting (22%), and fatigue (9%). The results of this phase 2 study in MF were recently updated87 ; after a median time on study of 8.2 months (range, 0.5-12.1 months), 17 (50%) had discontinued treatment, mainly because of adverse effects or disease progression. Spleen response rate was 44% by physical examination and 32% by MRI (≥ 35% reduction in splenic volume). The authors reported that 2 of their patients met conventional criteria for anemia response.
LY2784544
In a phase 1 study of patients with MF, PV, or ET,88 19 patients have so far been accrued, including 18 MF and 1 PV patient. DLT included increases in uric acid and potassium, occurring at 200 to 240 mg/day dose levels and MTD was declared at 120 mg/day. The patient with PV was reported to experience 100% reduction in spleen size. In MF, 4 (22%) patients have so far achieved spleen response per conventional criteria, and the authors expect this number to increase with time.88 No responses have so far been noted in terms of JAK2V617F allele burden reduction. Side effects have included diarrhea (42%), nausea (37%), anemia (21%), and transient increases in serum creatinine, uric acid, and potassium, some of which has been attributed to tumor lysis syndrome.
XL019
In a phase 1 study involving 21 MF patients, 7 of 9 patients receiving XL019 at daily doses of 100 mg or more experienced grade 1 or 2 peripheral or central neuropathy.89 Lower doses (≤ 50 mg/day) were used in the remaining patients who reportedly fared better in terms of neuropathy. Favorable effects were seen in JAK2 or MPL mutated patients (but not in unmutated patients) where 5 (42%) of 12 evaluable patients experienced a spleen response per conventional criteria and the majority reported improvement in constitutional symptoms (overall spleen response rate, including JAK2/MPL-unmuated cases was 33%). No drug-induced myelosuppression was reported, whereas improvements in leukocytosis and blast count were mentioned. Regardless, further development of clinical trials using XL019 has been halted because of neurologic toxicities.
BMS911543, NS-018, and AZD1480
There is currently no information in the public domain regarding preliminary results from clinical trials involving BMS911543, NS-018, or AZD1480.
Perspective: unabridged
True targeted therapy assumes that the pathogenetically relevant mutation or pathway has been identified and that the therapeutic agent in question is specific to that target. Neither of these 2 conditions applies to JAK inhibitor therapy in MPN. The study of molecular pathogenesis in MPN is a work in progress. Apart from JAK2V617F, the mutational frequencies of MPN-associated mutations are very low (often < 10%), and most of them also occur in myelodysplastic syndromes and acute myeloid leukemia.29 Even if one were to focus on JAK2 mutations, it has become evident that they are not necessarily the culprit for either disease initiation or clonal progression in MPN. There is now abundant evidence for other molecular events that can antedate acquisition of JAK2V617F and for leukemic transformations that involve JAK2-unmutated clones.28 It is therefore not a big surprise that currently available JAK inhibitors do not engender histologic, cytogenetic, or molecular remissions.58,59 Furthermore, considering the pathogenetic complexity of BCR-ABL1–negative MPN, I doubt that the situation will change much with the use of more JAK2V617F-specific drugs.
What then explains the palliative benefit of JAK inhibitors in the absence of drug-induced clonal suppression? JAK-STAT mediates both inflammatory cytokine activity and JAK2/MPL-driven myeloproliferation. It is currently assumed that certain aspects of the clinical phenotype in MF result from dysregulated cytokine expression and are therefore amenable to anticytokine therapy. This might partly explain the therapeutic activity of immune modulatory drugs and TNF inhibitors in MF.90-92 Accordingly, the broad cytokine-suppressive effect of ruxolitinib might constitute its primary mechanism of action, in terms of alleviating constitutional symptoms, cachexia, and splenic EMH in MF.58 However, cytokine abnormalities in MF are reactive in nature and are therefore probably correct with effective control of the underlying neoplastic process. Therefore, an alternative JAK inhibitor with more potent antimyeloproliferative activity should do more than simply treat the paraneoplastic effects of the disease.
Another important question regarding the therapeutic role of JAK inhibitors in MPN is whether or not disease modification is possible, considering their inability to induce selective clonal suppression. Based on recent information on the prognostic relevance of inflammatory cytokines and the host immune response in MF,39,40 it is conceivable for JAK inhibitors to favorably alter the disease microenvironment and promote clonal stability. The anti-inflammatory property of these drugs might also contribute to decreased mortality from comorbid conditions. However, such a possibility needs to be addressed in a properly designed, adequately powered, and stratified randomized study with best available therapy as the control arm. In other words, it cannot be inferred from a placebo-controlled study that was not risk-stratified at time of randomization and further confounded by a crossover design.72 Furthermore, the use of placebo versus a drug that is known to cause rapid and objective responses is unlikely to accomplish the intended objectives of a “double-blind” treatment design.
How do ruxolitinib and other JAK inhibitors fit into current MPN therapy? In general, the therapeutic role of currently available JAK inhibitors in PV or ET is dubious and is being promoted more for expanding the pharmaceutical market rather than for its scientific merits. A JAK1-mediated mechanism of action cannot be used to justify the use of a non-JAK2–selective JAK inhibitor in PV, where JAK2V617F-driven clonal myeloproliferation constitutes the predominant pathogenetic component. Furthermore, patients with PV or ET enjoy a median survival that is close to 20 years and disease complications are safely and effectively managed by hydroxyurea (first-line cytoreductive drug of choice) or interferon-α or busulfan (second-line cytoreductive drugs of choice).23,24
In the context of the aforementioned background, it is hardly rational for anyone to be excited about an alternative drug in PV or ET that lacks intrinsic disease-modifying effect. Incidentally, I find it comical to use spleen response or phlebotomy independence as primary endpoints for phase 3 studies in PV, considering the prognostic irrelevance of these parameters. It is to be recalled that anagrelide was FDA-approved for use in ET based on its platelet-lowering property,93,94 but the drug was later shown to have inferior antithrombotic value, compared with hydroxyurea, and to be more detrimental to patient outcome.4 Nevertheless, there are certain circumstances (eg, intractable pruritus, hydroxyurea-refractory symptomatic splenomegaly) where it is reasonable to prospectively evaluate the value of JAK inhibitor therapy in PV. In this regard, it makes more sense to choose a JAK2-selective drug, which might also be reasonable to consider in the setting of congenital polycythemia associated with dysregulated JAK2 activity.95
As for MF, Dynamic International Prognostic Scoring System-plus74 low or intermediate-1 risk patients may risk more harm (eg, anemia, thrombocytopenia) than benefit from receiving JAK inhibitor therapy (Figure 1). Life expectancy in such patients could exceed 15 years,96 and one must recognize the absence of long-term safety data for these drugs. In addition, patients with “very high risk” disease might be better served by pursuing allogenic stem cell transplantation sooner than later.96 On the other hand, it is reasonable to continue evaluating the therapeutic value of JAK inhibitors in high or intermediate-2 risk MF; and in this regard, I prefer drugs with prominent antimyeloproliferative as opposed to anticytokine activity.
In routine clinical practice, I strongly discourage the indiscriminate off-label use of ruxolitinib in non-MF patients (eg, patients with PV or ET) and in MF patients whose symptoms are not severe enough to justify the drug's short-term and unknown long-term ill effects. In other words, use of the drug where it is not indicated might make matters worse for the patient. In addition, patients and physicians must recognize the fact that ruxolitinib does not treat the disease but only its symptoms. These points should be clearly communicated to patients, who should also be advised that ruxolitinib is just the tip of the iceberg in JAK inhibitor therapeutics. In other words, we should not deprive our patients of the opportunity to participate in clinical trials involving possibly better drugs, especially in view of the fact that previous exposure to ruxolitinib might make them protocol ineligible.
Authorship
Contribution: A.T. designed the study, reviewed the literature, and wrote the manuscript.
Conflict-of-interest disclosure: The author has served as a principle or coinvestigator in a number of drug trials, including JAK inhibitors (ruxolitinib, SAR302503, CYT387, BMS911543) and others (pomalidomide).
Correspondence: Ayalew Tefferi, Division of Hematology, Department of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: tefferi.ayalew@mayo.edu.