Abstract
Based on small numbers, recent reports from 3 randomized trials have consistently demonstrated more hematologic malignancies in patients treated with lenalidomide as maintenance (vs placebo). This fact has prompted concern and highlighted the association between multiple myeloma and second malignancies. Furthermore, an excess of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) after multiple myeloma has been known for over 4 decades. Most prior studies have been restricted because of small numbers of patients, inadequate follow-up, and limitations of ascertainment of second malignancies. Although the underlying biologic mechanisms of AML/MDS after multiple myeloma are unknown, treatment-related factors are presumed to be responsible. Recently, an excess risk of AML/MDS was found among 5652 patients with IgG/IgA (but not IgM) monoclonal gammopathy of undetermined significance, supporting a role for disease-related factors. Furthermore, there is evidence to suggest that polymorphisms in germline genes may contribute to a person's susceptibility to subsequent cancers, whereas the potential influence of environmental and behavioral factors remains poorly understood. This review discusses current knowledge regarding second malignancies after multiple myeloma and gives future directions for efforts designed to characterize underlying biologic mechanisms, with the goal to maximize survival and minimize the risk for second malignancies for individual patients.
Introduction
After decades of virtually no progress, multiple myeloma survival has improved significantly in the last 10 years, in younger patients even 2- to 3-fold.1-3 Indeed, multiple myeloma has seen more remarkable progress in treatment and patient outcomes than any other cancer in the last decade. With improvements in survival, a relatively new clinical challenge that has emerged is the risk of second malignancies. This pattern of increase in second malignancies has been observed in other cancers with available curative therapies and favorable outcomes. Survivors of testicular cancer are at up to 3-fold higher risk of developing a second malignancy than the general population.4 Survivors of Hodgkin lymphoma have more than 3 times greater risk of solid tumors. Fifteen years after diagnosis, the cumulative mortality from second malignancies exceeds cumulative mortality from Hodgkin lymphoma.5,6 In the United States alone, the number of cancer survivors has tripled since 1971 and is growing by 2% each year; cancer survivors constitute 3.5% of the United States population.7 Indeed, second- or higher-order cancers account for 18% of incident cancers in the United States, making them the third most common cancer diagnosis.7 Based on the National Cancer Institute Surveillance, Epidemiology and End Results (NCI SEER) database, compared with the general population, cancer survivors have a 14% increased risk of developing a malignancy.7
In the late 1960s, based on a restricted number of patients, an association between multiple myeloma and leukemia was first reported.8-10 In 1979, based on a clinical trial, including 364 multiple myeloma patients, Bergsagel et al reported a greater than expected incidence of all forms of acute leukemia for patients treated with low-dose melphalan containing combinations of alkylating agents.11 In the era where low-dose melphalan was the mainstay of multiple myeloma therapy, because of poor overall survival rates, the absolute number of multiple myeloma patients at risk for acute leukemia was small. Although the use of low-dose melphalan declined substantially with the advent of high-dose melphalan followed by autologous stem cell transplantation (ASCT) in the late 1980s, melphalan-based combinations continue to be used in ASCT-ineligible patients.12 In the posttransplant era, several studies found that conventional chemotherapy preceding the transplant played a greater role in the development of myelodysplastic syndromes (MDSs) and acute leukemia than myeloablative therapy used in conjunction with ASCT. In the last decade, agents with new mechanisms of action (such as thalidomide, bortezomib, and lenalidomide), and continuing improvements in supportive care have further improved response rates, progression-free survival, and overall survival in multiple myeloma. Recent preliminary reports of increased risk of second malignancies, predominantly MDS/acute leukemia, with lenalidomide have further highlighted this challenge in multiple myeloma patients.
Larger population-based studies support and expand on findings from smaller clinical studies showing that multiple myeloma patients have an increased risk of developing MDS/acute leukemia compared with the general population.13-15 Based on the NCI SEER database, among 23 838 multiple myeloma diagnosed between 1973 and 2000, leukemia accounted for the largest cancer excesses, with acute myeloid leukemia (AML) constituting 80% of leukemia cases. Increased risks were also noted for Kaposi sarcoma and chronic myeloid leukemia.7 However, the overall risk of developing any type of a subsequent primary cancer was not increased. The increased risk of developing a new malignancy was limited to persons diagnosed with multiple myeloma at ages younger than 70 years; subsequent cancer risk did not differ by sex, race, or initial therapy. It is to be noted that NCI SEER database did not capture information on MDS until the introduction of International Classification of Diseases for Oncology, 3rd edition in 2001.16
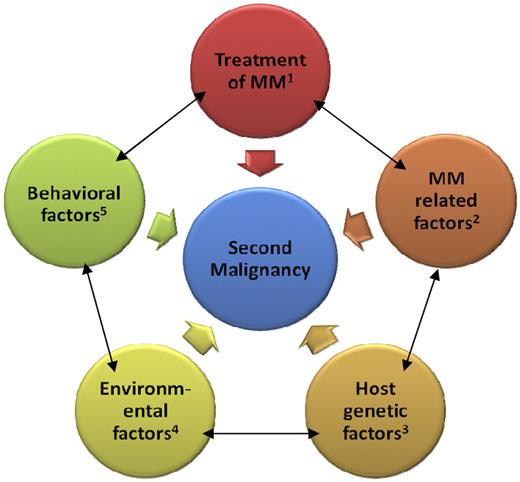
Overall, based on a restricted number of investigations, most prior studies implicate treatment-related factors as the main contributing factor to development of second malignancies after multiple myeloma. However, the lack of molecular markers that are specific for therapy-induced cancer and inability to compare different treatment durations in a clinical trial setting limits our ability to define the impact of prior therapy in the etiology of a second malignancy. Indeed, it seems reasonable to propose that second malignancies in multiple myeloma may not be attributable solely to prior treatment. Rather, the development of second malignancies may reflect combinations of influences, including treatment-related, multiple myeloma-related, host-related, environmental, and behavioral factors (Figure 1).17 In this article, we review and discuss our current understanding of second malignancies after multiple myeloma.
Proposed model of second malignancies after multiple myeloma. Examples for the categories include the following: (1) alkylating agents, immunomodulatory agents, autologous stem cell transplant, and radiation; (2) molecular subtypes of disease, biclonal disease, and bone marrow microenvironment; (3) polymorphisms in germline genes (eg, drug-metabolizing genes, erythropoietin promoter gene), chronic antigenic stimulation, and genetic susceptibility with other malignancies; (4) occupation, pesticides, and chlorinated solvents; and (5) tobacco, obesity, alcohol, and diet.
Proposed model of second malignancies after multiple myeloma. Examples for the categories include the following: (1) alkylating agents, immunomodulatory agents, autologous stem cell transplant, and radiation; (2) molecular subtypes of disease, biclonal disease, and bone marrow microenvironment; (3) polymorphisms in germline genes (eg, drug-metabolizing genes, erythropoietin promoter gene), chronic antigenic stimulation, and genetic susceptibility with other malignancies; (4) occupation, pesticides, and chlorinated solvents; and (5) tobacco, obesity, alcohol, and diet.
Treatment-related factors
The effects of treatment-related factors, including oral alkylating therapy on the development of malignancies after multiple myeloma, have been assessed (Table 1).11,13,14,18-22 Bergsagel et al conducted the first prospective clinical study evaluating the value of a combination of 3 alkylating agents in the treatment of multiple myeloma: melphalan, cyclophosphamide, and carmustine. In their study, the observed versus expected incidence of all forms of acute leukemia was increased for all age groups.11 Indeed, the patterns are quite similar to investigations focusing on non-Hodgkin lymphoma23 and Hodgkin lymphoma,24 showing MDS/acute leukemia to be associated with long-term alkylating therapy, and with a cumulative dose-response effect. Since these early observations, treatment-related factors, including melphalan, have been considered the main cause of excess of MDS/acute leukemia in multiple myeloma patients, although the biologic mechanisms were not well defined. In a subsequent study, Cuzick et al reported a positive association between the duration of melphalan treatment and the subsequent risk of developing leukemias.22 In that study, the cumulative dose of melphalan given up to 3 years before leukemia diagnosis was reported to be the most important determinant of risk. However, this association has not held true in all studies. For example, a retrospective cohort study from the Finnish Leukemia Group found no significant association between the duration and cumulative doses of melphalan and AML risk subsequent to multiple myeloma.13 In addition, in another study, cyclophosphamide was found to be less leukemogenic than melphalan.22,24
Selected studies focusing on second malignancies after multiple myeloma
| Reference . | Study design (study period) . | No. of patients . | Any second malignancy, % . | Multiple myeloma to second malignancy, median time . | Hematologic malignancy, n (%) . | Solid tumor, n (%) . |
|---|---|---|---|---|---|---|
| 15 | Population-based registry study (1986-2005) | 8740 | 6.6 | 45.3 mo (AML/MDS) | 69 (0.8) | 508 (5.8) |
| 18 | Retrospective study, single institution (1997-2008) | 589 | 3 | 35 mo | 6 (1.0) | 12 (2.0) |
| 30* | Randomized phase 3 trial, maintenance lenalidomide vs placebo after high-dose melphalan/ASCT | 614 | 5.5 (lenalidomide maintenance); 1 (placebo) | 44 mo | Lenalidomide maintenance: † 11 (1.8); placebo arm:† 3 (0.5) | Lenalidomide maintenance:† 12 (2.0); placebo arm:† 3 (0.5) |
| 31* | Randomized phase 3 trial, maintenance lenalidomide vs placebo after high-dose melphalan/ASCT | 460 | 6.5 (lenalidomide maintenance); 2.6 (placebo) | 17.5 mo after ASCT | Lenalidomide maintenance:† 8 (1.7); placebo arm:† 0 (0) | Lenalidomide maintenance:† 10 (2.2); placebo arm:† 4 (0.9) |
| 32* | Randomized phase 3 trial, maintenance lenalidomide vs placebo after low-dose melphalan/prednisone with or without lenalidomide | 459 | 3.9 (lenalidomide maintenance); 1.3 (placebo) | 25 mo | MPR-R arm:† 7 (1.5); MPR arm:† 5 (1.1); MP arm:† 1 (0.2) | MPR-R: 5 (1.1);† MPR:† 4 (0.9); MP:† 3 (0.7) |
| 19 | Retrospective study, single institution (1989-2007) | 2418 | 1.1 | NR | 26 (1.1) | NR |
| 20 | Retrospective study, single institution (1996-2005) | 82 | 12.2 | 50 mo | 10 (12.2) | NR |
| 14 | Population-based registry study (1958-1996) | 8656 | 5.5 | 2.9 y | 83 (1.0) | 392 (4.5) |
| 13 | Retrospective study based on patients from clinical trials (1979-1985) | 432 | 9.2 | 37 mo (solid tumors) 56 mo (acute leukemia) | 17 (3.9) | 23 (5.3) |
| 21 | Prospective study (NR) | 188 | 3.8 | 63 mo | 7 (3.8) | NR |
| 22 | Retrospective study based on patients from clinical trials (1964-1975) | 648 | 1.9 | 82 mo | 12 (1.9) | NR |
| 11 | Prospective study (1973-1977) | 364 | 3.8 | NR | 14 (3.8) | NR |
| 10 | Case series (1965-1966) | 3 | NA | 45 mo | 3 (NA) | NR |
| 71 | Case series (1950-1966) | 6 | NA | 10 y | 1 (NA) | NR |
| 8 | Retrospective study, multi-institution (1932-1963) | 310 | 2.3 | NR | 7 (2.3) | NR |
| Reference . | Study design (study period) . | No. of patients . | Any second malignancy, % . | Multiple myeloma to second malignancy, median time . | Hematologic malignancy, n (%) . | Solid tumor, n (%) . |
|---|---|---|---|---|---|---|
| 15 | Population-based registry study (1986-2005) | 8740 | 6.6 | 45.3 mo (AML/MDS) | 69 (0.8) | 508 (5.8) |
| 18 | Retrospective study, single institution (1997-2008) | 589 | 3 | 35 mo | 6 (1.0) | 12 (2.0) |
| 30* | Randomized phase 3 trial, maintenance lenalidomide vs placebo after high-dose melphalan/ASCT | 614 | 5.5 (lenalidomide maintenance); 1 (placebo) | 44 mo | Lenalidomide maintenance: † 11 (1.8); placebo arm:† 3 (0.5) | Lenalidomide maintenance:† 12 (2.0); placebo arm:† 3 (0.5) |
| 31* | Randomized phase 3 trial, maintenance lenalidomide vs placebo after high-dose melphalan/ASCT | 460 | 6.5 (lenalidomide maintenance); 2.6 (placebo) | 17.5 mo after ASCT | Lenalidomide maintenance:† 8 (1.7); placebo arm:† 0 (0) | Lenalidomide maintenance:† 10 (2.2); placebo arm:† 4 (0.9) |
| 32* | Randomized phase 3 trial, maintenance lenalidomide vs placebo after low-dose melphalan/prednisone with or without lenalidomide | 459 | 3.9 (lenalidomide maintenance); 1.3 (placebo) | 25 mo | MPR-R arm:† 7 (1.5); MPR arm:† 5 (1.1); MP arm:† 1 (0.2) | MPR-R: 5 (1.1);† MPR:† 4 (0.9); MP:† 3 (0.7) |
| 19 | Retrospective study, single institution (1989-2007) | 2418 | 1.1 | NR | 26 (1.1) | NR |
| 20 | Retrospective study, single institution (1996-2005) | 82 | 12.2 | 50 mo | 10 (12.2) | NR |
| 14 | Population-based registry study (1958-1996) | 8656 | 5.5 | 2.9 y | 83 (1.0) | 392 (4.5) |
| 13 | Retrospective study based on patients from clinical trials (1979-1985) | 432 | 9.2 | 37 mo (solid tumors) 56 mo (acute leukemia) | 17 (3.9) | 23 (5.3) |
| 21 | Prospective study (NR) | 188 | 3.8 | 63 mo | 7 (3.8) | NR |
| 22 | Retrospective study based on patients from clinical trials (1964-1975) | 648 | 1.9 | 82 mo | 12 (1.9) | NR |
| 11 | Prospective study (1973-1977) | 364 | 3.8 | NR | 14 (3.8) | NR |
| 10 | Case series (1965-1966) | 3 | NA | 45 mo | 3 (NA) | NR |
| 71 | Case series (1950-1966) | 6 | NA | 10 y | 1 (NA) | NR |
| 8 | Retrospective study, multi-institution (1932-1963) | 310 | 2.3 | NR | 7 (2.3) | NR |
MPR-R indicates melphalan/prednisone, revlimid (lenalidomide), with revlimid maintenance; MPR, melphalan/prednisone, revlimid (lenalidomide), without revlimid maintenance; MP, melphalan/prednisone, without revlimid maintenance; NR, not reported; and NA, not applicable.
These results come from interim analyses presented at the American Society of Hematology meeting, Orlando, FL, December 4-7, 2010.
Updated numbers from presentations at the International Myeloma Workshop in Paris, France, May 3-6, 2011. At this time, the final analyses and written reports have not yet been published.
After the introduction of high-dose melphalan/ASCT, several studies addressed the relative contribution of myeloablative therapy used in conjunction with ASCT and conventional chemotherapy preceding the transplant toward development of MDS/AML. Govindarajan et al21 compared 2 groups of patients with different exposure to alkylating agents preceding transplant. Group 1 had received no more than 1 cycle of standard alkylating therapy and group 2 had significantly prolonged exposure to chemotherapy, including alkylators before transplant. Both groups were treated with 1 course of high-dose cyclophosphamide (CTX) to mobilize stem cells followed by 2 courses of high-dose melphalan with autologous stem cell support. Despite a longer follow-up (36 months vs 29 months; P = .05), none of the patients in group 1 developed MDS, compared with 7 patients in group 2.21 Other studies also demonstrated that conventional chemotherapy before ASCT is a more likely contributing factor of MDS/acute leukemia, rather than pretransplant myeloablative therapy, maintenance therapy, or additional treatment after transplantation.19 Furthermore, a recent population-based study, based on 8740 myeloma patients diagnosed in Sweden (1986-2005), found the rates of MDS/AML before and after introduction of high-dose melphalan/ASCT to be very similar, further supporting that the introduction of high-dose melphalan as pretransplant myeloablative therapy has not increased the risk of subsequent MDS/AML, beyond that of lower doses of melphalan.15
Radiotherapy may also have a potential role in development of second malignancies after multiple myeloma. Indeed, approximately 40% of patients with multiple myeloma may require treatment with radiotherapy at some time during their illness.25 Studies focusing on Hodgkin lymphoma and breast cancer have found an increased risk of second malignancies after radiotherapy, with a dose-response relationship between risk of second malignancy and radiation dose to the surrounding tissues, including the bone marrow.17,26,27 For example, malignancies associated with locoregional radiation for breast cancer include sarcomas, lung and esophageal cancers, and AML.27-29 At this time, to our knowledge, there is limited information on the association between radiotherapy and risk of subsequent malignancies in multiple myeloma.
Maintenance therapy has been evaluated in relation to risk of second malignancies in 3 recently reported multicenter randomized phase 3 trials (IFM 2005-02, CALGB 100104, and MM-015)30-32 (Table 1). IFM 2005-02 and CALGB 100104 explored the role of lenalidomide maintenance therapy after high-dose melphalan/ASCT.30,31 In both trials, lenalidomide at a dose of 10 to 15 mg given within 3 to 6 months of autologous transplantation was compared with placebo until disease progression. Unlike CALGB 100104, patients in the IFM trial received lenalidomide induction for 2 months before maintenance dosing, had a longer follow-up, and no crossover was permitted to the lenalidomide arm at progression.32 In the IFM 2005-02 and CALGB 100104 trials, 5.5% and 6.5% of lenalidomide-treated patients developed second malignancies compared with 1% and 2.5% in the respective control arms. The second malignancies reported include AML/MDS, Hodgkin lymphoma, B-cell acute lymphoblastic leukemia, colon, prostate, breast, and esophageal cancers. MM-015 evaluated maintenance lenalidomide after combination of lenalidomide with melphalan and prednisone (MP) versus fixed MP duration regimens in 65-year-old (or older) transplant-ineligible patients with newly diagnosed multiple myeloma.32 This study also found an increase in the number of second cancers in lenalidomide-treated patients, notably AML, which was associated with complex baseline cytogenetics: 2 cases each were observed in the lenalidomide-treated arms and none in the MP arm (0.7% vs 0%). The standardized incidence ratio for AML in the MP-lenalidomide followed by lenalidomide maintenance and placebo maintenance was 4.46 and 4.65, respectively, compared with the NCI SEER database.32 At this time, IFM 2005-02 and MM-015 have demonstrated a progression-free survival benefit, although there was no improvement in overall survival for patients who received lenalidomide. CALGB 100104 demonstrated an overall survival improvement in lenalidomide-treated patients with an overall survival rate at 23 months of 90% in the continuous lenalidomide arm compared with 83% in the placebo arm (P < .018), despite 80% of patients crossing over to receive continuous lenalidomide.31 Maintenance lenalidomide was discontinued in the IFM 2005-02 trial, whereas patients on the other trials continue to receive lenalidomide with enhanced monitoring while an ongoing safety review is completed.35
Among patients with relapsed/refractory multiple myeloma, 2 retrospective studies have evaluated the role of lenalidomide in relation to the risk of second malignancies.33,34 Based on 230 relapsed/refractory multiple myeloma patients treated with lenalidomide-based regimens, Reece and Goswami found MDS/AML in 2.6% (6 patients) at a median of 76 months from the time of diagnosis of multiple myeloma and 61 months from the time of initiation of lenalidomide.33 Although the prior exposure to alkylating agents was similar in both groups, patients who developed AML/MDS were older (median, 68 years; range, 54-76 years; vs median, 61 years; range, 32-80 years), less likely to have had high-dose melphalan/ASCT (2, 33%; vs 149, 82%) and had longer duration of treatment with lenalidomide (median number of cycles, 21; range, 9-35; vs median number of cycles, 9; range, 1-50.33 A posthoc analysis of pooled data from phase 3 MM-009 and MM-010 trials revealed 2 MDS, 8 solid tumors, and no leukemias. Using NCI SEER data, no increase in incidence of solid tumors was noted compared with the general population.34
In parallel with the aforementioned studies reporting on lenalidomide maintenance and excess MDS/AML development in multiple myeloma, other investigations have been evaluating the role of lenalidomide treatment in the setting of MDS. For example, a recent study reported that lenalidomide used as treatment for 5q− MDS was not associated with AML progression.35
Taken together, mostly based on small numbers, prior studies have found various types of therapies (eg, oral alkylating therapy, myeloablative therapy used in conjunction with ASCT, radiotherapy, and lenalidomide) to be associated with an excess of second malignancies after multiple myeloma. Yet the exact underlying mechanisms remain to be determined, and several studies to elucidate the underlying mechanisms are ongoing.
Multiple myeloma-related factors
Although presenting with the same histologic picture, multiple myeloma displays a broad molecular range characterized by subgroups with unique gene expression profiles, which correlate with clinical characteristics and patient survival. Moreover, additional molecular events, including epigenetic changes and activation of molecular pathways, occur during multiple myeloma progression and treatment.36,37
In a recent population-based study from Sweden, based on 5652 patients with multiple myeloma precursor disease, monoclonal gammopathy of uncertain significance (MGUS), an 8-fold increased risk of developing MDS/AML was observed.15 The elevated risk was confined to those with IgG/IgA (and not IgM) MGUS. Interestingly, MGUS patients with M-protein concentrations more than 1.5 g/dL (standardized incidence ratio = 11.12) had higher risk than those with less than or equal to 1.5 g/dL (standardized incidence ratio = 4.67), suggesting that more active precursor disease has similar baseline risk for AML/MDS to that of active multiple myeloma.15 Overall, these observations are important in that they support a role for disease-related factors in MDS/AML after multiple myeloma and raise the question whether underlying molecular heterogeneities in multiple myeloma may be related to the risk of developing second malignancies. It is possible that certain molecular multiple myeloma subgroups are at a higher risk than others. For example, a potential mechanism could be selective pressure (ie, a pre-existing nondominant clone, unresponsive to treatment) leading to an increased susceptibility to developing second malignancies. A better understanding of underlying molecular mechanisms across multiple myeloma subgroups and risk of second malignancy will form the basis for modification and targeting therapies to specific subgroups, with the overall goal to minimize the risk of second malignancies.
Host-related factors
Although we lack large well-designed studies at this time, based on work done on other cancer types, it seems reasonable to propose that host-related (including both genetic and nongenetic) factors may play a role in the development of second malignancies after multiple myeloma. Indeed, it has been estimated that genetic variations can account for up to 95% of variability in drug disposition and effects.38 In addition to drug disposition and response to treatment, polymorphisms in genes encoding drug-metabolizing enzymes, DNA repair pathways, drug transporters, and targets may also contribute to a person's susceptibility for subsequent malignancies as well.17,39 For example, decreased production of glutathione S-transferase enzymes, GSTM1 and GSTT1, results from polymorphisms of respective genes, which may be associated with an increased MDS risk in the presence of environmental mutagens and/or carcinogen exposure.40 Similarly, polymorphisms in genes that regulate cellular responses to DNA damage can affect the risk of developing MDS/AML, presumably by influencing the survival of hematopoietic cells with proleukemogenic mutations.41 Nongenetic host factors, which can modulate treatment effects, include age, race, organ function, concomitant therapy, drug interactions, and myeloma itself.
Two studies (n = 2418 and 82) observed that patients who eventually develop MDS or MDS-associated cytogenetic abnormalities have a lower CD34 yield at collection, suggesting a preexisting marrow abnormality probably a result of host or host-myeloma interaction.11,20 Similar observations have been reported in Hodgkin lymphoma and non-Hodgkin lymphoma, where cytogenetic abnormalities observed at the diagnosis of MDS/AML were already present in the morphologically normal pretransplant bone marrow.42,43 Furthermore, the bone marrow microenvironment may be important in the pathogenesis of MDS/AML. MGUS and multiple myeloma are dependent on mutual interactions with cells and extracellular components of the bone marrow for survival and growth. Interactions of multiple myeloma cells with the bone marrow microenvironment activate a pleiotropic proliferative and antiapoptotic cascade, including the NFκB signaling pathway resulting in multiple myeloma cell growth, survival, drug resistance, and migration. Moreover, many of the growth factors secreted by multiple myeloma and bone marrow stromal cells stimulate osteoclastogenesis and angiogenesis.36 It is conceivable that the resultant changes in bone marrow microenvironment may play a role in development of MDS/AML after multiple myeloma. Chromosome 5 abnormalities and clinical phenotype consistent with 5q− syndrome have been described in some patients with lenalidomide-associated MDS.33,44 5q− syndrome is a disorder of the human hematopoietic stem cell with a combined lympho-myeloid potential and is known to represent an early event in MDS pathogenesis. Lenalidomide is approved for use in selected patients with 5q− with or without additional cytogenetic abnormalities. Rare and phenotypically distinct 5q− hematopoietic stem cells that are selectively resistant to lenalidomide have been identified in MDS patients during complete clinical and cytogenetic remission.45,46 It is plausible that a subclone of lenalidomide-resistant hematopoietic stem cells may expand during treatment, resulting in MDS/AML. 5q− has also been described as part of a complex karyotype in secondary leukemias.47,48
Recently, we found the G/G phenotype of single nucleotide polymorphism rs1617640 in the erythropoietin promoter gene, which is associated with decreased erythropoietin expression, to be more common (27% vs 12%) in multiple myeloma patients who developed MDS compared with patients who did not.49 This suggests a role for susceptibility genes in the development of second malignancies after multiple myeloma. These results need to be confirmed in larger studies on a wider panel of genes.
To better understand the role of host genetics in defining susceptibility to second malignancies, it is important to identify susceptibility loci and alleles and establish how these interact with exposure to affect cellular response to therapeutic exposures and the subsequent risk of disease.41 Genome-wide association studies and gene expression microarray analysis of groups of patients with and without second malignancies have identified several candidate single nucleotide polymorphisms, which are associated with acute leukemia after other malignancies.50-52 Identifying patients at risk for second malignancies at the time of diagnosis of multiple myeloma would enable personalizing treatment and posttherapy surveillance options to minimize this risk.
Environmental factors
Several proposed environmental risk factors are shared between multiple myeloma and second malignancies. For cancers that share etiologic factors with multiple myeloma, the pertinent genetic traits will probably have low to moderate penetrance and be driven by multiple gene-environment and gene-gene interactions.17 For example, some, but not all, prior studies indicate that exposure to ionizing radiation, especially at younger ages and at higher doses, increases the risk of developing multiple myeloma and MGUS in addition to leukemias, MDS, and solid tumors.53-57 In addition, prior studies have suggested that exposure to chlorinated solvents is associated with development of non-Hodgkin lymphoma, leukemia, and multiple myeloma.58,59 Chronic antigen stimulation from prior autoimmune, infectious, inflammatory, allergic disorders and immune dysregulation may play a role in pathogenesis of both multiple myeloma and AML/MDS.60-62 Recently, solid organ transplant patients receiving immunosuppressive therapy have been reported to be at risk for the development of AML.63 In addition, socioeconomic status has been shown to influence survival in both multiple myeloma and AML, suggesting that lifestyle factors in these disorders are of importance.64
Behavioral factors
Tobacco use and alcohol intake are causally related to multiple primary cancers. Multiple myeloma may share behavioral risk factors with other malignancies and multiple myeloma survivors exposed to these risk factors at a higher risk of subsequent malignancies. Interestingly, the commonly proposed behavioral risk factors (eg, tobacco, alcohol, and diet) for various types of cancers have not been associated with multiple myeloma.65,66 Nevertheless, obesity has been associated with an increased risk for both multiple myeloma and MGUS,67,68 and a slightly decreased risk for multiple myeloma has been reported to be associated with the consumption of cruciferous vegetable and fish.69
Discussion
Despite being known for several decades, accurate estimates of incidence and pathogenesis of second malignancies after multiple myeloma are lacking. Current literature focusing on second malignancies after multiple myeloma is limited and should be interpreted with caution. For example, most prior studies are restricted because of small numbers of patients, inadequate follow-up, and limitations of ascertainment of second malignancies (Table 2). Largely because of insufficient data and a small number of studies, most of our current understanding of malignancies after multiple myeloma is modeled on experiences with other malignancies, such as Hodgkin lymphoma, and emphasizes the role of treatment.11,19-22 Based on current knowledge, it seems reasonable to propose that the development of second malignancies after multiple myeloma, is most likely a multifactorial process. Contributing factors probably include various multiple myeloma treatments, multiple myeloma-related factors, host-related factors, as well as environmental and behavioral factors (Figure 1). Early works in this area and subsequent efforts have focused on the role of treatment-related factors, such as alkylating agents.11,19-22 Because of the insufficient data, the role of non–treatment-related factors remain largely unexplored. For example, based on small numbers of patients, there are indications that host genetic polymorphisms may play a role in pathogenesis of second malignancies.49 In addition, recent population-based data suggest that IgG/IgA MGUS patients may also be at an increased risk for AML/MDS.15 These results support a role for host- and disease-related factors and, if validated in larger studies, they set the stage for future investigations designed to define underlying molecular mechanisms. Other non–treatment-related factors, such as environment and behavior, are also not well understood.
Selected study-related factors that may bias the estimated risk of second malignancies after multiple myeloma
| Short-term follow-up |
| Small sample sizes |
| Combinations and interactions between multiple drugs |
| Inadequate control group |
| Retrospective data collection |
| Under-reporting by clinicians |
| Survival difference (person-years) between experimental and surveillance arms |
| Short-term follow-up |
| Small sample sizes |
| Combinations and interactions between multiple drugs |
| Inadequate control group |
| Retrospective data collection |
| Under-reporting by clinicians |
| Survival difference (person-years) between experimental and surveillance arms |
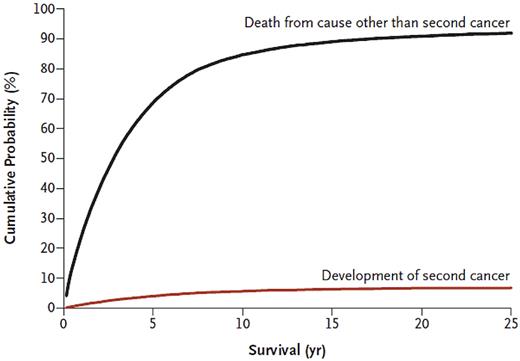
Based on small numbers, recent reports from 3 randomized trials have consistently demonstrated more hematologic malignancies in patients treated with lenalidomide as maintenance (vs placebo).30-32 Further studies are needed to better characterize underlying mechanisms of these observations. Beyond the underlying biology, the clinical implications of excess of second malignancies in multiple myeloma patients who receive lenalidomide need to be interpreted in the context of competing risks. On a clinical note, for most patients, multiple myeloma still remains an incurable malignancy and, on average, the general risk of dying is substantially higher than the risk of developing a second cancer (Figure 2).70 That being said, although numbers are small, for individual patients who do develop AML/MDS after multiple myeloma, the outcomes are devastating. These 2 parallel perspectives (“on average” vs “individual patients”) highlight the complexity of clinical medicine in the era of modern therapy and correlative science. Furthermore, progression-free survival was significantly prolonged in all 3 studies of lenalidomide maintenance; and in one of these, the CALGB 100104 trial of lenalidomide maintenance after high-dose melphalan/ASCT, there was also a significant overall survival benefit. In summary, these facts are tightly intertwined, and there are multiple aspects to consider. Although there are few clear answers available at this time, in our opinion, clinicians need to discuss the risks and benefits with patients and stay updated as more data become available. Until we have access to better knowledge, in our opinion, in circumstances where the benefit of maintenance therapy in terms of overall survival is not well established, the risks versus any possible benefit should be taken more cautiously.
Cumulative incidence of developing a second cancer and cumulative probability of death because of competing causes, after multiple myeloma. Data, which are based on 33 229 patients who received a diagnosis of multiple myeloma between 1973 and 2008 in the United States, are from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Reprinted from Landgren et al with permission.70
Cumulative incidence of developing a second cancer and cumulative probability of death because of competing causes, after multiple myeloma. Data, which are based on 33 229 patients who received a diagnosis of multiple myeloma between 1973 and 2008 in the United States, are from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Reprinted from Landgren et al with permission.70
Future directions
In the context of increasing overall survival in multiple myeloma and the recently reported increase of second malignancies associated with use of lenalidomide,30-32 it is imperative that we readdress the association between multiple myeloma and leukemia, which was first reported in the late 1960s.8-10 Importantly, because of inherent problems related to the small number of cases, collaborative efforts are needed to better characterize molecular features of patients who develop second malignancies after multiple myeloma. Such efforts would allow us to better define the role of treatment- and non-treatment–related factors, and how they may influence each other. Ultimately, we could use such knowledge to identify high-risk and low-risk patients, and to tailor therapy, with the goal to maximize survival and minimize the risk for second malignancies for the individual patient.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: A.T. and O.L. drafted the manuscript; and all authors were involved in the interpretation of the data and reviewed and approved the submitted version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ola Landgren, Multiple Myeloma Section, Metabolism Branch, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Bldg 10, Room 13N240, Bethesda, MD 20892; e-mail: landgreo@mail.nih.gov.